Abstract
The physiological role of the adenosine A3 receptor is being investigated using newly synthesized, selective ligands. Recently, in addition to agonists, selective antagonists have been developed that belong to three distinct, non-purine chemical classes: flavonoids, 1,4-dihydropyridine derivatives (e.g. MRS1191, which is 1300-fold selective for human adenosine A3 vs A1/A2A receptors, with a Ki value of 31 nM) and the triazoloquinazolines (e.g. MRS1220, which has a Ki value of 0.65 nM). The A3 receptor has proven enigmatic in terms of antagonist ligand specificity, coupling to second messengers, and biological effects in the CNS, inflammatory system and cardiovascular system. A3 receptors are also potentially involved in apoptosis. It appears that intense, acute activation of A3 receptors acts as a lethal input to cells, while low concentrations of A3 receptor agonists protect against apoptosis. Here, Kenneth Jacobson describes how A3 receptor agonists might be useful in treating inflammatory conditions, possibly through their inhibition of tumour necrosis factor α (TNF-α) release, which has been shown in macrophages. A3 receptor antagonists might be useful in treating asthma or acute brain ischaemia. Recently, the versatility of A3 receptor agonists, administered either before or during ischaemia, in eliciting potent cardioprotection has been shown.
Adenosine has been shown to be a critical modulator of a vast array of physiological functions, through activation of one or more of the four known receptor subtypes: A1, A2A, A2B and A3 (Refs 1, 2). The adenosine A1 and A2A receptors, pharmacologically well characterized through the use of selective ligands, generally have a protective role, i.e. in decreasing energy demand and increasing energy supply, respectively, under conditions of stress. Relatively recently identified through cloning1,2, the A3 receptor (Fig. 1) has provided a new challenge to medicinal chemists in search of selective ligands3 and to pharmacologists in defining its role in vivo. With the recent availability of selective agonists and antagonists, both protective and lethal effects of A3 receptor activation have been discovered.
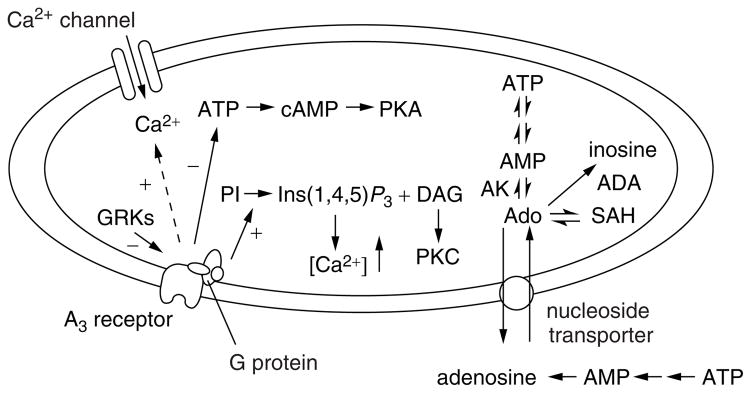
Fig. 1.
Processes of formation and degradation of adenosine in the cell and actions at A3 adenosine receptors. The dashed line indicates activity only at micromolar concentrations of IB-MECA. ADA, adenosine deaminase; AK, adenosine kinase; GRKs, G protein-coupled receptor kinases; Ins(1,4,5)P3, inositol (1,4,5)-trisphosphate; PI, phosphatidylinositol; PKA, cAMP-dependent protein kinase; SAH, S-adenosyl homocysteine.
In humans, A3 receptors are found in the lungs, liver, heart and kidneys, with a lower density being found in the brain and testes2. A3 receptors are found in both neurones4 and astrocytes5. Although the density of A3 receptors in the brain is possibly too low for mapping using either autoradiography with a high-affinity agonist, [125I]N6-(4-aminobenzyl)-adenosine-5′-N-methyluronamide {[125I]I-AB-MECA} (Fig. 2)6,7, or in situ hybridization8, a role for this subtype in the CNS has been proposed9,10.
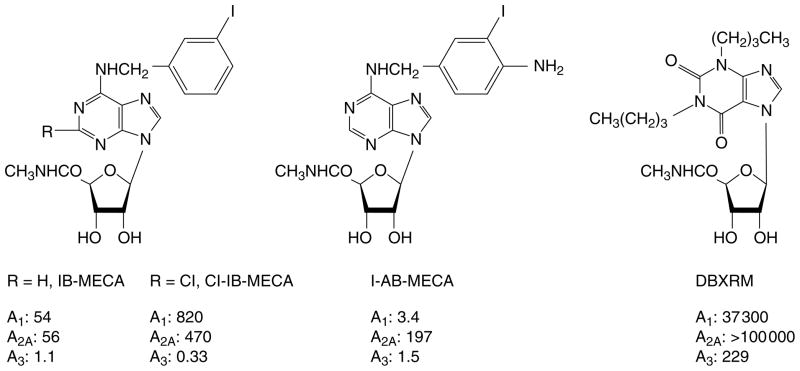
Fig. 2.
