Abstract
Purpose
Aim of this study was to evaluate morphometric parameters of metaphase II oocytes, including cytoplasm diameter (CD), zona pellucida thickness (ZPT) and width of the perivitelline space (PS), in relation with zona pellucida birefringence, spindle presence and age of the woman.
Methods
Oocytes were classified into groups according to zona birefringence (low or high zona birefringence, LZB and HZB, respectively) and presence or absence of a visible spindle (SP and aSP, respectively).
Results
HZB oocytes showed a thicker zona (17.7 ± 0.3 μm) than LZB oocytes (16.7 ± 0.3 μm, p < 0.01). Moreover, PS was narrower in HZB and SP oocytes than in LZB (p < 0,001) and aSP (p < 0,05) oocytes. Finally, we found that CD and ZPT linearly decrease with age of the woman (CD r = 0.028: p < 0.01; ZPT r = 0.050: p < 0.0001).
Conclusion
Our results evidence an association in human oocytes between zona pellucida and spindle birefringence and defined morphometric parameters and a decrease of oocyte size and ZPT as a function of women’s age.
Keywords: Birefringence, Morphometry, Oocyte aging, Zona pellucida
Introduction
Morphological oocyte evaluation, while highly subjective, is still the standard criterion for routine work in intracytoplasmic sperm injection (ICSI) [1]. Morphological evaluation before ICSI helps to identify metaphase II (MII) oocytes with higher developmental potential [2]. The gross morphology of MII oocytes includes variations of oocyte shape, color, granularity and homogeneity of cytoplasm, width of perivitelline space, debris in the perivitelline space, vacuolization, inclusions and abnormalities of the first polar body (1st PB) or of the zona pellucida (ZP) [3]. For example, it has been reported that oocyte selection based on 1st PB morphology results in higher fertilization rates and better embryo quality [4], while a large perivitelline space has been related to a lower fertilization rate [3]. However, the influence of all these oocyte features on post-ICSI fertilization rate and embryo development is still controversial [5].
Objective assessment of oocyte quality is actually an important matter of investigation in assisted reproduction technology (ART) because the use of ICSI is increasing also for non andrologic infertility and for some center represents nowadays the only ART performed.
Since in several countries, for bioethical reasons, it is not possible to discard or freeze embryos, the interest for oocyte selection is growing. On the other hand, thought many embryo or pronuclear score systems have been proposed for quality evaluation [6], their predictivity is still limited. Moreover, oocyte freezing technique performances improved recently [7] and it is becoming more and more used, also for egg donation procedures. However results of oocytes freezing/thawing ART cycles still relay on the choice of good quality oocytes [8]. All these reasons indicate the urgency of determining objective criteria for oocytes selection.
New methodologies, including polar body diagnosis (PBD) [9], metabolomics [10], polarization light microscopy [11–15] and pattern recognition of processed images by special algorithms [16], have been proposed as methods to improve the objective selection of good quality oocytes. In particular, the PolScope was used in a semiquantitative mode to evaluate the birefringence of the ZP [17] and the meiotic spindle (SP) of metaphase II oocytes [12]. The zona pellucida exhibits optical anisotropy and it was demonstrated that the filaments in the inner and outer layers exhibit different orientations of their axis [17]. Softwares are available (i.e. OCTAX PolarAIDE™) that automatically calculate the zona birefringence and display in real time objective and user-independent zona scores based on the intensity and distribution of the birefringence [18]. High zona pellucida birefringence (HZB) has been associated with a significantly higher rate of embryo implantation and development to term, as compared with low ZP birefringence (LZB) [14, 15]. In addition, oocytes with high fertilization rate show in general a birefringent SP [12, 15]. Thus, there is a broad agreement that the analysis of the birefringence of the oocyte ZP and SP represents a reliable, objective criterion for good quality oocyte selection.
Since only a few quantitative morphometric studies have been performed on human oocytes, in the present work we have systematically analyzed several morphometric parameters of MII oocytes including perivitelline space size, ZP thickness, and width of the perivitelline space. These parameters were then correlated to the birefringence of the zona pellucida and spindle. A further correlation was evaluated between the morphometric parameters and the patients’age together with FSH levels, conditions associated to decreased fertility [19, 20] and poor oocyte quality [21, 22], respectively.
