Abstract
The ability to learn, remember, and respond to emotional events is a powerful survival strategy. However, dysregulated behavioral and physiological responses to these memories are maladaptive. To fully understand learned fear and the pathologies that arise during response malfunction we must reveal the environmental variables that influence learned fear responses. Light, a ubiquitous environmental feature, modulates cognition and anxiety. We hypothesized that light modulates responses to learned fear. Using tone-cued fear conditioning, we found that light enhances behavioral responses to learned fear in C57BL/6J mice. Mice in light freeze more in response to a conditioned cue than mice in darkness. The absence of significant freezing during a 2-wk habituation period and during intertrial intervals indicated that light specifically modulates freezing to the learned acoustic cue rather than the context of the experimental chamber. Repeating our assay in two photoreceptor mutant models, Pde6brd1/rd1 and Opn4−/− mice, revealed that light-dependent enhancement of conditioned fear is driven primarily by the rods and/or cones. By repeating our protocol with an altered lighting regimen, we found that lighting conditions acutely modulate responses when altered between conditioning and testing. This is manifested either as an enhancement of freezing when light is added during testing or as a depression of freezing when light is removed during testing. Acute enhancement, but not depression, requires both rod/cone- and melanopsin-dependent photoreception. Our results demonstrate a modulation by light of behavioral responses to learned fear.
Keywords: learning, Pavlovian, posttraumatic stress disorder, retina, retinal ganglion cell
Light is a pervasive feature of the environment and exerts broad effects on behavior and physiology via two parallel pathways (1). The familiar image-forming visual pathway allows discernment of objects in the environment according to physical qualities: their color, form, texture, and motion. The parallel non-image forming (NIF) pathway enables light to exert numerous effects on physiology and behavior independently of image formation, such as pupil constriction, modulation of heart rate, and the synchronization of circadian rhythms and sleep–wake cycles to the daily light–dark cycle (2). In addition to the effects of light on basic physiological functions, light also modulates higher-order cognitive processes, including anxiety, mood, and alertness/awakeness (3, 4). The retina, the sole photosensory organ in mammals, projects directly to brain regions involved in emotional responses. Among these are the amygdala, the bed nucleus of the stria terminalis, and the periaqueductal gray (5). Activity in some of these regions is known to be acutely modulated by light in a wavelength-dependent manner (3, 6), whereas the link between photoreception and function in other retino-recipient emotional processing areas remains to be elucidated.
Brain sites involved in emotional processing participate in the critical function of learning and remembering emotionally arousing events. This function enables an organism to deal effectively and efficiently with similar situations when they arise again. Although fear of learned stimuli can be advantageous, disproportionate fear can lead to pathological states in humans. In fact, an estimated 40 million Americans over the age of 18 y suffer from an anxiety disorder, a hallmark of which is dysregulation of fear (7). A complete understanding of fear and fear-related pathologies requires an accounting of environmental factors that modulate fear responses.
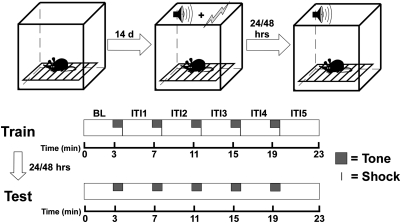
On the basis of the known role of light in modulating cognitive function and the suggestive anatomical evidence, we hypothesized that light may influence learning, memory, and the expression of fear. To determine whether light has an effect on learning, remembering, and responding to learned emotionally arousing events, we used a well-established assay for the assessment of learning and memory, tone-cued fear conditioning (Fig. 1). Tone-cued fear conditioning consists of repeated presentations of a tone (the conditioned stimulus), paired with a mild shock (the unconditioned stimulus). After several presentations of the tone–shock pair, the subject learns to associate the shock with the tone and will subsequently respond to presentations of the tone alone as though the shock were imminent (8). In rodents, the response is a complete cessation of locomotor activity, termed “freezing” (9). Freezing is robust and readily quantifiable. The percentage of time spent freezing during the tone presentation is a reliable indicator of the strength of the association that the subject has formed between the tone and shock, or, in more general terms, of the subject's learning and memory.
Fig. 1.
Tone-cued fear conditioning protocol. Mice were preexposed to the conditioning chamber for 30 min per day for 14 d before conditioning. On the day of conditioning (Train) activity was recorded for a 3-min baseline period (BL). At 3 min, the first of five tone–shock pairs was presented. The tone–shock pair consisted of a 60-s tone, the final 2 s of which were paired with a shock. Each tone–shock pair was separated by a 3-min intertrial interval (ITI). A fifth ITI followed the final tone–shock pair. Twenty-four hours and 48 h after testing mice were returned to the conditioning chamber and monitored during a tone-only test (Test). The tone-only test was identical to the tone–shock conditioning, except that no shock was paired with the tone.