Structures of highly potent adenosine A3 receptor agonists. Abbreviations used in the text and receptor binding affinities at rat A1/A2A/A3 receptors (nM) are indicated. Cl-IB-MECA, chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide; DBXRM, 1,3-dibutylxanthine-7-riboside-5′-N-methyl-carboxamide; I-AB-MECA, [125I]N6-(4-aminobenzyl)-adenosine-5′-N-methyluronamide; IB-MECA, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide.
The A3 receptors are under scrutiny in relation to potential therapeutic approaches for treating inflammatory and neurodegenerative diseases, asthma and cardiac ischaemia10–17. A3 receptor ligands are protective in cerebral ischaemia models in gerbils10. In the heart, both A1 and A3 receptor agonists appear to protect cardiac myocytes14–17, but the latter do not cause the hypotension and hypothermia associated with agonists for the other adenosine receptors. Several years ago, a commentary by Beaven et al.11 suggested, on the basis of studies on a rat basophilic cell line, that a then hypothetical A3 receptor antagonist could be a useful anti-asthmatic drug. Indeed, eosinophils are the only cells in which native human A3 receptors have been characterized using radioligand binding18. The occurrence of A3 receptors on these cells is consistent with the proposed relevance of this subtype to asthma, in which eosinophils may be activated.
Unique pharmacological properties within the adenosine receptor family
The concentration of endogenous adenosine required for half-occupancy of A1 and A2A receptors is in the range of 10−8 to 10−7 M (Refs 1, 9), concentrations that might be achieved in the basal, resting state of an organ. The Ki value of adenosine in binding to the rat A3 receptor has not been determined directly, but has been estimated to be 10−6 M (Ref. 9). Thus, activation of this subtype may require a relatively high concentration of adenosine, such as would occur during hypoxic stress and other cellular damage. Therefore, the pathophysiological role of the A3 receptor might be very different from the role of the A1 and A2A subtypes, in that it would act as an endogenous regulator under conditions of more severe challenge.
The low affinity of xanthines, the classic antagonists of the A1, A2A and A2B subtypes, at rat A3 receptors is striking1,2,9. At human, dog and sheep A3 receptors19–21, 8-phenyl-substituted xanthines bearing acidic groups are of higher potency as antagonists; however, none have been found to be highly selective. These differences in xanthine affinity among species and the relatively low degree of homology between human and rat receptor sequences (72%) have raised the hypothesis of two potential subtypes of A3 receptors, although this remains unsubstantiated. The dramatic species differences in antagonist affinity and in pharmacological responses make the extrapolation of studies of A3 receptors in rodents to the potential treatment of human disease more challenging. In general, one must be be cautious in comparing responses between species in which tissue and cellular expression of A3 receptors might be different.
In addition to a unique structure–activity profile for agonists, and particularly for antagonists, activation of the A3 receptor has a characteristic second messenger profile (Fig. 1), in that it has been shown to stimulate directly phospholipases C (Refs 1, 22) and D (Ref. 23) and to inhibit adenylate cyclase1. In HL-60 cells, activation of A3 receptors results in the influx of Ca2+ and its release from intracellular stores24. In addition, in the RBL-2H3 basophilic cell line, the potency of adenosine receptor agonists in raising [Ca2+]i but not IP1 levels parallels A3 receptor affinity25. In the human eye, A3 receptors also regulate chloride channels of non-pigmented ciliary epithelial cells [M. M. Civan et al., in abstracts from the XIII International Congress of Eye Research (in press)]. There is a strikingly large potency differential among various functional activities of A3 selective agonists (Table 1), i.e. the same agonists might act functionally in the low nanomolar range (consistent with their affinity in competitive binding assays) for some functional responses, while in other activities, even within the same species, micromolar concentrations of the agonists are needed. Although for many receptors, the measured affinity is typically lower than EC50 values in functional assays, the wide range of these values for A3 receptors, i.e. spanning ≥4 orders of magnitude, is unusual. The role of spare receptors in this phenomenon has not been explored.
Table 1.
