Abstract
Objectives
To test the effects of walking, light exposure, and a combination intervention (walking plus light plus sleep education) on the sleep of persons with Alzheimer’s disease (AD).
Design
Randomized, controlled trial with blinded assessors.
Setting
Independent community living.
Participants
132 AD patients and their in-home caregivers.
Interventions
Participants were randomly assigned to one of three active treatments (walking, light, combination treatment) or contact control. Participants received three or six in-home visits.
Measurements
Primary outcomes were patient total wake time based on wrist actigraphy, and caregiver ratings of patient sleep quality on the Sleep Disorders Inventory (SDI). Secondary sleep outcomes included additional actigraphic measurements of patient sleep percent, number of awakenings, and total sleep time.
Results
Patients in walking (p<.05), light (p<.04), and combination treatment (p<.01) had significant improvements in total wake time at post-test (effect size 0.51 – 0.63) compared to control subjects, but no significant improvement on the SDI. Moderate effect size improvements in actigraphic sleep percent were also observed in active treatment subjects. There were no significant differences between active treatment groups, and no group differences for any sleep outcomes at six months. Patients with greater adherence (4+ days/week) to walking and light exposure recommendations had significantly (p<.05) less total wake time and better sleep efficiency at post-test than those with lesser adherence.
Conclusion
Walking, light exposure and the combination are potentially effective treatments for improving sleep in community-dwelling persons with AD, but consistent adherence to treatment recommendations is required.
Keywords: Sleep, Alzheimer’s disease, walking, light, adherence
INTRODUCTION
Sleep disturbances in persons with dementia increase risk for physical and psychological morbidity in both patients and caregivers, and are associated with patient institutionalization.1–7 Further, they are difficult to treat. Sedative medications, as well as “off-label” agents such as antidepressants and antipsychotics, are widely prescribed but are of questionable efficacy and have significant side effect risks.8–10 Several studies have shown that actigraphic ratings of sleep in cognitively impaired individuals taking sedating medications are no different than those who are taking no medication.11,12
Two alternatives to pharmacotherapy for treating sleep disturbances in dementia are increased daytime activity and bright light therapy. Daytime activation, including physical exercise, adult day programs, and individualized social activity, has been reported to improve nighttime sleep in older adults with dementia.13–17 Light therapy has been shown to increase total nocturnal sleep time, decrease daytime napping, reduce behavioral outbursts, and increase stability of rest-activity rhythms.18–22 However, most research examining the efficacy of activity and light to improve sleep in dementia has been conducted in nursing homes. We previously reported that a combination of walking and light exposure to improve sleep was feasible and efficacious in community settings,23 but that study provided no information on the efficacy of walking or light exposure alone.
The current randomized controlled trial examined the efficacy of walking, light exposure, and a combination treatment (walking plus light plus guided sleep education) for improving sleep in persons with AD, compared to a contact control. Primary outcomes were actigraphic measures of patient total wake time at night, and caregiver reports of patient nighttime behavioral disturbances. It was hypothesized that active treatment would be more efficacious than control at post-test (2 months) and 6-month follow-up. We also sought to examine whether there would be treatment differences between the three active conditions, and in particular, whether the combination intervention would be more efficacious than walking or light exposure alone.
METHODS
Design overview
One hundred thirty-two persons with AD and their caregivers were recruited between November 2005 and March 2009 through community advertisements and from Group Health Cooperative (GHC), a non-profit integrated health care plan in Washington State. The study was approved by GHC and University of Washington IRBs. Written consent was obtained from caregivers; patients provided written consent or verbal assent, which was reaffirmed at each assessment point. Additionally, caregivers (next of kin or legal guardians) provided consent on behalf of patients. The study was registered at ClinicalTrials.gov (Identifier: NCT00183378).
Participants
Patient eligibility criteria were: (1) two or more sleep problems occurring several times a week measured by the 7-item Sleep Disorders Inventory;24 (2) diagnosis of probable or possible AD25 confirmed either by GHC medical record or in writing by participants’ primary care physicians; (3) no previously diagnosed primary sleep disorder (sleep apnea, restless legs, periodic leg movements syndromes, REM behavior disorder); (4) no significant vision impairment or medical contraindications to bright light exposure; (5) ability to walk across a room; (6) living with a caregiver who could monitor sleep and implement treatment recommendations; (7) score less than 32 on the Sleep Apnea subscale of the Sleep Disorders Questionnaire (SDQ);26 and (8) agree to make no changes in sedating medication use (type or dose) during the 2-month active treatment period. Eligible patients wore wrist actigraphs (see Measures) for one week. Patients whose wake time averaged one hour/night or greater on actigraphy were invited to participate.
Randomization
Participants were randomized into one of four treatment conditions: Walking (N=32), Light (N=34), combination walking, light, and guided sleep education (Nighttime Insomnia Treatment and Education in Alzheimer’s Disease; NITE-AD) (N=33), or Contact control (N=33). Patients were stratified by baseline sleep medication use. The random allocation sequence was obtained from a computer program that blocked in groups of 12 subjects. Treatment conditions were assigned by a research coordinator via sealed envelopes containing the random assignment.
Interventions
Overall intervention design
Subjects randomized to the Walking, Light, and Control conditions participated in three one-hour in-home training visits (Weeks 1, 2, and 8). They also received 2 brief phone calls (Weeks 4 and 6) to reinforce caregiver use of the daily log. Subjects randomized to the combination NITE-AD condition participated in six one-hour in-home visits (4 weekly, 2 biweekly). To control for general sleep knowledge across groups, all participants received handouts describing sleep changes associated with aging and dementia, and basic sleep hygiene guidelines (Table 1).27,28 All caregivers kept a daily patient sleep log; in the three active conditions, this log included questions about walking and/or light exposure.
