Abstract
Context
Although the cross-sectional association between cancer-related pain and disability is well-established, their longitudinal relationship has been less studied.
Objectives
Data from the Indiana Cancer Pain and Depression (INCPAD) trial was analyzed to determine whether baseline cancer-related pain and changes in pain over time predict disability over 12 months.
Methods
A total of 274 cancer survivors with cancer-related pain were accrued in the INCPAD trial. Data were collected at baseline, 1, 3, 6, and 12 months by interviewers blinded to treatment arm. Disability outcomes included a continuous measure (Sheehan Disability Scale score) and a categorical measure (≥ 14 days in the past four weeks with a ≥ 50% reduction in usual activities). Predictor variables, operationalized by the Brief Pain Inventory, included baseline pain severity and changes in pain severity scores between each time point. Multivariable analyses were conducted adjusting for treatment group, baseline disability, and selected covariates including depression.
Results
Baseline pain severity did not predict disability outcomes at 12 months. However, improvement in pain severity predicted less disability over 12 months both in terms of Sheehan Disability Scale scores (b = −0.17, t = −5.33, P< 0.001) and ≥ 14 disability days in the past month (odds ratio = 0.85; 95% confidence interval, 0.79–0.93; P< 0.001).
Conclusion
Disability over 12 months in patients with cancer-related pain is predicted by changes in pain severity over time. Results suggest that effective pain management may reduce subsequent disability among cancer survivors.
Keywords: Cancer-related pain, disability, longitudinal study
Introduction
The National Cancer Institute has determined that an individual becomes a cancer survivor at the time of cancer diagnosis and remains a cancer survivor through the balance of his or her life.1 In 2006, it was estimated that there were 11.4 million cancer survivors, representing approximately 3.8% of the U.S. population.2 Cancer survivors are at risk for disability because they are more vulnerable to other cancers, cardiovascular diseases, osteoporosis, diabetes mellitus, and accelerated functional decline.3
Comprehensive assessment of the impact of cancer and its treatment typically includes symptoms experienced, physical functioning, and quality of life.4–7 However, little attention has been paid to factors that influence disability after cancer is diagnosed. Previous cancer survivorship studies have defined disability in a limited way, such as physical activity limitation8–11 or work impairment.12, 13 It is necessary to understand how cancer survivors can function reasonably well both socially and at work. Disability can be broadly defined as impairment in family roles, social roles, and work roles and responsibilities.14, 15 While some cancer survivors, even those who experience severe symptoms, are able to maintain, or return to, their usual roles and responsibilities, others with less severe symptoms can be quite disabled.16, 17 Therefore, treatment must focus not only on control of symptoms for cancer survivors, but also on reducing morbidity resulting from disability.
Research has shown that symptoms are positively related to disability.15, 17–19 Pain, a highly prevalent symptom in cancer survivors, has the potential to be linked with disability. From 33% to 64% of cancer survivors experience pain, and studies have shown that cancer pain is undertreated in up to 82% of survivors.20, 21 It also has been shown that cancer survivors with more severe pain have lower Karnofsky Performance scores.22, 23, 24
The Indiana Cancer Pain and Depression (INCPAD) study is a randomized clinical trial implemented among survivors who have various types and phases of cancer, with a 12-month telephone care management intervention targeted to improve pain and/or depression.25 Cross-sectional analyses of baseline data showed that participants with more severe cancer-related pain reported greater disability.26 However, the relationship between changes in pain over time and subsequent disability is less well-established. The most recent symptom management model has shown that the temporal aspect of symptoms must be embraced in future research.27 The repeated assessment of outcomes over 12 months in the INCPAD trial provided a unique opportunity to examine the longitudinal relationship between cancer-related pain and disability. Therefore, we conducted this secondary analysis to address the following research questions:
Does cancer-related pain in cancer survivors on entry to the INCPAD trial (baseline) predict disability at 12 months?
Do changes in their cancer-related pain predict subsequent disability over 12 months?