Functional effects of adenosine A3 receptor agonists
| Site | Action | Agonist | Approximate EC50 (nM) | Refs |
|---|---|---|---|---|
| Primary tissue/culture | ||||
| Rat hippocampal slicesa | PLC activation | Cl-IB-MECA | 50 | 22 |
| PLC activation | IB-MECA | 180 | 22 | |
| Rat hippocampal slicesa | Inhibition of A1 effects | Cl-IB-MECA | 100–1000 | 4 |
| Rat hippocampal slices | Potentiation of Ca2+ current | APNEA | 20 | 27 |
| Rat cerebellar granule cells | Death | |||
| Increased cAMP | Cl-IB-MECA | 15 000 | 26 | |
| Chick ventricular myocytesa | Inhibition of cAMP | Cl-IB-MECA | 0.6 | 15 |
| Stimulation of PLD | Cl-IB-MECA | 2 | ||
| Preconditioning | Cl-IB-MECA | 8 | ||
| Protection during prolonged hypoxia | Cl-IB-MECA | 0.2 | 16 | |
| Rat cardiac myocytes | Apoptosis | IB-MECA | ≥10 000 | 28 |
| Human eosinophils | Apoptosis | |||
| Elevation of Ca2+ | Cl-IB-MECA | ≥10 000 | 18 | |
| Inhibition of chemotaxis | Cl-IB-MECA | 0.1 | 13 | |
| Neutrophils | Degranulation | IB-MECA | 1 | 29 |
| Cell lines | ||||
| Rat RBL (mast) cellsa | Elevation of Ca2+ | Cl-IB-MECA | 70 | 25 |
| IB-MECA | 110 | 25 | ||
| Human leukaemia (HL-60) | Apoptosis | |||
| Elevation of Ca2+ | Cl-IB-MECA | ≥10 000 | 24 | |
| IB-MECA | ≥10 000 | 24 | ||
| Protection against apoptosis | Cl-IB-MECA | 10 | 24 | |
| Human U937 macrophagea | Inhibition of TNF-α | IB-MECA | 3000 | 30 |
| Cl-IB-MECA | 3600 | 31 | ||
| Human ADF (astroglial) cellsa | Changes in cytoskeleton, Bcl-XL, Rho | Cl-IB-MECA | 100 | 5 |
| Recombinant receptors | ||||
| Rat A3–CHO cellsa | Inhibition of cAMP | Cl-IB-MECA | 70 | 9 |
| Inhibition of cAMP | IB-MECA | 90 | 9 | |
| Human A3–CHO cellsa | Inhibition of cAMP | IB-MECA | 59 | 31 |
| Inhibition of growth | Cl-IB-MECA | ≥10000 | 32 | |
Activity was antagonized either by a nonselective or selective adenosine A3 receptor antagonist. APNEA, N6-[2-(4-aminophenylethyl)adenosine], a nonselective agonist; IB-MECA, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide; PLC, phospholipase C; PLD, phospholipase D; TNF-α, tumour necrosis factor α.
Rat A3 receptors can interact with Giα2, Giα3 and to a lesser extent Gq (Ref. 33). Agonist-induced desensitization of recombinant human A3 receptors occurs within 20 min, and this is associated with specific down-regulation of Giα3 and β subunits. The mechanism of desensitization involves phosphorylation of the C-terminal segment of the receptor by G protein receptor-coupled kinases (GRKs), such as GRK 2, 3 and 5 (Ref. 34).
Selective agonists and antagonists
To obtain high potency agonists that selectively activate A3 receptors, modifications at two sites in the adenosine structure, the N6- and 5′-positions, are required9. N6-(3-iodobenzyl)-adenosine-5′-N-methyl-uronamide (IB-MECA; Fig. 2) was the first highly potent and selective A3 agonist, both in vitro, in species as diverse as human31, dog21 and chick16, and in vivo9,10. It is approximately 50-fold selective in binding assays for rat A3 vs either A1 or A2A receptors. The radio-ligand [125I]I-AB-MECA (Fig. 2) is widely used as a high-affinity radioligand for A3 receptors6, although it is not as selective as IB-MECA (Ref. 7). [125I]I-AB-MECA bound to cloned human A3 receptors expressed in HEK293 cells with a Kd value of 0.59 nM (Ref. 35), but also bound to other subtypes in autoradiographic studies7. Substitution at the 2-position of adenosine in combination with modifications at the N6- and 5′-positions further enhanced A3 affinity and selectivity. Thus, 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (2Cl-IB-MECA; Fig. 2)9 displayed a Ki value of 0.33 nM at A3 receptors and is 2500- and 1400-fold selective for rat A3 vs A1 and A2A receptors, respectively.
A3 receptor antagonists, which have been introduced only recently35–38, were previously hypothesized10,11 to act as potential anti-asthmatic1, anti-inflammatory or cerebroprotective agents. Selective antagonists are needed, especially as most of the effects of high concentrations of A3 agonists (Table 1) have not been ascribed unequivocally to activation of A3 receptors. Attempts to find leads for selective xanthine-based antagonists were unproductive9,20. An approach, suggested through molecular modelling, to increase potency of xanthines in A3 receptor binding by forming the 7-riboside derivative, did indeed enhance subtype selectivity.9 Thus, 1,3-dibutylxanthine-7-riboside-5′-N-methylcarboxamide (DBXRM; Fig. 2) is 140-fold selective in binding to rat A3 vs A1 receptors. However, as the affinity increased at A3 receptors, so did the agonist efficacy, and DBXRM proved to be a full agonist at recombinant rat A3 receptors.