Responses to light in mammals are mediated by the three photoreceptor classes of the retina: the rods, the cones, and the intrinsically photosensitive retinal ganglion cells (ipRGCs) (1). The rods and cones are the primary photoreceptors for image formation, whereas the ipRGCs are implicated primarily in NIF responses. To identify the retinal photoreceptors mediating observed light modulation of fear responses, we performed our assay in three lines of mice all on the C57BL/6J strain: wild-type (WT) mice, which have the full complement of photoreceptors; Pde6brd1/rd1 mice, which lack functional rods and cones (10) but retain intrinsic ipRGC photoresponses; and Opn4−/− mice, which have rods and cones but lack intrinsic ipRGC photoresponses owing to loss of the photopigment melanopsin (11).
Here, using a tone-cued fear conditioning assay, we show that light does indeed modulate behavioral responses to conditioned fear stimuli. In mice light causes an increase in the percentage of time spent freezing to a conditioned fear stimulus. This enhancement is specific to responses to the learned stimulus. Furthermore, using several retinal mutant mouse models we demonstrate that the rods and/or cones are the dominant photoreceptors driving light enhancement of conditioned fear. Finally, by repeating our fear conditioning assay with an altered lighting regimen we show that light or darkness can acutely modulate responses to a conditioned fear stimulus previously acquired under a different lighting condition. This work has far-reaching implications for the treatment of fear and anxiety disorders and for practical applications for the modulation of learning and memory.
Results
Light Enhances Behavioral Responses to Conditioned Fear.
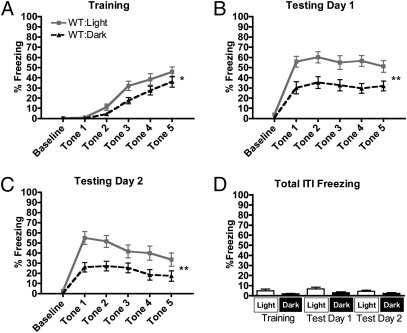
We initially performed our experiments in both light and darkness in WT C57BL/6J mice, which have normal retinas. Before conditioning, mice were preexposed to the fear conditioning chamber for 30 min each day for 2 wk (Fig. 1). This 2-wk latent inhibition period was sufficient to minimize contextual fear associations, as discussed below. During conditioning (Training) there was an increase in freezing in WT mice in both light and darkness across the session, revealed by a main effect of trial, indicating successful conditioning to the tone (Fig. 2A) [F(4,128) = 83.74, P < 0.0001]. Freezing during conditioning was enhanced in WT mice in light, as revealed by a main effect of light [F(1,128) = 4.16, P = 0.0498]. This light-dependent increase in freezing did not vary across trial number, as shown by the absence of a significant light × trial interaction [F(4,128) = 1.88, P = 0.1173]. The enhancing effect of light persisted over 2 subsequent days of testing, as revealed by a main effect of light on testing day 1 (Fig. 2B) [F(1,128) = 11.55, P = 0.0018] and testing day 2 (Fig. 2C) [F(1,128) = 8.09, P = 0.0077]. Extinction was minimal in both groups on testing day 1, as shown by the absence of a main effect of trial on freezing [F(4,128) = 1.36, P = 0.2519]. Extinction was observed on testing day 2, however, revealed by a main effect of trial [F(4,128) = 15.99, P < 0.0001]. Interestingly, the rate of extinction on testing day 2 was influenced by lighting conditions, revealed by a light × trial interaction [F(4,128) = 2.90, P = 0.0245]. Our results therefore demonstrate that light can indeed modulate behavioral responses to learned fear in WT mice.
Fig. 2.
Light enhances conditioned fear responses in C57BL/6J WT mice. (A–C) In WT mice, light significantly enhances freezing to a conditioned cue during both conditioning (A) and 2 subsequent days of testing (B and C) (n = 17 in light, n = 17 in dark). (D) Freezing during ITIs was not significantly different between light and dark groups on any day, indicating that contextual fear associations were minimized. In A–C evaluations are repeated-measures ANOVA, and in D evaluations are t tests, with P < 0.05 considered significant. *P < 0.05, **P < 0.01. Data are presented as average percentage freezing ± SEM.