Methods
Sample and Setting
The design, intervention, and participant characteristics of the INCPAD study have been described in previous studies.25, 26 Briefly, patients from 16 urban and rural outpatient oncology clinics in Indiana from March 2006 through August 2008 were screened for the presence of cancer-related pain or depression. Eligibility criteria required that patients be experiencing moderately severe cancer-related pain (a Brief Pain Inventory [BPI] worst pain severity score ≥ 6), or depression (a Patient Health Questionnaire nine-item depression scale [PHQ-9] score ≥ 10, with depressed mood and/or anhedonia).28–31 Cancer-related pain was defined as pain occurring in the region of the primary tumor or cancer metastases and/or occurring after the onset of cancer treatment. This pain had to be persistent despite the patient trying at least one pain medication. Excluded were patients who: (a) did not speak English; (b) had moderately severe cognitive impairment as defined by a six-item cognitive screener;32 (c) had schizophrenia or other psychoses; (d) had a disability claim currently being adjudicated for pain; (f) were pregnant; (g) were in hospice care; or (h) had pre-existing pain conditions unrelated to cancer.
Of the 405 cancer patients enrolled in the INCPAD trial, 96 had cancer-related pain only, 131 had depression only, and 178 had both cancer-related pain and depression. Block randomization was stratified by symptom type (cancer-related pain only, depression only, or both cancer-related pain and depression) resulting in 202 being in the intervention group and 203 being in the control group. For the current secondary analysis, only data from 274 participants who had cancer-related pain (with or without depression) were included: 137 in the intervention group and 137 in the control group. The intervention group received telephone care management (telecare) focusing on optimizing medications to treat their cancer-related pain and/or depression, while the control group received usual oncology care.
Telecare Intervention
Details of the telecare intervention have been described previously.25 In brief, participants in the intervention group underwent a schedule of automated symptom monitoring by telephone or over the Internet for 12 months. Participants also received several scheduled calls from a centralized nurse care manager during treatment initiation as well as subsequent calls based upon automated monitoring trend reports. The care manager nurse was supervised by a physician specialist who met in weekly case management conferences to discuss treatment plans for newly-enrolled patients as well as patients who needed further adjustments in therapy. Recommendations for optimizing analgesics (for pain) or antidepressants (for depression) were provided to the participant’s primary oncologist who was responsible for prescribing all medications. The treatment goal for cancer-related pain was at least a 30% reduction in the BPI score. The effects of the telecare intervention have been published.33 Our current analysis controlled for intervention group assignment.
Data Collection
Data were collected through phone interviews at baseline (T0), one month (T1), three months (T3), six months (T6), and 12 months (T12). Research assistants conducting the interviews were blinded to group assignment.
Measures
Outcome Variables
Disability was measured using the Sheehan Disability Scale (SDS) and Total Disability Days (TDD). The SDS consists of three items asking how much the participant’s health condition has interfered with his/her family life, social life, and work over the past month on a scale of 0 (not at all) to 10 (unable to carry on any activities). The SDS score was a mean of these three items; higher scores reflect greater disability. The construct and criterion validity of the SDS are well established18 and internal reliability in the INCPAD study was 0.82.25 TDD was assessed with a single item that asked participants to indicate the number of days during the preceding four weeks that they were either in bed or had to reduce work or usual activities by 50% or more.34, 35
Independent Variables
Cancer-related pain was the major predictor variable of interest and was measured using the Brief Pain Inventory (BPI) severity scale, which asks participants to rate their pain at its worst, its least, and on average in the past week, as well as their current pain on a 0 (no pain) to 10 (pain as bad as you can imagine) scale.28,36 The mean of the four items was determined, with higher scores reflecting more severe pain. The BPI had good internal reliability (Cronbach’s α = 0.79) in the INCPAD study.25
Depression at baseline was measured as an important covariate using the Hopkins Symptom Checklist 20-item (HSCL-20) depression scale.37 The 20 items ask how much participants had been distressed by various symptoms of depression in the previous four weeks on a 0 (not at all) to 5 (extremely) scale. The mean of these items is determined, with higher scores reflecting more severe depression. The HSCL-20 had good internal reliability (α = 0.79) in INCPAD.25
Other potential covariates included demographics (age, gender, race, marital status, education, job status, and income) and baseline clinical factors (medical comorbidity, type of cancer, and phase of cancer). Age, gender, race, marital status, education, job status, and income were collected using a demographic survey. The Socioeconomic Disadvantage (SED) Index is a composite measure, which assigns one point each for low education (“less than high school” = 1 point), unemployment (“unable to work due to health or disability” = 1 point), or low income (“not enough to make ends meet” = 1 point).38 Medical comorbidity was assessed using a checklist of eight diseases. Type of cancer and phase of cancer were extracted from medical records. Phase of cancer was determined by if participants were newly-diagnosed, had maintenance or disease-free status, or had recurrent or progressive cancers.