Materials and methods
Patients and study design
This study involved 72 infertile couples included in the ICSI program at the Genesis Center for Reproductive Medicine between October 2008 and March 2009. For this prospective study women’s age ranged from 24 to 46 years (37.2 ± 5.0 years). FSH levels (2nd day of the cycle) ranged from 3.4 to 13.7 mIU/ml (7.2 ± 2.4 mIU/ml). A written informed consent was obtained, in which patients agreed to share the outcomes of their own cycles for research purposes, and the study was approved by the local review board.
Ovarian stimulation and oocytes retrieval and culture
Patients were stimulated with exogenous FSH (Gonal-F; Serono Europe, United Kindom). Monitoring of follicular development by real-time ultrasound scans was performed from day 2 of treatment until the day of follicular puncture. When the ovarian follicles reached 18–20 mm diameter, hCG (Ovitrelle; Serono Europe, United Kindom) was administered, followed 36 h later by follicular aspiration. All incubation steps were performed in an incubator Galaxy S + (Celbio, Italy) equilibrated to 37°C and 5% CO2. The collected cumulus-enclosed oocytes were maintained in 500 μl multidisches 4 wells (Nunclon Surface, Denmark) in medium (IVF, Vitrolife, Sweden) under oil (Ovoil-100, Vitrolife Sweden, Sweden) and manteined in the incubator for 2 h after pick-up. Then they were decumulated in hyaluronidase drops (Hyaluronidase, 80 U/ml, SAGE In-vitro Fertilization, USA). Oocytes were screened for the presence or absence of the 1st PB by conventional light microscopy, and immature oocytes (germinal vesicle, metaphase I) were excluded from the study. Finally, the oocytes were individually placed in droplets of medium (Gamete-30, Vitrolife, Sweden) under oil in a glass bottom dish (WillCo Wells BV, Netherlands) and cultured for 15 min prior to image capture.
Screening of oocytes and live zona and spindle birefringence analysis
Live imaging of individual oocytes was performed on a Nikon Diaphot-300 inverted microscope equipped with Hoffmann interference optics, stain-free objectives, a circular polarization filter and liquid crystal analyzer optics. The images were captured and saved for analysis of morphometric parameters (see below).
Each oocyte was also imaged by enhanced polarizing microscopy. The birefringence analysis, including autocalibration, was fully controlled by a polarization imaging software module (OCTAX ICSI Guard™, OCTAX Microscience GmbH, Altdorf, Germany) implemented with an imaging software system (OCTAX Eyeware™).
On the basis of zona birefringence, MII oocytes were classified as having a high zona pellucida birefringence (HZB) or low zona pellucida birefringence (LZB). More specifically, the software calculated a score based on intensity and uniformity around the entire cell; oocytes with a birefringence score ≥10 were considered as HZB, oocytes with a <10 score as LZB.
The presence of a birefringent meiotic spindle was analyzed and the oocytes were classified as having a visible spindle (SP) or absent spindle (aSP). The microscope was equipped with a motorized stage (OCTAX) containing a fully heated ceramic plate with a glass insert in the objective pathway. The temperature of the heated plate was adjusted with an external calibrated sensor to maintain 37.0 ± 0.5°C in the medium droplet during microscopic observation [21]. The birifringence data were used to select the three oocytes that Italian law allowed to be fertilized.