Table 1.
Sleep Hygiene Recommendations Provided to All Participants
|
Modified from Alzheimer’s Association 27
Walking
In Session 1, a caregiver-supervised, self-paced walking program was initiated, with a goal of 30 continuous minutes/day.29 Frail or sedentary patients started with less than 30 minutes and gradually increased walking duration to minimize risk of injury. In Sessions 2 and 3, trainers helped problem-solve difficulties implementing and adhering to the walking plan.
Light
In Session 1, subjects were given a SunRay light box (SunBox Company, Gaithersburg, MD) to use in light exposure sessions. Boxes were placed at eye level, 1 meter from the patient, within a 45° visual field (equal to approximately 2,500 lux of full spectrum light). Patients sat in front of the light box for one hour/day. Light sessions were supervised by caregivers and timed to be within a 2-hour window before the patient’s habitual bedtime, replicating procedures in our previous study with community-dwelling AD dyads.23 Trainers helped caregivers identify activities that could engage the patient during the light sessions (e.g., watching TV, looking at picture albums, etc.) Caregivers were also encouraged to reduce light levels in patients’ sleeping areas at night. In Sessions 2 and 3, trainers helped problem-solve difficulties implementing and adhering to the light exposure plan.
NITE-AD
In Session 1, caregivers and trainers together developed an individualized sleep plan for the patient, focusing on establishing a consistent bed and rising time, reducing daytime napping, and identifying potential triggers for nighttime awakenings. Patients were also placed on a daily walking program as described above.
In Session 2, trainers introduced the daily light exposure program as described above. Sessions 3–6 focused on helping caregivers identify activators and consequences for nocturnal arousals,30 and on problem-solving any adherence challenges to following the sleep, walking, and light exposure plans.
Contact control
At all three sessions, trainers offered nondirective dementia care support but provided no training or homework related to changing sleep/wake routines, implementing daily walking, increasing light exposure, or managing dementia-related nocturnal behaviors.
Study Trainers
Trainers were three licensed masters-level health care professionals (a family counselor, medical social worker, and registered nurse). Two of the trainers had worked on previous research studies with this team delivering behavioral interventions to caregivers of persons with dementia. All three trainers had multiple years of clinical experience working in health care settings (assisted living and nursing homes, hospital acute care) with cognitively impaired older adults.
Trainers received an initial 2-hr orientation with the Principal Investigator (SMM) who described the study aims, treatment protocols, and outcome measures. Written treatment manuals and handouts for each intervention were reviewed (manuals are available from the senior author upon request). After trainers familiarized themselves with the manuals, a second training session was held to answer questions, review use of special equipment (e.g., actigraphs and the light boxes), and discuss treatment logistics.
All trainers delivered all interventions. All sessions were audiotaped and reviewed by the Principal Investigator (SMM), who provided weekly feedback throughout the study period regarding adherence to the treatment protocols.
Measures
Assessments were conducted at baseline, 2 months (immediately post-treatment), and 6-month follow-up by interviewers blind to treatment assignment.
Primary objective sleep outcome: Actigraphy
One week of sleep-wake activity was measured at each assessment using a Micro-Mini Motionlogger actigraph (Ambulatory Monitoring, Inc., Ardsley, NY). Actigraphs were worn on patients’ nondominant wrist. Data were collected in one-minute recoding epochs using the Proportional Integrating Measure (PIM, low sensitivity) operation mode. The Action4 software package (Ambulatory Monitoring, Inc.), which incorporates Cole and Kripke’s31 sleep scoring algorithm, was used to score sleep/wake.
The primary actigraphy outcome was patient total wake time at night. The night (in-bed) period was defined as “lights out” at bedtime until the final morning rising. Bed and rising times were derived from a daily sleep log kept by caregivers. Secondary actigraphic sleep outcomes included number of awakenings, total sleep time, and sleep percent. Information on nightly time in bed and daytime sleep/inactivity was also gathered.
Primary subjective sleep outcome: Sleep Disorders Inventory (SDI)24
The SDI is a semi-structured caregiver interview developed to rate the frequency, severity, and caregiver distress about seven patient nocturnal behaviors over the past two weeks. The SDI was developed for use in the Alzheimer’s Disease Cooperative Study (ADCS) clinical study of melatonin for treating sleep disturbances in persons with AD.32 Total SDI scores are computed as the product of the average frequency and severity ratings for the seven behavior items.
Covariate measures
Covariates hypothesized to relate to patient sleep were also collected. These included the Cornell Scale,33 a 19-item, clinician-rated scale of depression symptoms designed for use with dementia patients; the Self-Administered Comorbidity Questionnaire (SCQ),34 used to rate whether any of 13 common medical comorbidities limited patient activities; the Sleep Apnea subscale of the SDQ;26 and patient age, gender, and MMSE score.35
Treatment adherence
Daily logs were used to calculate frequency and duration of walking and light exposure sessions. Distance from the light box and timing of light exposure sessions were measured in Week 1, and each subsequent assessment using a Micro Light Sensor actigraph (Ambulatory Monitoring, Inc). The sensor, which measures 0 to 4,000 LUX in 16 LUX increments, was worn around the neck facing the light box during light exposure sessions.
A subset of recorded treatment session audiotapes (N=72, representing one session from 55% of cases) was randomly selected for blinded evaluation. Using a checklist designed for this project, two independent judges (a research nurse and a social work doctoral student) reviewed these tapes and rated nine treatment components related to sleep education and scheduling, walking, light exposure, and homework.
Treatment satisfaction
After the 6-month assessment, caregivers rated study burden and how much treatment helped them manage patient sleep problems.