Statistical Analysis
The two disability outcome variables in the analysis were the SDS score and TDD. The SDS was a continuous variable. The total number of disability days in the past four weeks, TDD, ranged from 0 to 28. However, the distribution of TDD was bimodal (U shaped). We, therefore, recoded TDD as a binary variable (< 14 days = 0; ≥ 14 days =1).
The first research question asked whether cancer-related pain at baseline of the INCPAD trial predicted disability at 12 months. The predictor variable was the baseline BPI severity score. Of the 274 participants who provided baseline data, 180 completed 12-month interviews (the remainder had died, dropped out, or were unable to be contacted). Data from these 180 participants were used to develop two models for the first research question. A multivariable linear regression analysis was performed to examine the SDS at 12 months. We initially conducted bivariate analyses to determine which covariates (i.e., those with a P-value of less than 0.20) to adjust for in the multivariable model. We then ran the full model with baseline BPI severity as a predictor while adjusting for the covariates, baseline SDS, and intervention group factor. For TDD at 12 months, a logistic regression model was used. We followed the same two steps described above for this second model as well.
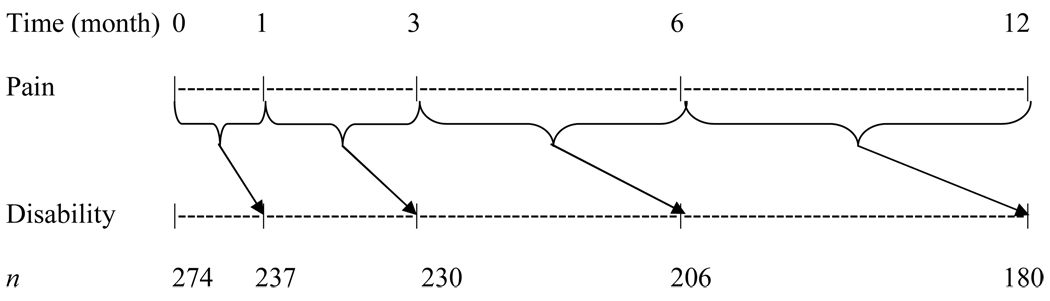
The second research question asked whether changes in cancer-related pain predicted disability at the subsequent time point over the 12 months. Figure 1 diagrams the repeated measures modeling. The outcome variables were SDS and TDD at one month, three months, six months, and 12 months. Predictor variables were BPI severity changes between each time point over 12 months (T0-T1: between baseline and one month; T1-T3: between one month and three months; T3-T6: between three months and six months; and T6-T12: between six months and 12 months). Data from available participants at each time point were examined in the linear mixed effects repeated measures for SDS and generalized linear mixed effects repeated measures for TDD. In this analysis, random subject effect was incorporated into the model to accommodate the potential correction among the repeatedly measured outcomes within the subject. For model selection, we initially performed bivariate analyses to determine which covariates were potentially significant (i.e., P-value < 0.20). Then we developed a full model by adjusting for the covariates, baseline disability, and intervention group factor. All analyses were performed using SAS Version 9.1 (SAS Institute, Cary, NC).