Because xanthines tended to bind only weakly to A3 receptors, an alternative strategy of screening diverse molecules in chemical libraries for leads was adopted in the design of selective A3 receptor antagonists. Once the structural principles of A3 receptor selectivity were discovered in these novel antagonist classes, the leads could be optimized through iterative cycles of chemical synthesis and pharmacological testing. Promising leads for A3 receptor antagonists appeared among non-xanthine heterocycles (Fig. 3). For example, 1,4-dihydropyridines, known as potent blockers of L-type Ca2+ channels and used widely in treating coronary heart disease, were found to bind to human adenosine A3 receptors. Common dihydropyridine drugs typically bound either non-selectively (for example, nifedipine, with a Ki value of 8.3 μM) or in some cases with selectivity for the A3 vs other adenosine receptor subtypes (for example, S-niguldipine, with a Ki value of 2.8 μM)36.
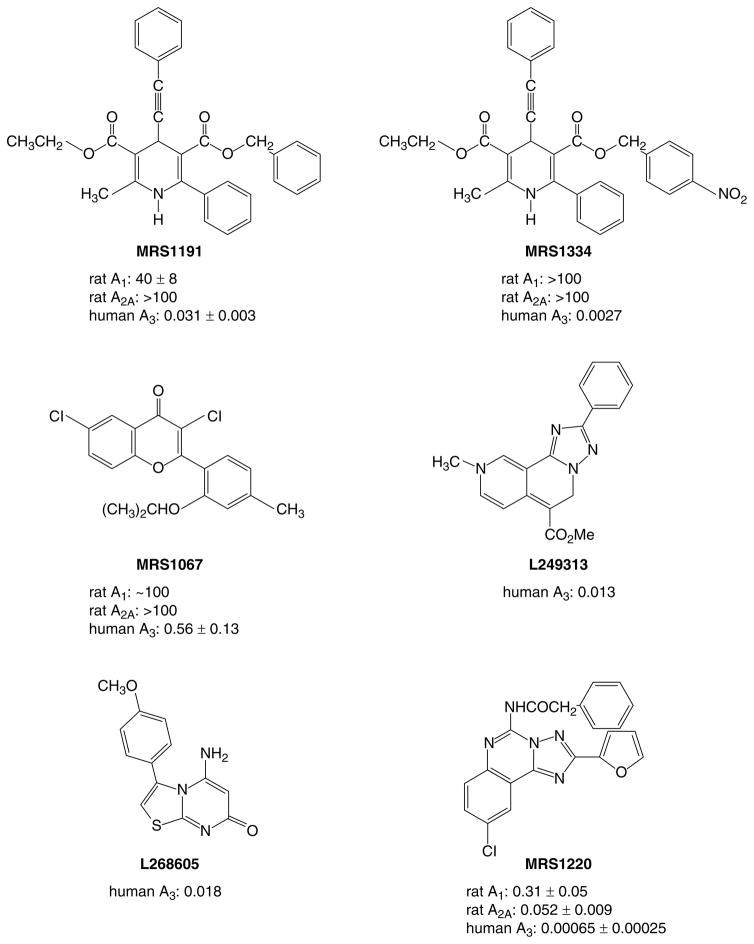
Fig. 3.
Structures of selective A3 adenosine receptor antagonists. Abbreviations used in the text and receptor binding affinities (μM) are indicated.
We have used the 1,4-dihydropyridine core as a template, in which it has been possible to select for affinity at adenosine receptors and completely deselect for affinity at L-type Ca2+ channels (Ki <100 μM), principally through introduction of a 6-phenyl group. At human A2B receptors, such 1,4-dihydropyridines are similarly inactive4. For example, a trisubstituted 1,4-dihydro-6-phenylpyridine analogue, MRS1191 (Fig. 3)36, has been found to inhibit radioligand binding at the human A3 receptor with a Ki value of 31 nM, while the same derivative was nearly inactive in binding at A1 and A2A receptor sites (i.e. >1300-fold selective). Even in the rat, MRS1191 was selective pharmacologically in various paradigms; it bound with 28-fold higher affinity for A3 (Ki value of 1.42 μM) vs A1 receptors, and at a 10 μM concentration it antagonized only the A3 subtype in the CA1 region of the hippocampus4,36. Furthermore, MRS1191 antagonized the effects of the A3 receptor-selective agonist IB-MECA on inhibition of adenylate cyclase via recombinant human or rat A3 receptors31. In chick ventricular myocyte cultures, MRS1191 antagonized the anti-ischaemic effects of Cl-IB-MECA (Ref. 16). Thus, dihydropyridine derivatives, such as MRS1191, appear to be useful as A3 receptor antagonists across species, although there is still a need for high-affinity antagonists of rat A3 receptors.