Statistical Methods
The study was designed to have 80% power to detect an effect size of 0.65, based on comparing each active treatment group to Contact control, on the basis of our previously published data.23 We assumed a 10% drop-out at post-test and 20% at 6 months. Attrition prior to post-test exceeded expectations, so there was less power to detect differences than anticipated. We used Stata (version 11.1, StataCorp LP, College Station, Texas) for all analyses. A p-value less than 0.05 (two-sided) was considered statistically significant for the two primary outcomes since each were conceptually unique (actigraphically measured nocturnal wake time and caregiver subjective report about patient sleep on the NPI.) For analyses of the five secondary sleep outcomes, a Bonferoni correction for multiple comparisons was utilized, with alphas set at p=.01.
Descriptive statistics are presented as means and standard deviations or as means and standard errors for difference scores and imputed data. Quantile-normal graphs were used to assess normality of continuous variables. Total wake time was log-transformed. Baseline values between the four groups were compared using one-way analysis of variance, Kruskal-Wallis, or chi-square tests, as appropriate.
The primary pre-post analyses were based on intention-to-treat using all randomized patients. The primary outcomes were change in total wake time and change in SDI between baseline and 2 months. Pre-post change scores for each active treatment versus control were compared using t-tests. To control for possible confounders in the pre-post analyses, we used generalized linear models with appropriate link functions and robust standard errors. The post-test outcome was regressed on active treatment group, controlling for baseline value and including patient age, gender, depression, comorbidity limitations, MMSE, and sleep apnea score as covariates.
Longitudinal analyses used mixed models and we modeled the non-linear effect of time using splines.36 Linear splines break curvilinear trajectories into separate linear components. We used two splines to examine sleep outcomes from baseline to 2 months (post-test) with a knot at post-test and from 2 months to 6 months (follow-up).
We used multiple imputation under the multivariate normal regression model to replace missing data. Thirty-one percent of patients had missing sleep outcome data across the two primary outcomes and the three assessment periods due to loss to actigraph failure, study discontinuation, or assessment non-completion. Eight percent were missing some covariate data. We conducted sensitivity analyses using intent-to-treat, complete case analyses to compare to imputed results.
RESULTS
Participant flow
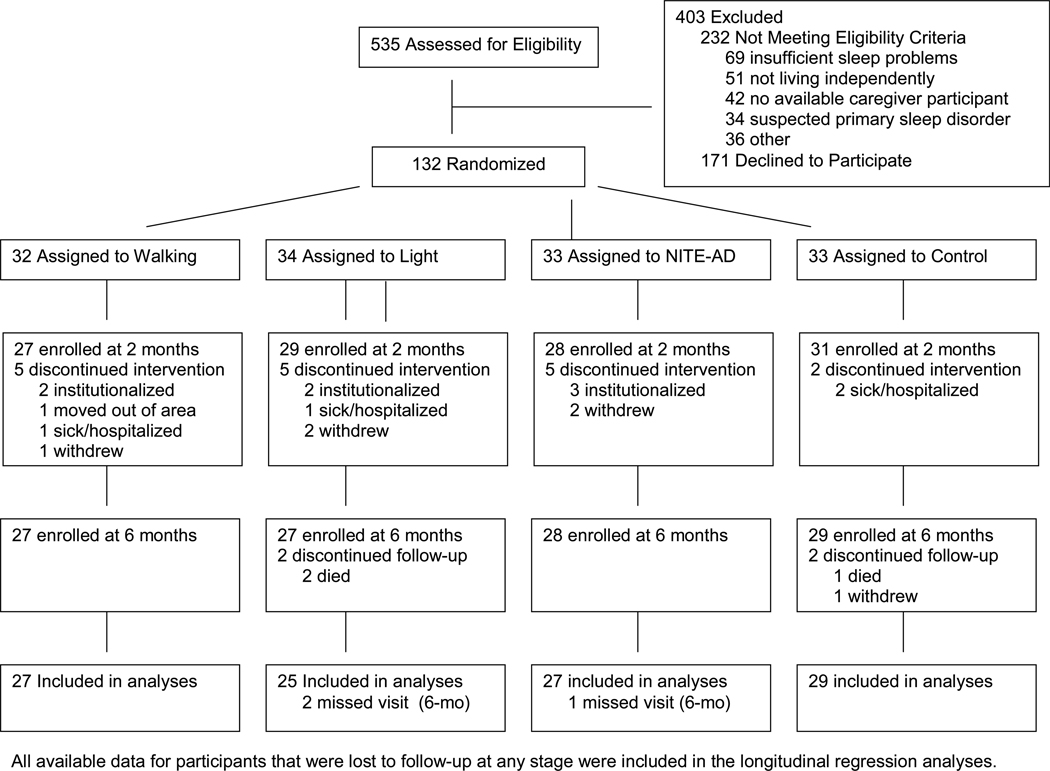
The flow of subjects is shown in Figure 1. Of 132 patients who began the study, 115 (87%) completed post-test, and 110 (83%) completed the 6-month assessment. There were no significant between-group differences in attrition (5 withdrawals each from walking, NITE-AD, and control, 7 withdrawals from light). At 6 months, nine patients had been institutionalized. Of these, none had completed active treatment (one was a control subject who dropped during post-treatment follow-up, and eight [3 walk, 2 light, 3 NITE-AD] dropped within the first two treatment weeks).
Figure 1.
Flow of Participants Through the Trial
Demographics and Baseline Scores
There were no significant pretreatment group differences on any patient or caregiver demographic variables (Table 2). There were also no significant group differences in any baseline actigraphic or subjective measurements of patient sleep, or in any other covariate measures.