Fig. 1.
Repeated measures for examining whether preceding cancer-related pain change predicts subsequent disability over 12 months.
Results
Characteristics of the Sample
Table 1 shows baseline characteristics of the 274 participants with cancer-related pain, and also compares those who completed the 12-month follow-up interview (completers) and those who did not (non-completers). Completers were 66% in the 274 participants. Non-completers were similar to completers except for being more socioeconomically disadvantaged (P< 0.001), more likely to have lung or gastrointestinal cancer (P< 0.001), and more likely to have a newly diagnosed or recurrent/progressive cancer (P< 0.001). Importantly, baseline disability (SDS and TDD), pain (BPI severity), and depression (HSCL-20) were not different between completers and non-completers. The completers reported a mean of 4.31 (± 3.85) on the SDS at 12 months, and 50% (n = 90) reported TDD ≥ 14 days.
Table 1.
Baseline Characteristics of Participants with Cancer-Related Pain (T0)
|
n =274 (T0) |
n =180 (12-month Completers) |
n = 94 (12-month Non-completers) |
Pa | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Age | 58.08 (10.54) | 58.06 (10.39) | 58.14 (10.87) | 0.95 |
| SED Index (0–3 points) |
1.30 (0.98) | 1.15 (0.96) | 1.60 (0.95) | < 0.001 |
| Medical Comorbidity (No. of diseases) |
2.09 (1.68) | 2.17 (1.63) | 1.93 (1.78) | 0.25 |
| Cancer-related Pain (BPI, 0–10 scale) |
5.22 (1.82) | 5.18 (1.80) | 5.30 (1.85) | 0.59 |
| Depression (HSCL-20, 0–5 scale) |
1.41 (0.76) | 1.40 (0.78) | 1.43 (0.74) | 0.75 |
| SDS (0–10 scale) |
5.43 (2.93) | 5.28 (2.99) | 5.72 (2.79) | 0.24 |
| n (%) | n (%) | n (%) | ||
| Group | ||||
| Intervention | 137 (50) | 91 (50.56) | 46 (48.94) | 0.80 |
| Symptom Group | ||||
| Pain Only | 96 (35.04) | 66 (36.67) | 30 (31.91) | 0.43 |
| Sex | ||||
| Female | 181 (66.06) | 126 (70.00) | 55 (58.51) | 0.06 |
| Race | ||||
| White | 212 (77.37) | 142 (78.89) | 70 (74.47) | 0.41 |
| Marital Status | ||||
| Married | 130 (47.45) | 85 (47.22) | 45 (47.87) | 0.92 |
| Type of Cancer | ||||
| Breast | 70 (25.55) | 60 (33.33) | 10 (10.64) | < 0.001 |
| Lung | 53 (19.34) | 24 (13.33) | 29 (30.85) | |
| Gastrointestinal | 51 (18.61) | 28 (15.56) | 23 (24.47) | |
| Lymphoma or Hematological |
40 (14.60) 27 (9.85) |
28 (15.56) 18 (10.00) |
12 (12.77) 9 (9.57) |
|
| Genitourinary | 33 (12.04) | 22 (12.22) | 11 (11.70) | |
| Other | ||||
| Phase of Cancer | ||||
| Newly-diagnosed | 104 (37.96) | 60 (33.33) | 44 (46.81) | < 0.001 |
| Maintenance or disease- free |
110 (40.15) | 88 (48.89) | 22 (23.40) | |
| Recurrent or progressive |
60 (21.90) | 32 (17.78) | 28 (27.79) | |
| TDD | ||||
| ≥ 14 days | 186 (67.88) | 124 (68.89) | 62 (65.96) | 0.62 |
| < 14 days | 88 (32.12) | 56 (31.11) | 32 (34.04) | |
BPI = Brief Pain Inventory; HSCL-20 = Hopkins Symptom Check List-20; SED Index = Socioeconomic Disadvantage Index; SDS = Sheehan Disability Scale; TDD = Total Disability Days.