Morphometric analysis of the oocytes
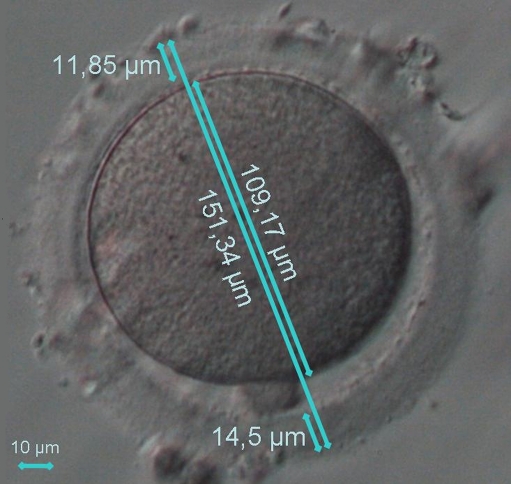
The captured images, as reported above, were used to obtain morphometric parameters. In particular, oocytes were analyzed for the total diameter, cytoplasm diameter and ZP thickness (Fig. 1) using the OCTAX Eyeware™ imaging software (OCTAX Microscience GmbH, Altdorf, Germany). To eliminate interpersonal variability, all measurements were taken by the same operator, who was unaware of age and FSH levels of the patients.
Fig. 1.
An example of the measurements taken on an oocyte: total diameter (151,34 μm), cytoplasm diameter (109,17 μm), zona pellucida thickness (11,85 μm + 14,5 μm = 26,35 μm/2 = 13,18 μm). The width of the perivitelline space is indirectly obtained by subtraction (151,34 μm–109,17 μm–(11,85 μm + 14,5 μm) = 16,32 μm)
Total and cytoplasm diameter of the oocyte and ZP thickness were measured in correspondence of a line drawn along the major axis of the oocyte in order to divide equally the oocyte cytoplasm in two semi-circle (Fig. 1). Among all possible cytoplasmic diameters we choosed the one that crossed the widest perivitelline space. Along this line we performed all the calculation: total and cytoplasm diameter of the oocyte and ZP thickness. Two ZP thickness measurements were taken at the opposites of the line, and the two measures were averaged. The width of the perivitelline space was indirectly obtained by subtracting the sum of cytoplasm diameter and zona pellucida thickness from the total diameter of the oocyte (Fig. 1).
Measurement procedures were performed by the same operator twice and the differences between two successive means in each examined parameter were analyzed by t-test. The average of the two measurements was used for calculation.
Statistical analysis
Unpaired t-test was used for paired groups statistical analysis (HZB vs LZB, SP vs aSP; HZB + SP vs LZB + aSP). Chi square test was used to compare HZB-LZB and SP-aSP groups. The results are expressed as the mean±standard error (SE). Linear regression analysis was used to correlate age and FSH levels to the morphometric parameters of the oocytes of the same patient. The results are expressed as the squared regression coefficient (r). Data analysis was carried out using the GraphPad Instat software.
Results
In the present study, we analyzed the morphometric characteristics of 314 MII oocytes obtained from stimulated ovaries of 72 patients enrolled in our Assisted Reproductive Technology (ART) program. For each oocyte the total diameter (zona pellucida + perivitelline space width + cytoplasm) (TD), the cytoplasm diameter (CD), the zona pellucida thickness (ZPT) and the width of the perivitelline space (PS) were measured (Fig. 1).
According to ZP birefringence, oocytes could be divided into two classes: those showing a high zona pellucida birefringence (HZB oocytes, 57%), and oocytes with low birefringence (LZB oocytes, 43%). On the basis of spindle birefringence, oocytes could be classified into two groups: with or without a visible spindle (SP = 61% and aSP = 39%, respectively).
No correlation was found between oocyte zona pellucida and spindle birefringence.
We analyzed if ZP birefringence was correlated with age or FSH (Table 1a). This analysis showed that HZB oocytes are significantly more numerous in younger women (p < 0,05), while FSH levels does not influenced the ZP oocytes birefringence.
Table 1.
The table show age and FSH mean values in relation to birefringence of zona pellucida (a) and spindle (b). In particular in the group of HZB oocytes the age mean resulted lower than LZB group. The values are expressed like mean in μm with ± standard error
| a | HZB | LZB | |
| AGE (years) | 36,7 ± 0,4* | 37,9 ± 0,4* | |
| FSH (mIU/ml) | 7,2 ± 0,2 | 7,1 ± 0,2 | |
| b | SP | aSP | |
| AGE (years) | 37,1 ± 0,3 | 37,4 ± 0,5 | |
| FSH (mIU/ml) | 7,1 ± 0,2 | 7,2 ± 0,2 |
*p < 0,05
In the same way, we searched a correlation between spindle birefringence and age or FSH levels (Table 1b). The spindle presence seemed not to be influenced neither by age or FSH.