Table 2.
Baseline Characteristics of Alzheimer’s Disease Patients and Caregivers*
| Characteristic | Walking (n = 32) |
Light (n = 34) |
NITE-AD (n = 33) |
Control (n = 33) |
|---|---|---|---|---|
| Patient | ||||
| Age, mean ± SD | 82.2 ± 8.5 | 80.6 ± 7.3 | 80.0 ± 8.2 | 81.2 ± 8.0 |
| Women, % | 53 | 56 | 61 | 51 |
| White, % | 84 | 91 | 88 | 81 |
| College graduate, % | 29 | 30 | 42 | 45 |
| MMSE score, mean ± SD | 19.2 ± 7.7 | 17.9 ± 7.0 | 19.1 ± 5.8 | 18.7 ± 6.9 |
| Duration of dementia, years, median (IQR) | 4.5 (3–8) | 3 (2.5–6) | 3 (1.5–5) | 4 (2–5) |
| Duration of sleep problems >1 year, % | 70 | 82 | 79 | 69 |
| Sleep Apnea score, mean ± SD | 23.6 ± 5.0 | 24.9 ± 5.3 | 23.7 ± 5.9 | 24.2 ± 5.3 |
| Sleep medication use, % | 25 | 32 | 30 | 33 |
| Cornell Depression score, mean ± SD | 7.7 ± 6.2 | 9.7 ± 6.2 | 7.6 ± 5.6 | 8.2 ± 5.7 |
| SCQ score, # limiting conditions, mean ± SD | 2.0 ± 1.5 | 2.4 ± 1.5 | 2.3 ± 1.9 | 2.3 ± 1.6 |
| Caregiver | ||||
| Age, mean ± SD | 70.4 ± 13.6 | 68.9 ± 14.4 | 73.3 ± 13.2 | 72.6 ± 11.5 |
| Women, % | 72 | 62 | 61 | 67 |
| White, % | 94 | 85 | 81 | 87 |
| College graduate, % | 59 | 32 | 53 | 39 |
| Spousal caregivers, % | 66 | 71 | 75 | 76 |
NITE-AD = combination sleep education, walk, and light therapy; SD = standard deviation, MMSE = Mini Mental Status Exam; IQR = interquartile range; SCQ = Self-Administered Comorbidity Questionnaire.
p > .05 for all comparisons.
Post-test (ITT) Outcome Analyses
Primary outcomes
All three active conditions showed a similar change score pattern in the post-test intent-to treat analyses. Patients in each active treatment had reduced actigraphic total wake time at post-test compared to control subjects (Table 3). Compared to control subjects, participants in the Walking condition were awake 33.1 fewer minutes/night (CI, −65.5 to −0.8 minutes; p =.05; effect size=.51); Light participants were awake 39.0 fewer minutes (CI, −76.0 to −1.9 minutes; p =.04; effect size=.53); and NITE-AD subects were awake 39.8 fewer minutes (CI, −69.9 to −9.6; p =.01; effect size=.63).There were no significant differences in total wake time change scores between the Walking, Light, and NITE-AD intervention groups (p =.92). Sensitivity analyses without imputation of missing data yielded a similar pattern of results, except for a slightly smaller difference in wake time for Light participants (−33.7 [CI, −68.2 to −0.9]; p =.06; effect size=.47) compared to Controls. Inclusion of potential confounders of treatment effect yielded similar findings with no effect of patient age, gender, depression, comorbidity, cognitive impairment, and sleep apnea on sleep outcomes.
Table 3.
Comparison of Primary Patient Sleep Outcomes at 2 Months and 6 Months for Contact Control vs Each Active Treatment Group*
| Mean ± (SE) | Mean ± (SE) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Baseline | 2 Months | Change at 2 Months |
p-Value | 6 Months | Change from 2 to 6 Months |
p-Value |
| Total wake time/night, minutes† | |||||||
| Control | 115.5 ± 12.9 | 122.9 ± 13.3 | 7.4 ± 12.0 | 122.0 ±15.1 | −0.9 ± 13.2 | ||
| Walking | 154.0 ± 16.5 | 128.2 ± 17.6 | −25.8 ± 11.0 | .05 | 146.5 ± 22.6 | 18.3 ± 15.2 | .97 |
| Light | 141.8 ± 14.1 | 110.2 ± 13.9 | −31.6 ± 13.5 | .04 | 123.3 ± 16.9 | 13.1 ± 13.5 | .60 |
| NITE-AD | 121.2 ± 10.8 | 88.8 ± 10.7 | −32.4 ± 9.9 | .01 | 115.0 ± 17.9 | 26.3 ± 13.5 | .67 |
| Sleep Disorders Inventory‡ | |||||||
| Control | 0.8 ± 0.2 | 0.6 ± 0.1 | −0.3 ± 0.2 | 0.5 ± 0.1 | −0.1 ± 0.2 | ||
| Walking | 1.0 ± 0.3 | 0.4 ± 0.1 | −0.6 ± 0.2 | .32 | 0.6 ± 0.2 | 0.2 ± 0.1 | .27 |
| Light | 1.3 ± 0.3 | 0.7 ± 0.1 | −0.6 ± 0.2 | .29 | 0.8 ± 0.2 | 0.1 ± 0.1 | .50 |
| NITE-AD | 1.1 ± 0.2 | 0.4 ± 0.1 | −0.6 ± 0.2 | .23 | 0.8 ± 0.2 | 0.3 ± 0.2 | .12 |
Intent-to-treat analyses, controlling for baseline values, multiple imputations. Control (n=33); Walking (n=32); Light (n=34); NITE-AD (n=33). SE = standard error; CI = confidence interval.