The structure–activity relationships of analogues of MRS1191, containing both subtle and drastic structural changes at various positions of the dihydropyridine ring (its 3- and 5-acyl substituents, the 4- and 6-aryl/alkyl substituents and the 2-methyl group), have been investigated systematically36. Substitutions of a 5-benzyl ester group provide the greatest versatility for achieving >30000-fold human A3 receptor selectivity and nanomolar potency. Affinity and selectivity for the human A3 receptor within this series was optimal in MRS1334 (Fig. 3), which has a Ki value of 2.7 nM (Ref. 36). These racemic dihydropyridines await optical resolution; however, side-by-side comparison of previously known dihydropyridine enantiomers shows that the stereoselectivity at A3 receptors favours the R-isomer, the opposite of the stereoselectivity at L-type Ca2+ channels.
Naturally occurring phenolic derivatives (e.g. the flavones and flavonols) provided another structural lead for development of A3 receptor antagonists35. The affinity of common phytochemicals at adenosine receptors suggests that a wide range of natural substances in the human diet might potentially antagonize the effects of endogenous adenosine, including those mediated via the A3 subtype. The flavonoid class has been chemically optimized in the form of MRS1067 (Ref. 35), which is 200-fold selective for human A3 vs A1 adenosine receptors. Other high-affinity A3 receptor-selective antagonists that have been reported recently include a triazolonaphthyridine (L249313; Fig. 3)38, thiazolopyrimidine (L268605; Fig. 3)38 and a derivative of the triazoloquinazoline CGS15943 (MRS1220; Fig. 3)37. L249313 binds to human A1 and A2A receptors with Ki values of 6.6 and 1.25 μM, respectively. The Ki value at rat A3 receptors is 33 μM. Although not as selective as MRS1191, MRS1220 is the antagonist with the highest affinity (Ki 0.65 nM) for human A3 receptors reported yet. MRS1220 is not A3-selective in the rat, further emphasizing the species differences in antagonist affinity.
Binding of MRS1067, MRS1191 and MRS1220 at human A3 receptors was shown to be competitive by Scatchard analysis versus binding of [125I]I-AB-MECA (Ref. 31). Antagonism was demonstrated in functional assays consisting of agonist-induced inhibition of adenylate cyclase and the stimulation of binding of [35S]GTP-γ-S to the associated G proteins. MRS1220 and MRS1191, with KB values of 1.7 and 92 nM, respectively, were highly selective for human A3 receptor vs human A1 receptor-mediated effects on adenylate cyclase.
Protective versus lethal effects of A3 receptor activation
The varied effects of A3 receptor agonists, in vitro and in vivo, appear to be dual and opposite, i.e. either cytoprotective or cytotoxic, depending on the level of receptor activation and the paradigm studied. The mechanisms involved in these opposite effects are not yet fully understood.
Nanomolar concentrations of selective agonists tend to protect cells, while micromolar concentrations are often toxic (Table 1)24. In certain cultured cell lines, antagonists alone are toxic39. The fact that A3 receptor antagonists representing three diverse chemical classes evoked the common biological effect of apoptosis (programmed cell death, see below) suggests that a tonic state of activation of the A3 receptor might exist, and that this possible low level of receptor activation has a protective role. If a tonic A3 receptor activation does exist, the apoptotic effects of A3 receptor antagonists might simply be explained on the basis of a block of a protective action induced by endogenous adenosine. To explain how very high doses of agonist alone might induce rather than prevent apoptosis, one could propose differential activation of different second messengers by the same receptor at low and high doses (Fig. 1). Such hypotheses will require further investigation, which would be greatly aided by the development of a high-affinity antagonist radioligand for the A3 receptor. The low density of A3 receptors has also made study difficult.
Central nervous system
The first cytoprotective effects of an A3 receptor agonist were shown following its chronic administration in gerbils in a model of stroke. In an in vivo gerbil model of global ischaemia, the acute administration of IB-MECA during ischaemia exacerbated histological and functional damage, clearly worsening the post-occlusive outcome10. However, chronic pre-administration of the same agent over several weeks had a highly neuroprotective, post-ischaemic effect, in which the agonist was highly cerebroprotective, preserved microtubule-associated protein 2 (MAP2) immunoreactivity, and depressed NO synthase. In primary astroglial cell cultures, nanomolar concentrations of selective A3 receptor agonists caused protection against cell death and induced differentiation, while high concentrations increased cell death32. In human ADF cells of astroglial lineage, 100 nM Cl-IB-MECA caused a marked reorganization of the cytoskeleton, with appearance of stress fibres and numerous cell protrusions (which became enriched in the anti-apoptotic protein Bcl-xL), accompanied by induction of the expression of Rho, a small GTP-binding protein5. A high concentration of Cl-IB-MECA (≥10 μM) was lethal to cultured rat cerebellar granule neurons, and the toxic effects of glutamate were also augmented27. In preliminary experiments, acute adminstration of the selective A3 receptor antagonist MRS1191 proved to be cerebroprotective in the gerbil global ischaemia model40.