Comparisons between 12-month follow-up and no 12-month follow-up groups. P-value < 0.05 is in boldface.
Research Question 1: Does Cancer-Related Pain in Cancer Survivors on Entry to the INCPAD Trial (Baseline) Predict Disability at 12 Months?
The results of the linear regression model and logistic regression model are summarized in Table 2. There was no relationship between baseline cancer-related pain and either of the disability variables (SDS or TDD) at 12 months after controlling for the intervention group factor, baseline disability, and covariates. Variables that did predict a higher SDS score at 12 months included greater baseline disability, worse depression, and more socioeconomic disadvantage, whereas being in the intervention group predicted a lower SDS score. Variables that predicted TDD ≥ 14 days at 12 months included greater baseline disability and greater socioeconomic disadvantage. Other potential covariates not shown in Table 2 that were not significant included age, medical comorbidity, sex, race, marital status, type of cancer, and phase of cancer.
Table 2.
Multivariate Predictors of Disability at 12 Months (n = 180)
| Outcome Variables | ||||||
|---|---|---|---|---|---|---|
| Independent Variables | Sheehan Disability Scale scorea | Total Disability Days in past 4 weeks ≥ 14b | ||||
| Beta | t | P | Odds Ratio | 95% CI | P | |
| Intervention Group | −1.04 | −2.66 | 0.009 | 0.62 | 0.31 – 1.25 | 0.18 |
| Baseline Disability | ||||||
| SDS | 0.20 | 2.29 | 0.02 | - | - | - |
| TDD | - | - | - | 1.08 | 1.04 – 1.13 | < 0.001 |
| Predictor Variable | ||||||
| Baseline Cancer-related Pain | −0.07 | −0.62 | 0.54 | 0.95 | 0.78 – 1.16 | 0.64 |
| Covariatesc | ||||||
| Depression | 1.21 | 3.52 | < 0.001 | 1.47 | 0.84 – 2.58 | 0.18 |
| SED Index | 0.49 | 2.14 | 0.03 | 1.72 | 1.14 – 2.60 | 0.009 |
SED Index = Socioeconomic Disadvantage Index; SDS = Sheehan Disability Scale; TDD = Total Disability Days; CI = confidence interval.
P-value < 0.05 is in boldface.
Multivariate linear regression model.
Logistic regression model.
Significant covariates from the models.
Research Question 2: Do Changes in Cancer Survivors’ Cancer-Related Pain Predict Subsequent Disability Over 12 Months?
Table 3 summarizes results from the linear mixed effects repeated measures and generalized linear mixed effects repeated measures analyses. Change in pain severity was a significant predictor of both disability outcomes. For each one-point decrease in BPI severity, the SDS score decreased by 0.17, and there was a 15% decrease (i.e., odds ratio [OR] = 0.85) in the likelihood of having 14 or more disability days in the past four weeks. Also, greater baseline disability and worse depression predicted greater disability over 12 months, whereas being in the intervention group predicted less disability.
Table 3.
Multivariate Predictors of Disability over 12 Months (n = 274)
| Outcome Variables | ||||||
|---|---|---|---|---|---|---|
| Independent Variables | Sheehan Disability Scale Scorea | Total Disability Days in past 4 weeks ≥ 14b | ||||
| Beta | t | P | Odds Ratio | 95% CI | P | |
| Intervention Group | −0.99 | −3.92 | < 0.001 | 0.60 | 0.38 – 0.94 | 0.03 |
| Baseline Disability | ||||||
| SDS | 0.24 | 4.37 | < 0.001 | - | - | - |
| TDD | - | - | - | 1.08 | 1.05 – 1.10 | < 0.001 |
| Predictor Variable | ||||||
| Cancer-related Pain Change | −0.17 | −5.33 | < 0.001 | 0.85 | 0.79 – 0.93 | < 0.001 |
| Covariatec | ||||||
| Depression | 1.16 | 5.49 | < 0.001 | 2.14 | 1.49 – 3.04 | < 0.001 |
SDS = Sheehan Disability Scale; TDD = Total Disability Days; CI = confidence interval.