To support the conclusion that light is the exclusive driver of the observed modulation of freezing, we repeated our experiment with a less intense light source (Dim Light; Materials and Methods). Dim light was not sufficient to enhance freezing levels relative to levels observed in darkness on any day of the protocol, as shown by an absence of a main effect of light on the day of conditioning [F(1,104) = 1.95, P = 0.1741], testing day 1 [F(1,104) = 0.22, P = 0.6396], and testing day 2 [F(1,104) = 0.15, P = 0.6983] (Fig. S1). Conversely, our standard light was sufficient to enhance freezing relative to dim light on all days of the protocol, as shown by a main effect of light during conditioning [F(1,104) = 8.15, P = 0.0084], testing day 1 [F(1,104) = 5.41, P = 0.0281], and testing day 2 [F(1,104) = 4.42, P = 0.0453] (Fig. S1). These data suggest that there is a threshold intensity for the enhancing effect of light that lies between the intensities tested here.
Rods and/or Cones Are the Dominant Photoreceptors for Driving Light Enhancement of Conditioned Fear.
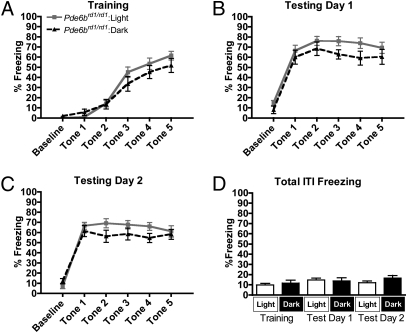
To determine the contributions of the rods, cones, and ipRGCs to the observed light-dependent enhancement of learned fear, we performed our fear conditioning assay in two mutant mouse lines: Pde6brd1/rd1 mice, which lack functional rods and cones beyond 4 wk of age (rodless-coneless) (10); and Opn4−/− mice, which have functional rods and cones but lack melanopsin and therefore lack intrinsic ipRGC photoresponses (melanopsin-knockout) (11). During conditioning, Pde6brd1/rd1 mice in both light (n = 14) and darkness (n = 10) successfully acquired a fearful association with the tone, indicated by a main effect of trial on freezing (Fig. 3A) [F(4, 88) = 86.69, P < 0.0001]. However, no main effect of light on freezing was observed during conditioning [F(1,88) = 0.87, P = 0.3610], nor was a main effect of light on freezing observed during testing day 1 (Fig. 3B) [F(1,88) = 2.11, P = 0.1605] or testing day 2 (Fig. 3C) [F(1,88) = 1.76, P = 0.1984]. Although rodless-coneless mice in light tended to freeze more than their counterparts in darkness during both conditioning and testing, the effect was not statistically significant during any portion of the protocol. The lack of an effect of light on freezing in Pde6brd1/rd1 mice indicates that the rods and/or cones play a critical role in the normal enhancing effect of light on learned fear. It should be noted that the lack of an observed difference in these mice is not due to saturated responses (i.e., a “ceiling effect”). When subjected to a more intense training protocol (0.75-mA foot shock under “bright light” conditions), Pde6brd1/rd1 mice exhibit significantly enhanced freezing during the testing phase, relative to Pde6brd1/rd1 mice conditioned with a 0.40-mA foot shock in darkness [F(1,48) = 6.48, P = 0.0257] (Fig. S2). The observation that freezing can be driven significantly higher in these mice indicates that the responses to our standard experimental paradigm are not saturated.
Fig. 3.
Light does not significantly enhance fear responses in Pde6brd1/rd1 mice. (A–C) In Pde6brd1/rd1 mice, light does not significantly enhance freezing to a conditioned cue during conditioning (A) or 2 subsequent days of testing (B and C) (n = 14 in light, n = 10 in dark). (D) Freezing during ITIs was not significantly different between light and dark groups on any day. In A–C evaluations are repeated-measures ANOVA, and in D evaluations are t tests, with P < 0.05 considered significant. Data are presented as average percentage freezing ± SEM.