Several possible explanations for the damaging effects of acute A3 activation during ischaemia have been offered. These include the detrimental effects seen on cerebral bood flow10 or the release of a cytotoxic agent. Alternatively, the effects might be via neuronal A3 receptors. In general, high concentrations of IB-MECA and Cl-IB-MECA directly cause influx of Ca2+ (Refs 24, 25); however, this may not be relevant to potent in vivo effects. In addition, examples of cross-talk between the A3 receptor subtype and other adenosine receptors are being discovered. For example, acute activation of presynaptic hippocampal A3 receptors antagonizes the action of metabotropic glutamate receptors, thus resulting in enhanced glutamate release41. Dunwiddie et al.4 found that A3 activation counteracts the protective effects of A1 receptor activation at the hippocampal synapse, i.e. the depression of excitatory transmission (EPSPs) elicited by A1 agonists is blunted by selective A3 agonists. In contrast, Mogul and coworkers27 have shown that A3 receptor activation increases cellular excitability in these neurones through a pathway independent of A1 receptors. Activation of A3 receptors in isolated CA3 pyramidal neurones from guinea-pig hippocampus by a low concentration of a selective agonist was also found to potentiate a Ca2+ current through a cAMP-dependent protein kinase (PKA)-dependent/protein kinase C (PKC)-independent mechanism27.
The immune system and inflammation
In a variety of human cell lines of the immune system, A3 agonists at high concentrations often prove lethal (Table 1). Apoptosis, with characteristic DNA fragmentation, has been shown to occur in human leukaemia HL-60 cells, MCF-7 breast cancer cells and in human peripheral blood eosinophils in response to high concentrations (≥10 μM) of A3 receptor-selective agonists24,39. A mediator of apoptosis, bak (pro-apoptotic Bcl-2 homology protein), is upregulated under these conditions39. The protective effects of A3 receptor activation might involve cytokines. Sajjadi et al.30 have shown that micromolar concentrations of the agonist IB-MECA in U937 cells inhibit the release of tumour necrosis factor α (TNF-α), which in turn might induce apoptosis. Clarification of the need for such high doses of agonists, thousands of times higher than the Ki values at A3 receptors, has awaited the introduction of selective A3 receptor antagonists, which are now available for the human A3 receptor. As discussed above, A3 receptor antagonists of diverse structures alone cause apoptotic cell death39, and cells may be rescued by subcytotoxic concentrations of selective A3 receptor agonists.
Walker et al.12 postulated a role for A3 receptors in lung inflammation, as adenosine leads to exaggerated airway narrowing in individuals with inflammatory airway disorders. Evidence was found that in humans A3 receptor gene expression is localized to inflammatory cells (eosinophils, but not mast cells) and that gene expression is upregulated in airway inflammation. Cl-IB-MECA inhibited eosinophil chemotaxic responses to PAF, RANTES or LTB, without affecting adhesion receptors CD18 and selectin, or assembly of F-actin. This effect was blocked by the selective A3 antagonist L249313 (Ref. 13). Based on this effect, it is not known whether an A3 agonist or antagonist would be more useful in treating asthma, as, theoretically, eosinophil activation could either augment (via migration to site) or counteract (via migration away from site) inflammation. However, other experiments suggest that an antagonist might be more useful. For example, Meade et al. found that in the BDE-strain rat model of airway disease, A3 receptor agonists induced bronchospasm via mast-type cells42. Although aerosol challenge of antigen-immunized rabbits with the non-selective agonist N6-[2-(4-aminophenylethyl)-adenosine] (APNEA) did not elicit dose-dependent changes in either airway resistance or dynamic compliance43, Ali et al. found that the agonists IB-MECA and Cl-IB-MECA caused bronchoconstriction44.
Selective activation of A3 receptors appears to inhibit human neutrophil degranulation, suggesting the anti-inflammatory potential of A3 receptor agonists in neutrophil-mediated tissue injury29.
There might be an involvement of A3 receptors in cancer45. Activation of A3 receptors reduced cytotoxic lymphocyte adhesion to tumour cells.
Cardiovascular and renal systems
The acute activation of A3 receptors in rodents leads to hypotension, exclusively via release of histamine and other mediators from peripheral mast cells46. However, canine and human mast cells do not react in this manner21. Shepherd et al.47 found that in microcirculation of the hamster cheek pouch, activation of A3 receptors results in vasoconstriction, which also occurs through activation of mast cells.
A3 receptors occur on ventricular, but not atrial, chick cardiac myocytes15. There are protective effects of A3 receptor activation in heart cells, administered both prior to15,17 and during16 an ischaemic episode (Fig. 4)17. IB-MECA also protects against myocardial stunning in conscious rabbits48. In cultured chick cardiac myocytes, a brief prior exposure to nanomolar concentrations of the A3 receptor agonist Cl-IB-MECA protected cells from damage induced by subsequent hypoxia14,15, thus simulating the protection afforded by a brief hypoxic period, a phenomenon termed ‘preconditioning’. Activation by endogenous adenosine of both A1 and A3 receptors is thought to mediate preconditioning. Because the culture consisted almost exclusively of ventricular myocytes, this was not an indirect effect of activation of A3 receptors on mast cells, as has been reported by Fozard and co-workers46 to explain hypotensive effects in vivo. Thus, an A3 agonist at low concentration is potentially useful therapeutically as a cardioprotective agent, having a more sustained duration of protection and fewer in vivo side-effects than other (e.g. A1-selective agonists) adenosine agonists14,16,49. However, high concentrations of the same agonists were shown to be damaging, i.e. they induce apoptosis in rat cardiac myocytes28.