Finally we studied the relationship between morphometric measures and other parameters, such as birefringence, age and FSH.
No significant differences were observed in total diameter and cytoplasm diameter of the oocytes of the HZB and LZB groups. However, the zona of HZB oocytes was significantly thicker than that of LZB oocytes (17.7 ± 0.3 μm vs 16.7 ± 0.3 μm, p < 0.01). Moreover, the HZB oocytes was associated to a significant (p < 0.001) smaller width of the perivitelline space in comparison to LZB oocytes (14.4 ± 0.5 μm vs 17.2 ± 0.6 μm) (Table 2 group a).
Table 2.
The table showed the mean values of the morphometric parameters in oocytes with high (HZB) or low (LZB) zona birefringence (a) and presence (SP) or absence (aSP) of the spindle (b). Data are expressed in μm with ± standard error. n indicates the number of oocytes that are classified in each group. TD: total diameter of oocyte; CD: cytoplasm diameter of oocyte; ZPT: zona pellucida thickness; PS: perivitelline space. n indicates th number of oocytes studied
| TD | CD | ZPT | PS | ||
|---|---|---|---|---|---|
| a | HZB (n = 179) | 163,6 ± 1,2 | 113,6 ± 0,7 | 17,7 ± 0,3 ** | 14,4 ± 0,5 *** |
| LZB (n = 135) | 164,7 ± 1,5 | 114,1 ± 1,0 | 16,7 ± 0,3 ** | 17,2 ± 0,6 *** | |
| b | SP (n = 192) | 163,8 ± 1,2 | 114,1 ± 0,7 | 17,4 ± 0,2 | 14,9 ± 0,4 * |
| aSP (n = 122) | 164,5 ± 1,5 | 113,4 ± 1,0 | 17,2 ± 0,3 | 16,7 ± 0,6 * | |
*p < 0,05, **p < 0,01, ***p < 0,001
The presence or absence of a spindle was not correlated with other morphometric parameters, except for width of the perivitelline space. Specifically, SP oocytes had a thinner perivitelline space (14.9 ± 0.5 μm) than aSP oocytes (16.7 ± 0.6 μm) (p < 0.05) (Table 2 group b).
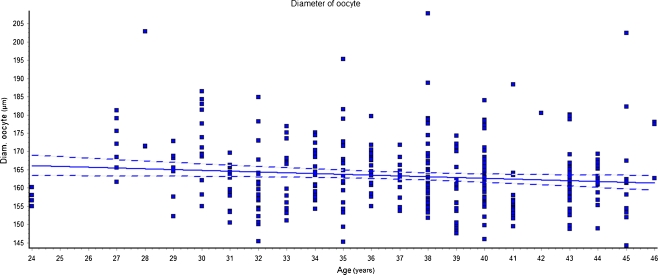
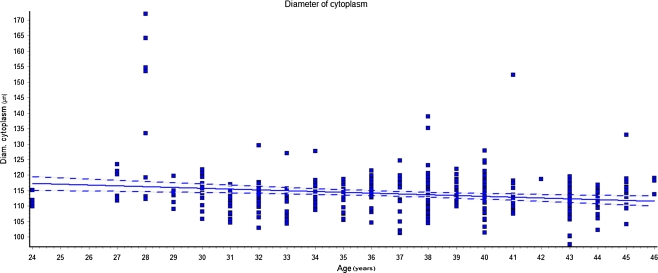
The morphometric parameters of the oocytes were then correlated with age of the women. Linear regression analyses showed that both total and cytoplasm diameter of the oocytes were significantly inversely related to the women’s age (Figs. 2 and 3).
Fig. 2.
Linear regression of total diameter (TD) of the oocytes vs women’s age (r = 0.013; p < 0.05). We observed that TD decreases linearly with the increase of the women’s age
Fig. 3.