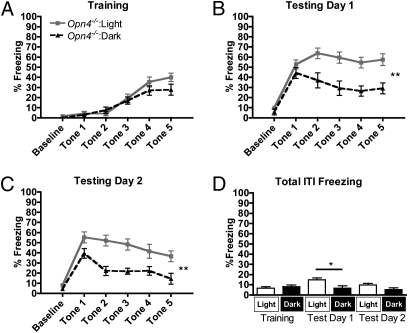
During conditioning, melanopsin-knockout mice (Opn4−/−) in light (n = 14) and darkness (n = 6) also successfully acquired a fearful association with the tone, as indicated by a significant main effect of trial on freezing [F(4,72) = 32.34, P < 0.0001]. Light did not significantly modulate freezing in Opn4−/− mice during conditioning, as indicated by the absence of a main effect of light on freezing (Fig. 4A) [F(1,72) = 0.90, P = 0.3553]. However, during the 2 subsequent days of tone-only testing, Opn4−/− mice in light did freeze more in response to the tone than mice in darkness, as indicated by a main effect of light on freezing during testing day 1 (Fig. 4B) [F(1,72) = 9.45, P = 0.0065] and testing day 2 (Fig. 4C) [F(1,72) = 7.87, P = 0.0117]. This indicates that the rods and/or cones are sufficient to drive this response during the recall testing phase. Furthermore, the rate of extinction was influenced by lighting conditions on testing day 1, as indicated by a significant light × trial interaction [F(4,72) = 3.46, P = 0.0122]. This effect was not observed on testing day 2 [Light × Trial, F(4,72) = 1.17, P = 0.3326]. In contrast to WT mice, extinction was apparent on both days of testing, as indicated by a main effect of trial on testing day 1 [F(4,72) = 2.72, P = 0.0362] and testing day 2 [F(4,72) = 9.54, P < 0.0001]. Taken together, these data indicate that the rods and/or cones are the dominant photoreceptor class(es) driving light enhancement of learned fear.
Fig. 4.
Light enhances conditioned fear responses in Opn4−/− mice. (A–C) In Opn4−/− mice light does not significantly enhance freezing to a conditioned cue during conditioning (A) but does enhance freezing during 2 subsequent days of testing (B and C) (n = 14 in light, n = 6 in dark). (D) Freezing during ITIs was not significantly different between light and dark groups during conditioning and testing day 2, but a slight elevation was observed during testing day 1. In A–C evaluations are repeated-measures ANOVA, and in D evaluations are t tests, with P < 0.05 considered significant. *P < 0.05, **P < 0.01. Data are presented as average percentage freezing ± SEM.
Light-Dependent Enhancement of Fear Is Specific to the Learned Cue.
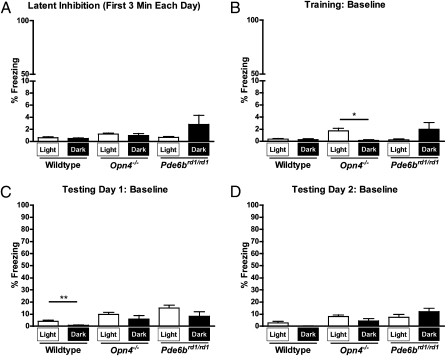
Importantly, freezing during a 2-wk preexposure period (Materials and Methods; Fig. 1) was found to be similar between WT mice in light (n = 17) and WT mice in darkness (n = 17), indicating that light alone does not cause freezing (Fig. 5A) (t test, P = 0.3733). Furthermore, on the day of conditioning and both days of testing, freezing during the 3 min before the first tone presentation (Baseline, Fig. 5 B–D) and during the intertrial intervals (ITIs) (Fig. 2D) was negligible, indicating that contextual fear associations with the fear conditioning chamber were successfully minimized by the preexposure period and that fear associations were predominantly made with the tone. Fear associations with the conditioning apparatus itself could reasonably have been expected to differ between the groups, owing to the differential light conditions, but the data indicate that this was not the case.
Fig. 5.
Light alone does not cause elevated freezing. (A) Freezing during the preexposure period was near zero for all genotypes, indicating that light alone does not induce freezing. (B) Freezing during the first 3 min (before tone presentation) on the day of conditioning was near zero for all genotypes. (C and D) Freezing during the first 3 min on both days of testing was negligible relative to tone-cued freezing, indicating that fear associations were made primarily with the tone. All evaluations are t tests, with P < 0.05 considered significant. *P < 0.05, **P < 0.01. Data are presented as average percentage freezing ± SEM.
Likewise, freezing during the preexposure period in both melanopsin-knockout and rodless-coneless mice was near zero, and freezing levels did not differ significantly between light and dark groups, demonstrating that light alone does not induce freezing in either of our mutant genotypes (Fig. 5A) (t test, Opn4−/− P = 0.5675; Pde6brd1/rd1 P = 0.1155). As with WT mice, freezing during the 3-min baseline period was negligible (Fig. 5 B–D), and freezing during the ITIs was low relative to tone-cued freezing in both lines during conditioning and both days of testing (Figs. 3D and 4D). Freezing was similar between light and dark groups in both genotypes during all baseline and ITI periods (t test, P > 0.05) with the exception of modest elevations in Opn4−/− mice in light during the baseline on the day of conditioning (t test, P = 0.0309) and during the ITIs during testing day 1 (t test, P = 0.0264).