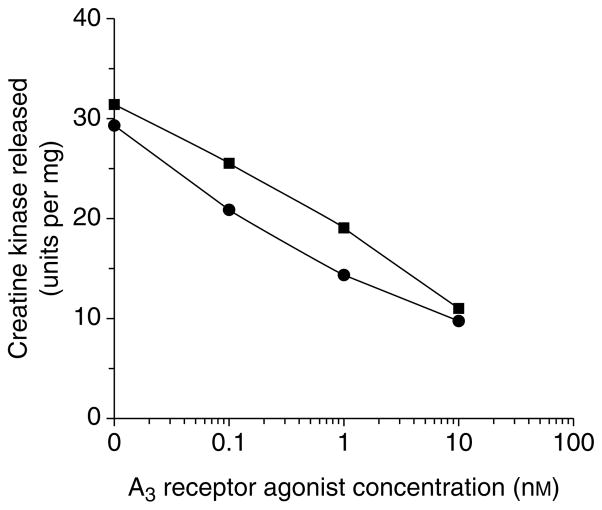
Fig. 4.
Cardioprotection elicited by the selective A3 adenosine receptor agonists, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA) and Cl-IB-MECA, during prolonged ischaemia (modified from Ref. 16). Cardiac ventricular myocytes were cultured from chick embryos 14 days in ovo, and cell injury was induced by a 90 min exposure of the culture to hypoxia with glucose deprivation. Release of creatine kinase into the medium was directly proportional to the degree of cell injury. Curves shown were measured in the presence of 1 μM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (selective A1 adenosine receptor antagonist), which had no effect on the cardioprotection observed. The selective A3 adenosine receptor antagonist MRS1191 antagonized the cardio-protection provided by 10 nM Cl-IB-MECA, with an IC50 of ~10 nM. A3 adenosine agonists were also cardioprotective following a brief exposure, prior to ischaemia (Ref. 15). Squares, IB-MECA; circles, Cl-IB-MECA.
Concluding remarks
By virtue of regulating programmed cell death, A3 receptors might play a critical role in human disease states. The relation of the A3 receptor to apoptosis suggests that both A3 receptor agonists and antagonists might be useful for treating diseases either in which cytotoxicity is undesirable, such as neurodegeneration, or desirable, such as cancer and inflammation. A3 receptor antagonists might also be useful in treating asthma. The acute administration of an A3 receptor antagonist or the chronic adminstration of an A3 receptor agonist appears to protect brain cells in a global ischaemia model, and thus are potential therapeutic approaches for preventing stroke damage. In the heart, because A3 receptor activation protects both in a preconditioning model and during prolonged ischaemia, selective agonists might be of great clinical importance.
Chemical names
- CGS15943
9-chloro-2-(2-furyl)[1,2,4]triazolo[1,5-c] quinazolin-5-amine
- L249313
6-carboxymethyl-5,9-dihydro-9-methyl-2-phenyl-[1,2,4]-triazolo[5,1-a][2,7]naphthyridine
- L268605
3-(4-methoxyphenyl)-5-amino-7-oxo-thiazolo[3,2]pyrimidine
- MRS1067
3,6-dichloro-2′-(isopropoxy)-4′-methylflavone
- MRS1191
3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenyl-ethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate
- MRS1220
9-chloro-2-(2-furyl)-5-phenylacetylamino [1,2,4]triazolo[1,5-c]quinazoline
- MRS1334
3-ethyl 5-(4-nitrobenzyl) 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate
References
- 1.Olah ME, Stiles GL. Annu Rev Pharmacol Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 2.Linden J. Trends Pharmacol Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson KA, Suzuki F. Drug Dev Res. 1997;39:289–300. doi: 10.1002/(sici)1098-2299(199611/12)39:3/4<289::aid-ddr8>3.0.co;2-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunwiddie TV, Diao L, Kim HO, Jiang J-l, Jacobson KA. J Neurosci. 1997;17:607–614. doi: 10.1523/JNEUROSCI.17-02-00607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbracchio MP, et al. Biochem Biophys Res Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. Mol Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- 7.Shearman LP, Weaver DR. Brain Res. 1997;745:10–20. doi: 10.1016/s0006-8993(96)01120-1. [DOI] [PubMed] [Google Scholar]
- 8.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson KA, et al. Drugs Future. 1995;20:689–699. doi: 10.1358/dof.1995.020.07.531583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Lubitz DKJE, Lin RCS, Popik P, Carter MF, Jacobson KA. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaven MA, Ramkumar V, Ali H. Trends Pharmacol Sci. 1994;15:13–14. doi: 10.1016/0165-6147(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 12.Walker BA, et al. Am J Respir Cell Mol Biol. 1997;16:531–737. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- 13.Knight DA, et al. J Leukocyte Biol. 1997;62:465–468. doi: 10.1002/jlb.62.4.465. [DOI] [PubMed] [Google Scholar]