Linear regression of cytoplasm diameter (CD) of the oocytes vs women’s age (r = 0.028; p < 0.01). The correlation shows that CD decreases significantly with the increase of the women’s age
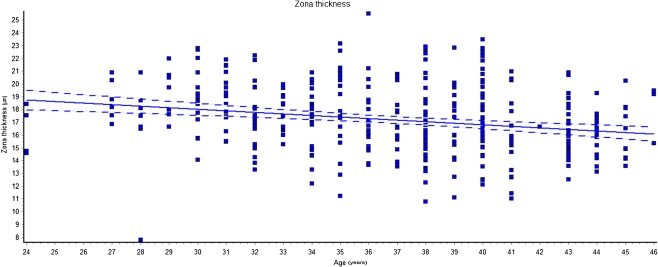
We also observed a significant (p < 0.0001) inverse relationship between zona thickness and patient’s aging (Fig. 4).
Fig. 4.
Linear regression of ZP thickness vs women’s age (r = 0.050; p < 0.0001). The ZPT decreases as a function of age
No significant linear correlation was observed between perivitelline space width and aging (data not shown).
FSH level on the 2° day of the cycle is considered as an indicator of the ovarian reserve, as FSH concentration increases when ovarian reserve declines. As expected, we observed that FSH levels increased with the women’s age (p < 0.01). No significant linear correlation was found between FSH levels and any of the examined morphometric parameters (data not shown).
Discussion
Oocyte quality is intensely investigated for its pivotal role in reproductive success or failure. Several strategies have been suggested to objectively identify the best oocytes [10]. Birefringence of the spindle and the zona of the metaphase II oocyte, as evaluated by polarized light microscopy, have been recently proposed as criteria of quality in live human oocytes [13, 23, 24]. Several studies have reported a positive correlation between high birefringence of the zona pellucida (HZB) and that of the spindle (SP) in metaphase II oocytes, and the main reproductive indexes such as fertilization, embryo implantation and women’s pregnancy rate [14, 15].
In the present paper we show that in MII oocytes HZB and the presence of SP are significantly related to some morphometric parameters (perivitelline space and zona pellucida thickness). Moreover, we have found that a significant correlation exists between the size of oocytes (like oocyte diameter, cytoplasm diameter and zona pellucida thickness) and the patient’s age.
We observed that HZB is positively correlated with zona pellucida thickness (ZPT) and inversely correlated with perivitelline space width (PS). On the contrary, LZP oocytes showed decreased ZPT and a wide PS. Moreover, the oocytes with visible spindle (SP) showed a significant smaller perivitelline space than oocytes without spindle (aSP). Because it is known that birefringence is an index of oocyte quality and the width of perivitelline space correlates both with zona pellucida and spindle birefringence, we could consider PS a good morphometric parameters to evaluate oocytes quality.
Previous studies on the structure of the zona pellucida with polarized optics demonstrated that the zona pellucida consists of a complex multilaminar structure. In particular, ZP appears to be divided into two birefringent layers separated by an anisotropic layer [17]. The inner layer (IL) is the most birefringent and thicker and it is the main responsible for the changes in birefringence and thickness of the entire zona [23, 25]. The birefringence of the zona pellucida has been reported to be positively correlated with zona thickness by some authors [13, 23, 25], but not by others [26]. The zona probably behaves as a whole functionally, though birefringence is seen only in its inner layer. Our data demonstrated a correlation between thickness of zona pellucida and birefringence of its inner layer. It is possible that inner zona is better investigated by birefringence and outer by morphometry. In other terms, the function of the zona might be better described by birefringence and thickness together. It is to be pointed out, however, that an apparent discrepancy about the average ZPT values exists among different works. This could be due to several factors including different scoring methods, high variability oocytes shape among patients and different culture conditions [26–28]. Moreover we have observed that zona thickness progressively decreases with women’s age. Such a decrease is associated with a reduction of the total and cytoplasmic diameter of the oocyte. Zona pellucida birefringence as a marker of oocyte quality might be quite dependent on structural modification caused by age, as our data demonstrated.