P-value < 0.05 is in boldface.
Linear mixed effects repeated measures.
Generalized linear mixed effects repeated measures.
The only significant covariate from the models.
Discussion
Our study findings can help inform cancer survivors and their family members, health care providers, and employers who are concerned about disability resulting from cancer-related symptoms such as pain. While baseline cancer-related pain was not a predictor of disability at 12 months, change in pain over the 12 months did predict disability. Specifically, improvement in pain resulted in less disability whether measured as a continuous disability score or as categorical outcome (i.e., ≥ 14 disability days in the past four weeks). Importantly, change in pain severity remained a predictor even after adjusting for baseline disability, depression severity, and the effects of the intervention.
Our study has several strengths. First, we focused on survivors with at least moderately severe cancer-related pain, so we could readily observe changes in pain over a period of time without a ceiling effect. Second, disability beyond physical dysfunction was measured. We broadened our conceptual definition of disability to difficulty performing roles and responsibilities in family, social life, and at work. We also used two measures to operationalize disability: a continuous disability score (SDS) as well as a binary variable of 14 or more days in the past four weeks with at least a 50% reduction in usual activities (TDD). Therefore, when a predictor variable or covariate predicted both outcomes, its relationship with disability could be considered more robust because it was associated with two perspectives on disability measures.
Not surprisingly, the intervention group, which received telecare management focused on optimizing pain therapy, had less disability. Also, baseline disability was a predictor of disability at 12 months as well as in the repeated measures analysis over the 12 months of the INCPAD trial. This result was similar to that of a prior study that showed baseline disability predicted disability at 24 months among older cancer survivors who had had no active treatment for three years.10
Two other predictors of disability over 12 months were depression and socioeconomic disadvantage. The substantial and pervasive effect of depression on disability is well-established.39, 40 Our findings suggest in addition to treating pain, treating comorbid depression may be beneficial in reducing disability. The reciprocal adverse effects of pain and depression on one another as well as upon functional status and quality of life41–43 make their co-management a clinical priority.
Some cancer studies have shown that lower educational attainment or household income was associated with working disability.11, 44–46 Socioeconomically disadvantaged patients may be more likely to use negative coping strategies, hold physically demanding jobs with limited autonomy, possess little or no health insurance, and have less social support, which may lead to greater disability.47 For cancer survivors who are disadvantaged socioeconomically, clear and appropriate pain education, additional care management, and supportive social services may be needed when a pain management intervention is implemented. Referrals to social workers, public welfare agencies, and resources for cancer survivors, such as the American Cancer Society, may be important to consider for this subgroup of the population.
There are some limitations in our study. First, we used a sample of cancer patients in the INCPAD study. This may limit the generalizability of the findings as patients had to meet study eligibility criteria and agree to participate in the study. Second, we lost one-third (34%) of participants over 12 months in the study. However, their baseline disability, pain, and depression were not different from those who completed the study interview at 12 months.
Between 33% and 50% of patients experience cancer-related pain at some point in the cancer trajectory.48 Our findings highlight the importance of effective pain management among cancer survivors in potentially reducing long-term disability. Patients with high disability at baseline may require extra efforts, as may those who are socioeconomically disadvantaged or suffer from clinical depression. Recognizing the value of effective pain treatment as well as clinical factors requiring special attention are significant steps towards the alleviating the burden of cancer-related symptoms.
Acknowledgments
This study was supported by a grant from the National Cancer Institute to Dr. Kroenke (R01 CA-115369) and by a grant from the National Institute of Nursing Research (T32 NR007066).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- 1.National Cancer Institute. About cancer survivorship research: Survivorship definition. 2006 Available from: http://cancercontrol.cancer.gov/ocs/definitions.html. Accessed January 25, 2010.