- 14.Liu GS, et al. Cardiovasc Res. 1994;28:1057–1061. doi: 10.1093/cvr/28.7.1057. [DOI] [PubMed] [Google Scholar]
- 15.Strickler J, Jacobson KA, Liang BT. J Clin Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stambaugh K, Jacobson KA, Jiang J-l, Liang BT. Am J Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tracey WR, et al. Cardiovasc Res. 1997;33:410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 18.Kohno Y, Ji Xd, Mawhorter SD, Koshiba M, Jacobson KA. Blood. 1996;88:3569–3574. [PMC free article] [PubMed] [Google Scholar]
- 19.Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Proc Natl Acad Sci U S A. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden J, et al. Mol Pharmacol. 1993;44:524–532. [PubMed] [Google Scholar]
- 21.Auchampach JA, et al. Mol Pharmacol. 1997;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 22.Abbracchio MP, et al. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- 23.Ali H, et al. J Pharmacol Exp Ther. 1996;276:837–845. [PubMed] [Google Scholar]
- 24.Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Biochem Biophys Res Commun. 1996;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin Y, Daly JW, Jacobson KA. Drug Dev Res. 1996;39:36–46. doi: 10.1002/(sici)1098-2299(19960901)39:1<36::aid-ddr5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sei Y, von Lubitz DKJE, Abbracchio MP, Ji Xd, Jacobson KA. Drug Dev Res. 1997;40:267–273. [Google Scholar]
- 27.Fleming KM, Mogul DJ. Neuropharmacology. 1997;36:353–362. doi: 10.1016/s0028-3908(97)83762-8. [DOI] [PubMed] [Google Scholar]
- 28.Shneyvays V, Jacobson KA, Shainberg A. J Mol Cell Cardiol. 1997;29:11A. doi: 10.1006/jmcc.2001.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouma MG, et al. J Immunol. 1997;158:5400–5408. [PubMed] [Google Scholar]
- 30.Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. J Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 31.Jacobson KA, et al. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbracchio MP, et al. Ann New York Acad Sci. 1997;825:11–22. doi: 10.1111/j.1749-6632.1997.tb48410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer TM, Gettys TW, Stiles GL. J Biol Chem. 1995;270:16895–16902. doi: 10.1074/jbc.270.28.16895. [DOI] [PubMed] [Google Scholar]
- 34.Palmer TM, Benovic JL, Stiles GL. J Biol Chem. 1996;271:15272–15278. doi: 10.1074/jbc.271.25.15272. [DOI] [PubMed] [Google Scholar]
- 35.Karton Y, et al. J Med Chem. 1996;39:2293–2301. doi: 10.1021/jm950923i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J-l, et al. J Med Chem. 1997;40:2596–2608. doi: 10.1021/jm970091j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YC, Ji Xd, Jacobson KA. J Med Chem. 1996;39:4142–4148. doi: 10.1021/jm960482i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobson M, Chakravarty PK, Johnson RG, Norton R. Drug Dev Res. 1996;37:131. [Google Scholar]
- 39.Yao Y, Sei Y, Abbracchio MP, Kim YC, Jacobson KA. Biochem Biophys Res Commun. 1997;232:317–322. doi: 10.1006/bbrc.1997.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Lubitz DKJE, Lin RCS, Jacobson KA. Soc Neurosci Abstr. 1997;23:1924. [Google Scholar]
- 41.Macek TA, Conn PJ. Soc Neurosci Abstr. 1997;23:1754. [Google Scholar]
- 42.Meade CJ, Mierau J, Leon I, Ensinger HA. J Pharmacol Exp Ther. 1996;279:1148–1156. [PubMed] [Google Scholar]
- 43.el-Hashim A, D’Agostino B, Matera MG, Page C. Br J Pharmacol. 1996;119:1262–1268. doi: 10.1111/j.1476-5381.1996.tb16031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali S, Jacobson KA, Mustafa SJ. FASEB J. 1997;11:A346. [Google Scholar]
- 45.MacKenzie WM, Hoskin DW, Blay J. Cancer Res. 1994;54:3521–3526. [PubMed] [Google Scholar]
- 46.Fozard JR, Pfannkuche HJ, Schuurman HJ. Eur J Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- 47.Shepherd RK, Linden J, Duling BR. Circ Res. 1996;78:627–634. doi: 10.1161/01.res.78.4.627. [DOI] [PubMed] [Google Scholar]
- 48.Auchampach JA, et al. Circ Res. 1997;80:800–809. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- 49.Liang BT, Jacobson KA. Proc Natl Acad Sci U S A. in press. [Google Scholar]