Based upon one week of wrist actigraphy; data presented are the mean ± SE of daily average.
The Sleep Disorders Inventory is based on a proxy-report and has a range of 0 to 7.
For the primary subjective outcome measure (SDI), there were no significant post-test differences in caregiver reports of patient sleep for any treatment group compared to Control.
Secondary actigraphy outcomes
After correcting for multiple comparisons, there were no significant differences between active treatment condition results for any secondary outcome. However, there were moderate effect size improvements in sleep percent for the three active treatment groups (NITE-AD, 5% improvement; effect size=.63; walking and light, 3.0 and 4.3% improvement, respectively; effect size=.45 and .48, respectively). There was also a trend (p =.07; effect size=.46) for Walking subjects to have fewer nighttime awakenings than those in Control (Table 4). Sensitivity analyses without imputation of missing data yielded a similar pattern of results. There were also no significant post-test differences between groups for total time in bed, suggesting that observed treatment effects were not secondary to restricted sleep schedules.
Table 4.
Comparison of Secondary Nighttime Patient Sleep Outcomes at 2 Months and 6 Months for Contact Control vs Each Active Treatment Group*
| Mean ± (SE) | Mean ± (SE) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Baseline | 2 Months | Change at 2 Months |
p-Value | 6 Months | Change from 2 to 6 Months |
p-Value |
| Sleep percent | |||||||
| Control | 79.9 ± 2.0 | 78.1 ± 2.2 | −1.9 ± 2.2 | 78.2 ± 2.4 | 0.2 ± 2.0 | ||
| Walking | 76.0 ± 2.2 | 79.0 ± 2.7 | 3.0 ± 1.6 | .07 | 77.0 ± 3.2 | −2.0 ± 2.2 | .48 |
| Light | 76.1 ± 2.2 | 80.4 ± 2.5 | 4.3 ± 2.3 | .07 | 78.9 ± 2.8 | −1.5 ± 2.0 | .61 |
| NITE-AD | 79.4 ± 1.6 | 84.4 ± 1.9 | 5.0 ± 1.6 | .02 | 80.5 ± 2.9 | −4.0 ± 2.0 | .16 |
| # Night awakenings | |||||||
| Control | 16.2 ± 1.6 | 17.6 ± 1.9 | 1.4 ± 1.3 | 18.4 ± 1.8 | 0.8 ± 1.5 | ||
| Walking | 18.7 ± 1.4 | 16.8 ± 1.6 | −1.9 ± 1.2 | .07 | 17.1 ± 1.8 | 0.3 ± 1.5 | .81 |
| Light | 18.5 ± 1.4 | 17.0 ± 2.0 | −1.5 ± 1.7 | .17 | 17.7 ± 1.9 | 0.7 ± 1.7 | .94 |
| NITE-AD | 15.2 ± 1.2 | 14.1 ± 1.5 | −1.0 ± 1.1 | .15 | 13.7 ± 1.5 | −0.4 ± 1.3 | .49 |
| Total sleep/night, mins | |||||||
| Control | 449.2 ± 18.8 | 438.3 ± 19.7 | −10.8 ± 15.6 | 435.6 ± 20.2 | −2.7 ± 17.0 | ||
| Walking | 468.1 ± 17.7 | 469.1 ± 19.8 | 1.0 ± 13.5 | .57 | 470.6 ± 21.2 | 1.5 ± 16.0 | .86 |
| Light | 454.1 ± 19.1 | 453.4 ± 19.3 | −0.6 ± 16.9 | .67 | 462.4 ± 22.1 | 9.0 ± 16.7 | .63 |
| NITE-AD | 460.4 ± 15.3 | 472.5 ± 15.7 | 12.2 ± 15.5 | .32 | 465.0 ± 19.7 | −7.6 ± 13.0 | .82 |
| Time in bed | |||||||
| Control | 564.7 + 19.4 | 561.2 + 20.5 | −3.5 ± 12.5 | 557.6 ± 21.8 | −3.6 ± 17.4 | ||
| Walking | 622.1 + 19.7 | 597.3 + 18.6 | −24.8 ± 14.7 | .27 | 617.1 ± 20.8 | 19.8 ± 15.8 | .29 |
| Light | 595.9 + 16.8 | 563.6 + 18.2 | −32.2 ± 13.0 | .11 | 585.7 ± 21.4 | 22.1 ± 18.1 | .28 |
| NITE-AD | 581.6 + 16.6 | 561.3 + 13.6 | −20.3 ± 14.9 | .42 | 580.0 ± 16.0 | 18.7 ± 12.7 | .31 |
| Daytime sleep/inactivity | |||||||
| Control | 203.9 + 20.8 | 217.0 + 24.2 | 13.1 ± 14.7 | 213.7 ± 23.5 | −3.4 ± 20.9 | ||
| Walking | 151.3 + 15.1 | 156.0 + 18.5 | 4.6 ± 12.9 | .67 | 174.2 ± 21.7 | 18.3 ± 19.7 | .42 |
| Light | 208.4 + 14.7 | 212.3 + 20.4 | 3.9 ± 16.1 | .70 | 205.3 ± 23.1 | −7.0 ± 21.6 | .89 |
| NITE-AD | 162.2 + 16.4 | 168.1 + 18.5 | 5.8 ± 13.8 | .73 | 186.9 ± 20.4 | 18.9 ± 19.2 | .38 |
Intent-to-treat analyses, controlling for baseline values, multiple imputations. Control (n=33); Walking (n=32); Light (n=34); NITE-AD (n=33). SE = standard error; CI = confidence interval.
Based upon one week of wrist actigraphy; data presented are the mean ± SE of daily average
6-Month Longitudinal Analysis
At six months, there were no significant differences in any treatment group relative to control for either the actigraphic or subjective sleep outcomes.