It is well known that oocyte aging is one of the most important factors involved in the failure of the assisted reproduction techniques. Oocyte aging has been associated with several morphological and functional alterations, including changes in structure of the plasma membrane, zona pellucida, cytoskeleton or mitochondria, displacement of the spindle, misalignment of chromosomes, dispersion of centrosomal material, displacement of first polar body and cortical granules [29]. Our results clearly show that aged oocytes are characterized by thinner zonae and a reduced total and cytoplasm diameter. The enlargement of PS did not result significantly depending on age, maybe because it could be mostly related to pathologic characteristic of some oocytes that are not necessarily associated with the aging process [2, 3].
The relationship between zona pellucida structure and aging is controversial. Some studies have suggested positive correlation between ZPT and age [26, 30] Our results, on the contrary, showed that there is a significant inverse correlation between ZPT and women’s age, between 24 and 46 years. The reduction of ZPT can be attributed to a progressive decrease of the capability of oocytes and granulosa cells to synthesize and properly assemble ZP proteins. In fact, it is known that defects in the synthesis and secretion of ZP proteins result in a thinner and more loosely organized zona pellucida [31]. Interestingly in mice lacking ZP1, one of the three proteins of the mouse zona, the diameter of ovulated oocytes is on average about a half that of wild type oocytes [32].
Similarly, the reduction of the oocyte cytoplasm size and total diameter can be a consequence of a decreased ability by the aged oocyte to synthesize protein or to maintain the proper cell volume. A reduced volume of intracellular compartments has been described with age in body composition studies [33]. Reduction of cell volume as a function of aging, due to change in the activity of ionic pumps or in the ability to adjust water uptake, has been described for other cell types such as blood red cells [34] and Leydig cells [35]. It is possible that reduction of water content in the zona might decrease its elasticity and impair the physiological hatching before implantation explaining a possible rationale for assisted hatching as suggested in more aged patients.
The decreased size of oocytes might represent a general biological phenomenon correlated with aging, quicker and more evident in the oocyte because of its large volume and limited life span.
The reduction of oocyte size, however, might as well be caused, in aging oocytes, by the in vitro isolation conditions. The volume changes of mammalian cells are induced by several factors including anisoosmolarity or, under isoosmotic conditions, by hormones and oxidative stress [36]. Reactive oxygen species (ROS) affect several physiological processes from oocyte maturation to fertilization, embryo development and pregnancy [37].
It is also possible that the changes of oocyte size might be influenced by apoptosis. It has been reported that apoptosis plays a critical role in maintaining oocyte quality. In oocytes with defect in apoptosis it is observed a decrease in oocyte quality that correlated with a decrease in oocyte size [38]. Another effect of women’s aging is the FSH level increase [20]. Although we observed that the FSH levels linearly increases with age, a significant correlation between FSH levels and morphometric parameters was not found, maybe because there are other factors that weight on this relationship.
In conclusion, our data show that certain morphometric parameters, such as the cytoplasmic diameter of the oocyte, are particularly affected by aging as a physiological process. Other measurements, such as perivitelline space width, showing a clear relation with birefringence, appear to be more related to individual oocyte quality; confirming findings by others [2, 3]. On the other hand zona pellucida thickness, and its age-related changes, might represent a useful index of oocyte quality that in itself may be used to increase the probabilities of identifying bad quality oocytes.
The evaluation of possible relationships between the morphometric parameters and implantation rates after ICSI will form the object of future investigations.
Acknowledgement
We wish to thank Prof. Gregorio Siracusa for helpful suggestions throughout the study and for discussion of the results and Prof. Alessandra Lumini for help in statistical analysis. This work was supported by grants of Junior Srl.
Footnotes
Capsule
A relationship between oocyte birefringence and morphometry has been investigated and found. Women’s age also was found to influence oocyte size and zona pellucida thickness.