Lighting Conditions Acutely Modulate Behavioral Responses to Fear Cues.
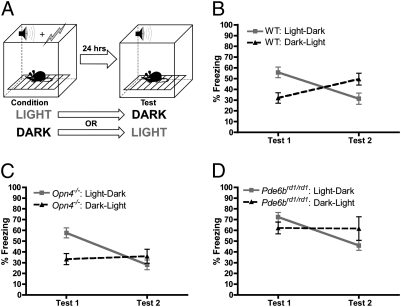
Our results demonstrate that light enhances learned fear responses when it is a constant feature of the conditioning environment. We then asked whether light could acutely enhance behavioral responses to fear cues learned in darkness and whether darkness could suppress responses to conditioned fear cues learned in light. To assess whether light could acutely enhance the behavioral expression of fear cues learned in darkness, we repeated our fear conditioning protocol with one important change: we preexposed and conditioned mice in darkness, then, during the testing period 24 h later, we exposed the mice to light (Fig. 6A). As shown in Fig. 6B, when WT mice were tested in light after conditioning in darkness (n = 10) their freezing was significantly elevated relative to freezing levels during testing when mice were preexposed, conditioned, and tested in darkness only (n = 17) (t test, P = 0.0198). This demonstrates that light does not have to be present during the acquisition of a fear association to have an enhancing effect on the response during subsequent testing in WT mice. In other words, light can acutely enhance freezing responses to cues learned in darkness. Freezing in WT mice tested in light was similar whether conditioning occurred in light (n = 17) or in darkness (n = 16) (Fig. 6B) (t test, P = 0.6197). This result shows that a maximal effect of light on freezing behavior can be attained regardless of whether light was present during conditioning and also reveals that light must be present during the testing phase to maintain high levels of freezing induced by light during the acquisition phase. Moreover, this result underscores that contextual conditioning is not behind the light enhancement of conditioned fear responses.
Fig. 6.
Lighting conditions acutely modulate conditioned fear responses during testing. (B–D) Light-Dark indicates mice conditioned in light. Dark-Light indicates mice conditioned in dark. Test 1 indicates that conditioning and testing occurred in the same lighting condition. Test 2 indicates that the lighting condition was switched between conditioning and testing. (A) Schematic of altered lighting protocol. Mice were conditioned in light and tested in dark, or conditioned in dark and tested in light. (B) In WT mice, removing the light during testing results in a significant decline in freezing, whereas adding light during testing results in a significant increase in freezing. In Opn4−/− mice (C) and in Pde6brd1/rd1 mice (D), removing the light during testing results in a significant decline in freezing, but adding light during testing does not increase freezing in either genotype. Data are presented as average percentage freezing ± SEM.
When we performed our altered lighting protocol with WT mice that had already been through the protocol once in all darkness (preexposure, conditioning, and testing, n = 16), we found that the responses of these mice during the testing portion of the altered lighting protocol were similar to responses of naïve mice (n = 10) during the same period (t test, P = 0.2872). Because no difference was observed in these mice, subsequent experiments using the altered lighting regimen were performed with mice already exposed to the original protocol.
To determine the precise roles of rods, cones, and melanopsin in mediating the acute effects of light on fear conditioning, we repeated our altered lighting regimen experiment in rodless-coneless and melanopsin-knockout mice. As mentioned above, the mice used in these experiments had previously been through the protocol once in the original lighting conditions. When either strain of mouse was conditioned in light then tested in darkness (Opn4−/− n = 14, Pde6brd1/rd1 n = 14), freezing levels during testing declined radically relative to freezing levels during testing of mice conditioned and tested in light (Opn4−/− n = 14, Pde6brd1/rd1 n = 14) (Fig. 6 C and D) (t test, Opn4−/− P < 0.0001; Pde6brd1/rd1 P = 0.0003). However, when mice were conditioned in darkness and tested in light, freezing was not significantly affected in either genotype relative to conditioning and testing in darkness (Opn4−/− n = 6, Pde6brd1/rd1 n = 3) (Fig. 6 C and D) (t test, Opn4−/− P = 0.7570; Pde6brd1/rd1 P = 0.9623). Taken together, these data indicate that photoreception by either the rods and/or cones or by melanopsin is sufficient to enhance behavioral responses to learned fear, but only when light is present during both the acquisition and recall phase. For light to acutely enhance fear responses during testing of a cue learned in darkness, both the rod–cone and melanopsin systems must be intact, suggesting a synergistic action by these two pathways.