Study Participation
Walking, Light, and Control subjects completed an average of 2.8 home visits (range, 1–3 visits), equalling 94.6% participation in the number of total possible sessions. NITE-AD subjects had an average of 5.4 visits (range, 1–6 visits), with an overall participation rate of 89.9%. There were no significant participation differences between treatment conditions.
Compliance with Actigraphy
The majority of patients provided 5+ days of actigraphy data for all three sampling periods (98%, 93%, 90%, respectively). Technical difficulties with equipment accounted for approximately two-thirds of the missing data, with the remainder due to loss or refusal to wear the actigraph.
Adherence to Walking and Light Recommendations
In Week 1, patients assigned to Walking significantly (p<.001) increased the number of days they walked per week compared to baseline proxy-reports (4.6 vs. 2.9 days/week, respectively). Median light exposure dose for Light and NITE-AD subjects was > 2500 lux at all sampling points. However, frequency and duration of daily walking and light box use declined over time. During the first week of treatment, 65.7% of participants in the active conditions adhered to walking and/or light box recommendations 4+ days/week. At post-test, 64.6% were at this level of adherence, and adherence dropped to 48.5% at 6-month follow-up. Subjects adhering 4+ days/ week had significantly (p<.05) less total wake time and higher sleep percent at post-test than those who were less adherent. At six months, there was no relationship between adherence and sleep outcomes.
Treatment Integrity and Satisfaction
Ratings of tape-recorded sessions indicated that, consistent with study protocol, 100% of Walking (n=16), Light (n=19), and NITE-AD (n=19) sessions contained only content that was permitted for inclusion. No Control sessions (n=18) included instruction regarding walking, light exposure, sleep scheduling, or homework.
Caregivers rated all treatment conditions highly in terms of how they were treated and how well information was explained. More than 92% of caregivers indicated they would refer the program to a friend. Caregivers in NITE-AD were significantly more likely to state they had benefited from treatment (χ2 = 8.17, p =.04). However, NITE-AD was also the condition that caregivers were most likely to rate as requiring too much work or effort (Control=4%, Walking=13%, Light=19%, NITE-AD=25%).
Adverse Reactions
Subject changes in health status (including medical visits, injuries, and falls) were collected at each assessment point and reviewed quarterly by a data safety committee consisting of two nurses and a neurologist unrelated to the study. No unexpected or serious adverse events were attributed to any intervention.
DISCUSSION
This study indicates that caregivers of community-dwelling persons with dementia can implement walking and light exposure protocols, singly and in combination, and that such interventions can improve objectively measured patient nighttime sleep. Patients in active treatment were awake for an average of 37 minutes/night less than control subjects at post-test, and were asleep for a proportionally greater percent of their time in bed compared to control subjects. Patients with better adherence to treatment recommendations had better sleep outcomes. Despite wide variability in dementia severity, type of sleep disturbance, age, medical co-morbidity, caregiver relationship, and home environments, caregivers overall rated the interventions positively and indicated they were not overly burdensome. Obtaining objective sleep improvements in this difficult and heterogeneous population supports the potential generalizability of study findings.
Significant post-test actigraphic improvements were not replicated on the SDI, which was surprising since subjective sleep outcomes are often greater than objective changes in sleep intervention studies. Given that actigraphy is a measure of movement, it is possible that the interventions reduced nighttime restlessness rather than improved sleep per se. This finding may also be due to low initial baseline scores on the SDI compared to those published for other sleep-disturbed AD populations.24 During the initial phone screening, caregivers reported a minimum of two sleep problems on the frequency subscale of the SDI occurring three or more times per week (mean=3.6). However, when the entire SDI was collected at the baseline assessment, mean frequency ratings were significantly lower (mean=2.6), leaving less room for treatment related change. Previous studies have shown that caregiver reports of AD patient sleep disturbances are not always congruent with actigraphy measurements.37 Additional research is needed to examine the accuracy and reliability of proxy sleep reports with dementia dyads.
Significant sleep improvements were also not sustained at six months. If trainers had met with participants between the post-test and 6-month follow-up assessments to encourage continued daily walking and light box use, longitudinal outcomes might have been improved. Feedback solicited after the 6-month follow-up revealed a number of factors that contributed to decreasing treatment adherence over time. Inclement weather, patient or caregiver frailty, and poor neighborhood walkability caused some participants to stop walking. Others had difficulty using the light box for an hour every day. Previous research has suggested that caregiver and patient factors such as depression and stress can also negatively impact adherence to behavioral protocols.29,38 Future studies could clarify the physical, environmental, or interpersonal characteristics of patients and caregivers that influence treatment adherence and subsequent sleep outcomes, as well as the amount of trainer contact (treatment “dose”) that is needed for dyads to develop sustainable daily walking and/or light exposure routines.
Although these findings are promising, the study had several methodological limitations. Due to a higher than planned attrition rate, we were unable to detect significant differences between the three active treatment conditions. Nevertheless, this is the largest study to date examining treatment of sleep disturbances in independently living AD patients, and provides a solid foundation for future investigations. Subjects in the combination NITE-AD condition had double the trainer contact time (six home visits versus three) compared to the other treatment groups. Although this raises concerns about the possibility of a Hawthorne effect influencing outcomes, data patterns did not suggest that NITE-AD participants had any greater treatment response than subjects assigned to walking or light exposure alone. Participants in all treatment groups (including Control) received written handouts about basic sleep hygiene such as are widely available in the popular literature (Table 1), and it is possible that results were confounded because dyads in the Walking, Light, or Control conditions made sleep hygiene changes without explicit instruction and assistance in doing so. However, research indicates that information-only interventions generally have limited benefit for improving sleep, and dementia caregivers need assistance implementing and maintaining sleep behavior change plans.39–41 Lastly, we did not conduct polysomnographic assessments, so cannot rule out the possibility that some subjects may have had undiagnosed primary sleep disorders. However, inclusion of polysomnography would have added considerable expense and burden to the study protocol, excluded cognitively impaired individuals unable to tolerate the procedure, and compromised the ecological validity of week-long in-home actigraphic assessments.