References
- 1.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 2.Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90:1692–1700. doi: 10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12(8):1750–1755. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 4.Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15(2):427–430. doi: 10.1093/humrep/15.2.427. [DOI] [PubMed] [Google Scholar]
- 5.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13(12):3431–3433. doi: 10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- 6.Baczkowski T, Kurzawa R, Głabowski W. Methods of embryo scoring in in vitro fertilization. Reprod Biol. 2004;4(1):5–22. [PubMed] [Google Scholar]
- 7.Tao T, Zhang W, Valle A. Human oocyte cryopreservation. Curr Opin Obstet Gynecol. 2009;21(3):247–252. doi: 10.1097/GCO.0b013e328329c2d2. [DOI] [PubMed] [Google Scholar]
- 8.Santis L, Cino I, Coticchio G, Fusi FM, Papaleo E, Rabellotti E, et al. Objective evaluation of the viability of cryopreserved oocytes. Reprod Biomed Online. 2007;15(3):338–345. doi: 10.1016/S1472-6483(10)60348-3. [DOI] [PubMed] [Google Scholar]
- 9.Verlinsky Y, Ginsberg N, Lifchez A, Valle J, Moise J, Strom CM. Analysis of the first polar body: preconception genetic diagnosis. Hum Reprod. 1990;5(7):826–829. doi: 10.1093/oxfordjournals.humrep.a137192. [DOI] [PubMed] [Google Scholar]
- 10.Patrizio P, Fragouli E, Bianchi V, Borini A, Wells D. Molecular methods for selection of the ideal oocyte. Reprod Biomed Online. 2007;15:346–353. doi: 10.1016/S1472-6483(10)60349-5. [DOI] [PubMed] [Google Scholar]
- 11.Oldenbourg R. A new view on polarized microscopy. Nature. 1996;381:175–2008. doi: 10.1038/381811a0. [DOI] [PubMed] [Google Scholar]
- 12.Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001;16(7):1464–1468. doi: 10.1093/humrep/16.7.1464. [DOI] [PubMed] [Google Scholar]
- 13.Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online. 2007;14(2):166–174. doi: 10.1016/S1472-6483(10)60784-5. [DOI] [PubMed] [Google Scholar]
- 14.Montag M, Schimming T, Koster M, Zhou C, Dorn C, Rosing B, et al. Oocyte zona birefringence intensity is associated with embryonic implantation potential in ICSI cycles. Reprod Biomed Online. 2007;16:239–244. doi: 10.1016/S1472-6483(10)60580-9. [DOI] [PubMed] [Google Scholar]
- 15.Madaschi C, Aoki T, Paes de Almeida Ferreira Braga D, Cassia Savio Figueira R, Semiao Francisco L, Iaconelli AJ, et al. Zona pellucida birefringence score and meiotic spindle visualization in relation to embryo development and ICSI outcomes. Reprod Biomed Online. 2009;18:681–686. doi: 10.1016/S1472-6483(10)60014-4. [DOI] [PubMed] [Google Scholar]
- 16.Manna C, Patrizi G, Rahmanm A, Sallam H. Experimental results on the recognition of embryos in human assisted reproduction. Reprod Biomed Online. 2004;8:460–469. doi: 10.1016/S1472-6483(10)60931-5. [DOI] [PubMed] [Google Scholar]
- 17.Keefe D, Tran P, Pellegrini C, Oldenbourg R. Polarized light microscopy and digital image processing identify a multilaminar structure of the hamster zona pellucida. Hum Reprod. 1997;12:1250–1252. doi: 10.1093/humrep/12.6.1250. [DOI] [PubMed] [Google Scholar]
- 18.Montag M, Ven H. Oocyte assessment and embryo viability prediction: birefringence imaging. Reprod Biomed Online. 2008;17:454–460. doi: 10.1016/S1472-6483(10)60231-3. [DOI] [PubMed] [Google Scholar]
- 19.Gomes LM, Canha Ados S, Dzik A, Novo NF, Juliano Y, Dos Santos SI, et al. The age as a predictive factor in in vitro fertilization cycles. Rev Bras Ginecol Obstet. 2009;31(5):230–234. doi: 10.1590/S0100-72032009000500005. [DOI] [PubMed] [Google Scholar]
- 20.Burger HG. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition-an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol. 1994;130:38–42. doi: 10.1530/eje.0.1300038. [DOI] [PubMed] [Google Scholar]