- 2.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 3.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Cohen HJ. Promoting healthy lifestyles in older cancer survivors to improve health and preserve function. J Am Geriatr Soc. 2009;57 Suppl 2:S262–S264. doi: 10.1111/j.1532-5415.2009.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37(4):715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pockaj BA, Degnim AC, Boughey JC, et al. Quality of life after breast cancer surgery: what have we learned and where should we go next? J Surg Oncol. 2009;99(7):447–455. doi: 10.1002/jso.21151. [DOI] [PubMed] [Google Scholar]
- 6.Jensen K, Jensen AB, Grau C. A cross sectional quality of life study of 116 recurrence free head neck cancer patients. The first use of EORTC H&N35 in Danish. Acta Oncol. 2006;45(1):28–37. doi: 10.1080/02841860500417536. [DOI] [PubMed] [Google Scholar]
- 7.Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2382–2389. doi: 10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilt TJ. Clarifying uncertainty regarding detection and treatment of early-stage prostate cancer. Semin Urol Oncol. 2002;20(1):10–17. doi: 10.1053/suro.2002.30393. [DOI] [PubMed] [Google Scholar]
- 9.Satariano WA. Comorbidity and functional status in older women with breast cancer: implications for screening, treatment, and prognosis. J Gerontol. 1992;47:24–31. Spec No. [PubMed] [Google Scholar]
- 10.Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc. 2010;58(1):76–82. doi: 10.1111/j.1532-5415.2009.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 12.Short PF, Vasey JJ, Belue R. Work disability associated with cancer survivorship and other chronic conditions. Psychooncology. 2008;17(1):91–97. doi: 10.1002/pon.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberst K, Bradley CJ, Gardiner JC, Schenk M, Given CW. Work task disability in employed breast and prostate cancer patients. J Cancer Surviv. 2010;4(4):322–330. doi: 10.1007/s11764-010-0128-8. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11 Suppl 3:89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan DV, Raj AB, Harnett-Sheehan K, Soto S, Knapp E. The relative efficacy of high-dose buspirone and alprazolam in the treatment of panic disorder: a double-blind placebo-controlled study. Acta Psychiatr Scand. 1993;88(1):1–11. doi: 10.1111/j.1600-0447.1993.tb03405.x. [DOI] [PubMed] [Google Scholar]
- 16.Hou WK, Lam WW, Law CC, Fu YT, Fielding R. Measuring social relational quality in colorectal cancer: the Social Relational Quality Scale (SRQS) Psychooncology. 2009;18(10):1097–1105. doi: 10.1002/pon.1500. [DOI] [PubMed] [Google Scholar]
- 17.Pryce J, Munir F, Haslam C. Cancer survivorship and work: symptoms, supervisor response, co-worker disclosure and work adjustment. J Occup Rehabil. 2007;17(1):83–92. doi: 10.1007/s10926-006-9040-5. [DOI] [PubMed] [Google Scholar]
- 18.Leon AC, Shear MK, Portera L, Klerman GL. Assessing impairment in patients with panic disorder: the Sheehan Disability Scale. Soc Psychiatry Psychiatr Epidemiol. 1992;27(2):78–82. doi: 10.1007/BF00788510. [DOI] [PubMed] [Google Scholar]
- 19.Spelten ER, Verbeek JH, Uitterhoeve AL, et al. Cancer, fatigue and the return of patients to work-a prospective cohort study. Eur J Cancer. 2003;39(11):1562–1567. doi: 10.1016/s0959-8049(03)00364-2. [DOI] [PubMed] [Google Scholar]
- 20.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 21.Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miaskowski C, Dibble SL. The problem of pain in outpatients with breast cancer. Oncol Nurs Forum. 1995;22(5):791–797. [PubMed] [Google Scholar]
- 23.Lin CC, Lai YL, Ward SE. Effect of cancer pain on performance status, mood states, and level of hope among Taiwanese cancer patients. J Pain Symptom Manage. 2003;25(1):29–37. doi: 10.1016/s0885-3924(02)00542-0. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Dictionary of Cancer Terms. Karnofsky performance status. Available from http://www.cancer.gov/dictionary/?CdrID=44156. Accessed Feburary 8, 2010.