The study was designed to make treatment recommendations as feasible as possible given each dyad’s unique situation. In doing so, we had to sacrifice some experimental control of the interventions. For example, subjects were able to select their own walking pace, and future studies are needed to determine if greater sleep improvements could be obtained from a more vigorous exercise routine. Light exposure sessions were scheduled based upon each patient’s average baseline bedtime rather than using more sensitive biological markers of circadian rhythm (e.g., body temperature). Thus, some patients may not have received maximum benefit from exposure to light. However, scheduling sessions at alternative times matched to biological rhythms might not be feasible given the daily life routines of most community-dwelling dyads. We also did not ask walkers to avoid outside light exposure during the daily walks, which may have confounded the walking and light exposure results.
In conclusion, this study provides evidence that community-dwelling AD patients with sleep problems can benefit from walking and increased light exposure, either alone or in combination. However, these interventions must be implemented with caregiver assistance, and would likely be strengthened with booster sessions aimed at maintaining adherence over time. Future research is needed to determine characteristics of dyads most likely to benefit from one treatment versus another. In the meantime, non-pharmacological strategies should be considered as an alternative or supplement to pharmacotherapy in the management of this difficult problem in dementia care.
Appendix 1.
Overview and comparison of treatment protocol session content
| Week | Walk | Light | NITE-AD | Control |
|---|---|---|---|---|
| 1 | Introduction to sleep in aging and dementia Rationale for walking to improve sleep Development of daily walking plan |
Introduction to sleep in aging and dementia Rationale for light exposure to improve sleep Development of daily light exposure plan |
Introduction to sleep in aging and dementia Development of individualized participant sleep plan Rationale for walking to improve sleep Development of daily walking plan |
Introduction to sleep in aging and dementia |
| 2 | Problem-solving obstacles to walking adherence | Problem-solving obstacles to light exposure adherence | Problem-solving obstacles to walking and sleep plan adherence Rationale for light exposure to improve sleep Development of daily light exposure plan |
Caregiver nondirective support |
| 3 | Problem-solving obstacles to walking, light, and sleep plan adherence ABCs of behavior management |
|||
| 4 | Brief phone call: Use of daily sleep and walking log | Brief phone call: Use of daily sleep and light exposure log | Problem-solving obstacles to walking, light, and sleep plan adherence | Brief phone call: Use of daily sleep log |
| 5 | ||||
| 6 | Brief phone call: Use of daily sleep and walking log | Brief phone call: Use of daily sleep and light exposure log | Problem-solving obstacles to walking, light, and sleep plan adherence | Brief phone call: Use of daily sleep log |
| 7 | ||||
| 8 | Maintenance walking plan | Maintenance light exposure plan | Maintenance plan | Caregiver nondirective support |
|
Treatment Implementation Components |
Education Goal setting Problem-solving Homework |
Education Goal setting Problem-solving Homework |
Education Goal setting Problem-solving Homework |
Education Caregiver support |
ACKNOWLEDGMENTS
Appreciation is extended to study trainers (Maureen Brink-Lund, Cynthia Deschenes, David LaFazia), and staff at the University of Washington Northwest Research Group on Aging (Amy Moore, June van Leynseele, Thom Walton, Raymond Houle, Kendra Wight, Felicia Fleming, and Raquelle Williams) and Group Health Research Institute (Malia Oliver, Kathy Plant, Darlene White). We would also like to thank Drs. Sonia Ancoli-Israel and Charles F. Reynolds, III for their assistance as consultants on this grant.
This work was supported by the National Institute of Mental Health (Grant #MH072736).
Sponsor’s Role: The National Institute of Mental Health was not involved in study design, implementation, analysis, or manuscript preparation. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
Footnotes
Portions of this paper were presented at the Associated Professional Sleep Societies’ 24th annual meeting, June 5–9, 2010, San Antonio, TX.
Financial disclosures: None reported.
Author Contributions: Dr. McCurry, as principal investigator, had full access to all study data and takes responsibility for all aspects of the study, including the integrity of the data and the accuracy of the data analysis.