- 21.El-Toukhy T, Khalaf Y, Hart R, Taylor A, Braude P. Young age does not protect against the adverse effects of reduced ovarian reserve-an eight year study. Hum Reprod. 2002;17(6):1519–1524. doi: 10.1093/humrep/17.6.1519. [DOI] [PubMed] [Google Scholar]
- 22.Montag M, Schimming T, Ven H. Spindle imaging in human oocytes: the impact of the meiotic cell cycle. Reprod Biomed Online. 2006;12(4):442–446. doi: 10.1016/S1472-6483(10)61996-7. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum Reprod. 2005;20(6):1596–1606. doi: 10.1093/humrep/deh811. [DOI] [PubMed] [Google Scholar]
- 24.Ebner T, Balaban B, Moser M, Shebl O, Urman B, Ata B, et al. Automatic user-independent zona pellucida imaging at the oocyte stage allows for the prediction of preimplantation. Fertil Steril. 2010;94(3):913–920. doi: 10.1016/j.fertnstert.2009.03.106. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier C, Keefe D, Trimarchi JR. Non invasive polarized light microscopy quantitatively distinguished the multilaminar structure of the zona pellucid of living human eggs and embryo. Fertil Steril. 2004;81:850–856. doi: 10.1016/j.fertnstert.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Kilani SS, Cooke S, Kan AK, Chapman MG. Do age and extended culture affect the architecture of the zona pellucida of human oocytes and embryos? Zygote. 2006;14(1):39–44. doi: 10.1017/S0967199406003625. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J, Inge KL, Suzman M, Wider SR, Wright GR. Videocinematography of fresh and cryopreserved embryos: a retrospective analysis of embryonic morphology and implantation. Fertil Steril. 1989;51:820–827. doi: 10.1016/s0015-0282(16)60673-8. [DOI] [PubMed] [Google Scholar]
- 28.Garside WT, Mola JR Loret, Bucci JA, Tureck RW, Heyner S. Sequential analysis of zona thickness during in vitro culture of human zygotes: correlation with embryo quality, age, and implantation. Mol Reprod Dev. 1997;47(1):99–104. doi: 10.1002/(SICI)1098-2795(199705)47:1<99::AID-MRD13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:273–285. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- 30.Nawroth F, Müller P, Wolf C, Sudik R. Is the Zona pellucida thickness of metaphase-II oocytes in an IVF/ICSI program influenced by the patient’s age? Gynecol Obstet Investig. 2001;52(1):55–59. doi: 10.1159/000052942. [DOI] [PubMed] [Google Scholar]
- 31.Wassarman PM, Litscher ES. Mammalian fertilization: the egg's multifunctional zona pellucida. Int J Dev Biol. 2008;52(5–6):65–76. doi: 10.1387/ijdb.072524pw. [DOI] [PubMed] [Google Scholar]
- 32.Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 1999;126:3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- 33.Chernoff R. Protein and older adults. J Am Coll Nutr. 2004;23(6):627S–630S. doi: 10.1080/07315724.2004.10719434. [DOI] [PubMed] [Google Scholar]
- 34.Caprari P, Scuteri A, Salvati AM, Bauco C, Cantafora A, Masella R, et al. Aging and red blood cell membrane: a study of centenarians. Exp Gerontol. 1999;34(1):47–57. doi: 10.1016/S0531-5565(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15(6):551–557. [PubMed] [Google Scholar]
- 36.Schliess F, Reinehr R, Häussinger D. Osmosensing and signaling in the regulation of mammalian cell function. FEBS J. 2007;274(22):5799–5803. doi: 10.1111/j.1742-4658.2007.06100.x. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;14:3–28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andux S, Ellis RE. Apoptosis maintains oocytes quality in aging Caenorhabditis elegans females. PLoS Genet. 2008;4(12):e1000295. doi: 10.1371/journal.pgen.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]