- 25.Kroenke K, Theobald D, Norton K, et al. The Indiana Cancer Pain and Depression (INCPAD) trial. Design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants. Gen Hosp Psychiatry. 2009;31(3):240–253. doi: 10.1016/j.genhosppsych.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K, Theobald D, Wu J, et al. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J Pain Symptom Manage. 2010;40(3):327–341. doi: 10.1016/j.jpainsymman.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Brantes F, Rosenthal MB, Painter M. Building a bridge from fragmentation to accountability--the Prometheus Payment model. N Engl J Med. 2009;361(11):1033–1036. doi: 10.1056/NEJMp0906121. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS. Pain assessment in cancer. In: Osoba D, editor. Effect of cancer on quality of life. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 30.Williams LS, Jones WJ, Shen J, Robinson RL, Kroenke K. Outcomes of newly referred neurology outpatients with depression and pain. Neurology. 2004;63(4):674–677. doi: 10.1212/01.wnl.0000134669.05005.95. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA. 2010;304(2):163–171. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner EH, LaCroix AZ, Grothaus LC, Hecht JA. Responsiveness of health status measures to change among older adults. J Am Geriatr Soc. 1993;41(3):241–248. doi: 10.1111/j.1532-5415.1993.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 35.Rost K, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice: a randomized trial of the quEST intervention. Quality Enhancement by Strategic Teaming. J Gen Intern Med. 2001;16(3):143–149. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cleeland CS. Houston, TX: The University of Texas M. D. Anderson Cancer Center; 2009. The Brief Pain Inventory user guide. Available from: http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/symptom-research-brief-pain-inventory-user-s-guide.html. Accessed June 20, 2010. [Google Scholar]
- 37.Williams JW, Jr, Stellato CP, Cornell J, Barrett JE. The 13- and 20-item Hopkins Symptom Checklist Depression Scale: psychometric properties in primary care patients with minor depression or dysthymia. Int J Psychiatry Med. 2004;34(1):37–50. doi: 10.2190/U1B0-NKWC-568V-4MAK. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Zhong X, Theobald D, et al. Somatic symptoms in cancer patients with pain and/or depression prevelence, disability, and health care use. Arch Intern Med. 2010;170(18):1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitzer RL, Kroenke K, Linzer M, et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. JAMA. 1995;274(19):1511–1517. [PubMed] [Google Scholar]
- 40.Strine TW, Kroenke K, Dhingra S, et al. The associations between depression, health-related quality of life, social support, life satisfaction, and disability in community-dwelling US adults. J Nerv Ment Dis. 2009;197(1):61–64. doi: 10.1097/NMD.0b013e3181924ad8. [DOI] [PubMed] [Google Scholar]
- 41.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K. Somatic symptoms and depression: a double hurt. Prim Care Companion. J Clin Psychiatry. 2005;7(4):148–149. doi: 10.4088/pcc.v07n0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 44.Taskila T, Martikainen R, Hietanen P, Lindbohm ML. Comparative study of work ability between cancer survivors and their referents. Eur J Cancer. 2007;43(5):914–920. doi: 10.1016/j.ejca.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Drolet M, Maunsell E, Brisson J, et al. Not working 3 years after breast cancer: predictors in a population-based study. J Clin Oncol. 2005;23(33):8305–8312. doi: 10.1200/JCO.2005.09.500. [DOI] [PubMed] [Google Scholar]
- 46.Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol. 2006;24(3):345–353. doi: 10.1200/JCO.2004.00.4929. [DOI] [PubMed] [Google Scholar]
- 47.Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008;136(3):235–238. doi: 10.1016/j.pain.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGuire DB. Occurrence of cancer pain. J Natl Cancer Inst Monogr. 2004;(32):51–56. doi: 10.1093/jncimonographs/lgh015. [DOI] [PubMed] [Google Scholar]