Discussion
In these experiments we have shown that light alters behavioral responses to conditioned fear. This demonstration of light-dependent modulation of responses to learned fear cues represents an unappreciated effect of light on behavior. Light enhances freezing in WT mice in response to a conditioned fear cue during both acquisition and recall (Fig. 2). Furthermore, our experiments have shown that at the light intensities used here, the modulation of conditioned fear by light is driven primarily by the rods and/or cones (Figs. 3 and 4). A primacy of rod–cone input during conditioned responses is supported by previous work showing that rodless-coneless mice show deficits [rd/rd cl strain (12)] or are unresponsive [Pde6brd1/rd1strain (13)] to light as a conditioned stimulus in a learned avoidance task.
The elevated freezing levels seen in our experiments are not due to a general induction of freezing by light, as evidenced by the similar freezing levels observed in mice in light and in darkness during a 2-wk preexposure period (Fig. 5A). Freezing was also similar between light and dark groups during the ITIs in all genotypes on both days of testing, with the exception of a moderate elevation in Opn4−/− mice in light on the first day of testing (Figs. 2D, 3D, and 4D). Furthermore, freezing during the 3-min baseline period was negligible relative to tone-cued freezing on both days of testing (Fig. 5 C and D). These data support the hypothesis that light specifically enhances freezing to a conditioned cue. Finally, light enhanced freezing in WT mice during a recall test 24 h after tone-cued conditioning in darkness (Fig. 6A), demonstrating that light can acutely enhance behavioral responses to conditioned fear stimuli learned in darkness. Taken together, these data strongly argue against the interpretation that contextual conditioning is responsible for the increase in freezing seen in mice in light.
Light has long been recognized as an anxiogenic/aversive stimulus for nocturnal rodents. The simplest example is the suppression of locomotor activity exhibited by nocturnal mice during a nighttime light pulse (although under some circumstances light during the night may increase locomotor activity) (see ref. 14 for review). In a slightly more complex assay, the light/dark box, rodents are allowed to explore a divided chamber in which one half is illuminated and the other half is kept in darkness. Both mice and rats show a preference for the dark portion, avoiding the illuminated side (15). Light also potentiates the acoustic startle reflex, used as a measure of anxiety (4), an effect that the authors attribute to an anxiogenic effect of the illumination. Exploratory behavior in both rats and mice is decreased under high illumination in the open field test (16–18), a phenomenon also attributed to increased anxiety. A complementary effect is observed after exposure to sudden darkness, which induces a sudden decrease in measures of anxiety in the open field (19). Finally, darkness increases entry into the open arms of an elevated plus maze (19), whereas bright light causes a decrease in the frequency and duration of open arm exploration (20) (but see ref. 21 for a contradictory view).
The reduction of exploratory behavior in light has been suggested to be a mechanism for predation avoidance from the rodents’ highly visual predators (18). By avoiding brightly lit areas, prey avoid detection and therefore increase fitness. The freezing response measured in our study is one of a suite of behaviors and physiological responses initiated when an animal detects an imminent threat (22). In fact, a recent study showed that this behavior can be recapitulated in rats when faced with a predator-like robot in a seminaturalistic environment (23). The freezing response in particular serves to make the animal less detectable, thereby avoiding predation from the threat. It stands to reason that an enhanced freezing response, similar to decreased exploratory behavior, would be advantageous in light, when prey animals are easier to spot. Our results therefore expand on and extend prior reports of the anxiogenic/aversive effects of light by providing a definitive demonstration of light-dependent increase in vigilance and defensive behavior in response to a learned threat (a fear conditioned tone), a modulation of a basic survival strategy that has been conserved across animal species (22).
In humans, darkness, rather than light, causes an increase in anxiety. This is manifested in the laboratory as an enhancement of the acoustic startle reflex in darkness (24). This effect was enhanced further in patients with posttraumatic stress disorder (25). Furthermore, patients suffering from panic disorder exhibit amplification of freezing-like behaviors under stressful conditions (26). Although lighting conditions were not explored in this study, it stands to reason that darkness-induced anxiety would constitute a stressful condition. It follows from our results that lighting conditions will likely alter behavioral responses to stressful circumstances in these patients and others with disorders related to learned fear. Light already has demonstrated clinical benefits: bright light therapy is an accepted and widely used treatment for seasonal affective disorder (27). In combination with the known effects of light (or darkness) on affect and anxiety, our results raise the possibility that light therapy could be part of an effective treatment regimen for other affective disorders involving dysregulated fear, broadly classified as anxiety disorders.