Study concept and design: McCurry, Vitiello, Logsdon, Larson, Teri
Acquisition of data: McCurry, Larson
Analysis and interpretation of data: McCurry, Pike, Vitiello, Logsdon, Larson, Teri
Drafting of the manuscript: McCurry, Pike
Critical revision of the manuscript for important intellectual content: McCurry, Pike, Vitiello, Logsdon, Larson, Teri
Statistical analysis: Pike
Obtained funding: McCurry, Vitiello, Larson, Teri
Administrative, technical, or material support: McCurry, Vitiello, Logsdon, Larson, Teri
Study supervision: McCurry
REFERENCES
- 1.Gaugler JE, Edwards AB, Femia EE, et al. Predictors of institutionalization of cognitively impaired elders: Family help and the timing of placement. J Gerontol Psychol Sci. 2000;55B:P247–P255. doi: 10.1093/geronb/55.4.p247. [DOI] [PubMed] [Google Scholar]
- 2.Hope T, Keene J, Gedling K, et al. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Knopman DS, Kitto J, Deinard S, et al. Longitudinal study of death and institutionalization in patients with primary degenerative dementia. J Am Geriatr Soc. 1988;36:108–112. doi: 10.1111/j.1532-5415.1988.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 4.McCurry SM, Logsdon RG, Teri L, et al. Sleep disturbances in caregivers of persons with dementia: Contributing factors and treatment implications. Sleep Med Rev. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- 6.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58:480–486. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 7.Spira AP, Friedman L, Beaudreau SA, et al. Sleep and physical functioning in family caregivers of older adults with memory impairment. Int Psychogeriatr. 2010;22:306–311. doi: 10.1017/S1041610209991153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Jeste DV, Chung H, et al. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med. 2005 Spec No:6–22. [PubMed] [Google Scholar]
- 9.Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004;6 Suppl 1A:S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 10.Paniagua MA, Paniagua EW. The demented elder with insomnia. Clin Geriatr Med. 2008;24:69–81. doi: 10.1016/j.cger.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Alessi CA, Schnelle JF, Traub S, et al. Psychotropic medications in incontinent nursing home residents: Association with sleep and bed mobility. J Am Geriatr Soc. 1995;43:788–792. doi: 10.1111/j.1532-5415.1995.tb07052.x. [DOI] [PubMed] [Google Scholar]
- 12.Gehrman PR, Connor DJ, Martin JL, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessi CA, Yoon EJ, Schnelle JF, et al. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: Do sleep and agitation improve? J Am Geriatr Soc. 1999;47:784–791. doi: 10.1111/j.1532-5415.1999.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 14.Femia EE, Zarit SH, Stephens MA, et al. Impact of adult day services on behavioral and psychological symptoms of dementia. Gerontologist. 2007;47:775–788. doi: 10.1093/geront/47.6.775. [DOI] [PubMed] [Google Scholar]
- 15.Naylor E, Penev PD, Orbet L, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- 16.Sullivan SC, Richards KC. Predictors of circadian sleep-wake rhythm maintenance in elders with dementia. Aging Ment Health. 2004;8:143–152. doi: 10.1080/13607860410001649608. [DOI] [PubMed] [Google Scholar]
- 17.Richards KC, Beck C, O'Sullivan PS, et al. Effect of individualized social activity on sleep in nursing home residents with dementia. J Am Geriatr Soc. 2005;53:1510–1517. doi: 10.1111/j.1532-5415.2005.53460.x. [DOI] [PubMed] [Google Scholar]
- 18.Dowling GA, Burr RL, Van Someren EJ, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. J Am Geriatr Soc. 2008;56:239–246. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontana Gasio P, Krauchi K, Cajochen C, et al. Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Exp Gerontol. 2003;38:207–216. doi: 10.1016/s0531-5565(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 20.Riemersma-van der Lek RF, Swaab DF, Tiwsk J, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 21.Sloane PD, Williams CS, Mitchell CM, et al. High-intensity environmental light in dementia: Effect on sleep and activity. J Am Geriatr Soc. 2007;55:1524–1533. doi: 10.1111/j.1532-5415.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 22.van Someren EJW, Kessler A, Mirmiran M, et al. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 23.McCurry SM, Gibbons LE, Logsdon RG, et al. Nighttime insomnia treatment and education for Alzheimer's disease: A randomized, controlled trial. J Am Geriatr Soc. 2005;53:793–802. doi: 10.1111/j.1532-5415.2005.53252.x. [DOI] [PubMed] [Google Scholar]
- 24.Tractenberg RE, Singer CM, Cummings JL, et al. The Sleep Disorders Inventory (SDI): An instrument for studies of sleep disturbance in persons with Alzheimer's disease. J Sleep Res. 2003;12:331–337. doi: 10.1046/j.0962-1105.2003.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 27.Facts: About sleep changes in Alzheimer's disease. Chicago: IL: 2002. Alzheimer's Association. [Google Scholar]
- 28.Age Page: A good night's sleep. Washington, D.C.: U.S. Government Printing Office; 1990. National Institute on Aging. #PHS 242-787 00001. [Google Scholar]
- 29.McCurry SM, Pike KC, Logsdon RG, et al. Predictors of short and long-term adherence to a daily walking program in persons with Alzheimers disease. Am J Alzheimers Dis Other Demen. 2010;25:505–512. doi: 10.1177/1533317510376173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teri L, Logsdon R. Assessment and management of behavioral disturbances in Alzheimer's disease patients. Comprehensive Ther. 1990;16:36–42. [PubMed] [Google Scholar]
- 31.Cole RJ, Kripke DJ. Automatic sleep/wake identification from wrist actigraphy. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 32.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexopoulos GS, Abrams RC, Young RC, et al. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 34.Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 37.McCurry SM, Vitiello MV, Gibbons LE, et al. Factors associated with caregiver perceptions of sleep disturbances in persons with dementia. Am J Geriatr Psychiatry. 2006;14:112–120. doi: 10.1097/01.JGP.0000192499.25940.da. [DOI] [PubMed] [Google Scholar]
- 38.Dowling GA, Wiener CL. Roadblocks encountered in recruiting patients for a study of sleep disruption in Alzheimer's disease. Image J Nurs Sch. 1997;29:59–64. doi: 10.1111/j.1547-5069.1997.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 39.Edinger JD, Samson WS. A primary care "friendly" cognitive behavioral insomnia therapy. Sleep. 2003;26:177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 40.McCurry SM, Gibbons LE, Logsdon RG, et al. Training caregivers to change the sleep hygiene practices of patients with dementia: The NITE-AD Project. J Am Geriatr Soc. 2003;10:1455–1460. doi: 10.1046/j.1532-5415.2003.51466.x. [DOI] [PubMed] [Google Scholar]
- 41.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]