Anxiety and fear share overlapping but distinct pathways (28). Anxiety is hypothesized to derive from a state of heightened vigilance to a generalized, unperceived threat, whereas fear is a rapid-onset and -offset response to a specific threat (29). The phenomenon we report here, light modulation of conditioned fear, builds a bridge between the two well-studied, distinct fields. Similar to the light-modulation of anxiety-related behaviors mentioned above, we report an enhancement of fear-related behaviors in the presence of light. Our data cannot be explained simply by an increase in anxiety, however, because such an interpretation would imply increases in freezing during preexposure, baseline, and ITI periods. To the contrary, we have observed significant increases in freezing specifically during presentation of a learned fear-inducing cue.
Our results have far-reaching implications. Although further research is needed to determine the full extent of the effects of light on learning and memory, our results show that light can indeed modulate behavioral responses to learned stimuli. It is likely that light can modulate learning, memory, and behavioral responses to learned cues in other paradigms. Although there is discrepancy in the field, several independent research groups have posited that a switch from regular fluorescent lighting, which emits light at only a few wavelengths, to full-spectrum lighting, which emits light across the visual spectrum, can result in improved mood and performance in the workplace and classroom (30). It is within reason, given our present results, that such a switch could have similar effects on learning and memory performance.
Finally, our results also draw attention to the influence of lighting conditions in behavioral assays. Care should be taken in all behavioral assays to assess the intensity and spectral composition of environmental light sources to avoid unrealized confounding effects.
Prior evidence has hinted that light may influence learning, memory, and fear, including the modulatory effect of light on cognition, the role of light in anxiety, the role of light in circulating hormones that feed into memory systems, and the central projections of ipRGCs. Our results show definitively that a pervasive environmental variable, light, can modulate conditioned fear responses.
Materials and Methods
Animals.
Male mice on a C57BL/6J background were used in this study. WT mice were purchased from Jackson Labs. Pde6brd1/rd1 mice were purchased from Jackson Labs or bred at the University of Virginia. Opn4−/− mice were bred at the University of Virginia. Animals were housed in individual cages and kept under a 12-h light/dark cycle with lights on at 0500 hours (ZT0). All of the experimental procedures were carried out in accordance with Association for Assessment of Laboratory Animal Care policies and approved by the University of Virginia Animal Care and Use Committee.
Tone-Cued Fear Conditioning.
The fear conditioning and monitoring system used in these studies has been described in detail previously (31). Particulars of our study follow and are represented in Fig. 1. Before the first day of conditioning, mice were allowed to explore the conditioning apparatus for 30 min each day for at least 12 d. Preexposure days were not always consecutive. On the day of conditioning, mice were placed in the conditioning apparatus, and baseline activity was recorded for 3 min. At 3 min, the first of five tone presentations began (2,800-kHZ pure tone, 85 dB). The tone persisted for 60 s, the final 2 s of which were paired with a mild foot shock (0.4 mA). After the tone–shock there was a 3-min intertrial interval (ITI), followed by the second tone–shock. This pattern persisted through five tone–shock pairings. The final tone–shock was followed by 3 min of no stimulus, after which the mice were returned to home cages. Twenty four hours and 48 h later the mice were returned to the conditioning apparatus to undergo testing. The testing protocol was the same as the conditioning protocol except that no shocks were administered. Behavior was monitored at all times when the mice were in the chambers (preexposure, conditioning, and testing), and freezing was scored using the Video Freeze system (Med-Associates, described in ref. 31). Owing to previous reports of a circadian modulation on fear conditioning (32, 33), preexposure, conditioning, and testing were all performed between ZT7 and ZT12.
Lighting.
For mice in “light,” blue light-emitting diodes (LEDs) with a peak emission wavelength of ≈470 nm (Super Bright LEDs, catalog #E27-x8-G) were placed adjacent to the fear-conditioning chambers. A neutral density filter was used to achieve a light intensity inside the chamber of 0.7 μW/cm2 at the point nearest to the light and 0.4 μW/cm2 at the point farthest from the light, corresponding to a photon flux of ≈9.5 × 1011 to 1.6 × 1012 photons/s per cm2. For mice in “dark” the light fixtures were removed. The light intensity in the chambers in “dark” was <0.0001 μW/cm2. For mice in “dim light,” additional neutral density filters were used to achieve a light intensity of ≈0.01 μW/cm2 at the center of the chamber, corresponding to a photon flux of ≈3 × 1010 photons/s per cm2. For mice in “bright light” a blue LED (Super Bright LEDs, catalog #PAR20-B36) was used without neutral density filters to achieve a light intensity of 165 μW/cm2 at the center of the chamber, corresponding to a photon flux of ≈3.9 × 1014 photons/s per cm2.
Statistical Analyses.
Except where noted, all experiments were analyzed by repeated-measures ANOVA. Significance was defined as a P value <0.05.
Supplementary Material
Acknowledgments
We thank Kaycie Tayler for technical assistance. This work was supported by National Institutes of Health Grant NS052112 (to I.P.) and by the Biology (I.P.) and Psychology (B.J.W.) Departments at the University of Virginia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103214108/-/DCSupplemental.
References
- 1.Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: Melanopsin and the non-visual opsins. Philos Trans R Soc Lond B Biol Sci. 2009;364:2849–2865. doi: 10.1098/rstb.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16:435–446. doi: 10.1016/j.molmed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 5.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandewalle G, et al. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci USA. 2010;107:19549–19554. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 9.Bouton ME, Bolles RC. Conditioned fear assessed by freezing and by the suppression of three different baselines. Anim Learn Behav. 1980;8:429–434. [Google Scholar]
- 10.LaVail MM, Sidman RL. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974;91:394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- 11.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 12.Mrosovsky N, Salmon PA. Learned arbitrary responses to light in mice without rods or cones. Naturwissenschaften. 2002;89:525–527. doi: 10.1007/s00114-002-0369-0. [DOI] [PubMed] [Google Scholar]
- 13.Provencio I, Wong S, Lederman AB, Argamaso SM, Foster RG. Visual and circadian responses to light in aged retinally degenerate mice. Vision Res. 1994;34:1799–1806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 14.Mrosovsky N. Masking: History, definitions, and measurement. Chronobiol Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- 15.Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 16.Valle FP. Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psychol. 1970;83:103–111. [PubMed] [Google Scholar]
- 17.Eilam D. Locomotor activity in common spiny mice (Acomys cahirinuse): The effect of light and environmental complexity. BMC Ecol. 2004;4:16. doi: 10.1186/1472-6785-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alstott J, Timberlake W. Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free-standing central corners and without peripheral walls. Behav Brain Res. 2009;196:214–219. doi: 10.1016/j.bbr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Nasello AG, Machado C, Bastos JF, Felicio LF. Sudden darkness induces a high activity-low anxiety state in male and female rats. Physiol Behav. 1998;63:451–454. doi: 10.1016/s0031-9384(97)00462-9. [DOI] [PubMed] [Google Scholar]
- 20.Cardenas F, Lamprea MR, Morato S. Vibrissal sense is not the main sensory modality in rat exploratory behavior in the elevated plus-maze. Behav Brain Res. 2001;122:169–174. doi: 10.1016/s0166-4328(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 21.Becker A, Grecksch G. Illumination has no effect on rats’ behavior in the elevated plus-maze. Physiol Behav. 1996;59:1175–1177. doi: 10.1016/0031-9384(95)02224-4. [DOI] [PubMed] [Google Scholar]
- 22.Davis M. Neurobiology of fear responses: The role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 23.Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci USA. 2010;107:21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grillon C, Pellowski M, Merikangas KR, Davis M. Darkness facilitates the acoustic startle reflex in humans. Biol Psychiatry. 1997;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 25.Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effect of darkness on acoustic startle in Vietnam veterans with PTSD. Am J Psychiatry. 1998;155:812–817. doi: 10.1176/ajp.155.6.812. [DOI] [PubMed] [Google Scholar]
- 26.Lopes FL, et al. Freezing reaction in panic disorder patients associated with anticipatory anxiety. Depress Anxiety. 2009;26:917–921. doi: 10.1002/da.20593. [DOI] [PubMed] [Google Scholar]
- 27.Prasko J. Bright light therapy. Neuroendocrinol Lett. 2008;29(Suppl 1):33–64. [PubMed] [Google Scholar]
- 28.Veening JG, et al. Activation of the septohippocampal system differentiates anxiety from fear in startle paradigms. Neuroscience. 2009;163:1046–1060. doi: 10.1016/j.neuroscience.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 29.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veitch JA, McColl SL. A critical examination of perceptual and cognitive effects attributed to full-spectrum fluorescent lighting. Ergonomics. 2001;44:255–279. doi: 10.1080/00140130121241. [DOI] [PubMed] [Google Scholar]
- 31.Anagnostaras SG, et al. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Front Behav Neurosci. 2010;4:158. doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentinuzzi VS, et al. Effect of circadian phase on context and cued fear conditioning in C57BL/6J mice. Anim Learn Behav. 2001;29:133–142. [Google Scholar]
- 33.Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.