Abstract
Purpose
In oocyte in-vitro maturation (IVM) treatments, the chances to achieve a pregnancy are critically dependent on the retrieval of a suitable number of oocytes. In this study, we assessed the ability of circulating levels of anti-mullerian hormone (AMH) to identify normo-ovulatory women suitable for IVM treatment on the basis of the number of retrieved oocytes.
Method
Serum AMH was quantified in normo-ovulatory women younger than 39 years undergoing IVM treatment. After immature oocyte retrieval and IVM, maximum 3 mature oocytes were used for treatment and all resulting embryos were transferred, as established by law. From 177 cycles, 991 oocytes were recovered. Following IVM, 484 mature oocytes were obtained (50.1%).
Results
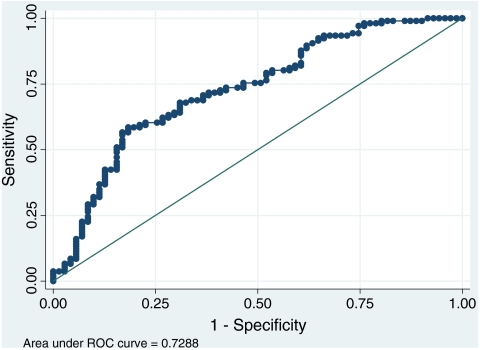
The overall pregnancy rate per embryo transfer was 16.6% (25/151) and the implantation rate was 10.9% (30/278). Linear regression and receiver operating characteristic (ROC) analyses were applied to identify independent variables and quantify a cut-off AMH value able to identify patients suitable for IVM treatment. An AMH value of 1.28 ng/ml was identified as a threshold for the prediction of the retrieval of at least 5 oocytes, with a sensitivity of 93.4% and a specificity of 33.8%. Positive and negative predictive values were 67.6% and 75.0%, respectively.
Conclusions
AMH can be adopted to identify women candidate for an IVM treatment from whom a suitable number of oocytes may be retrieved. This is of crucial significance during a non-stimulated cycle, in order to prevent an insufficient oocyte collection and rescue the treatment by implementing a conventional controlled ovarian stimulation.
Keywords: In vitro maturation, Oocytes, Anti-mullerian hormone, Female age, Pregnancy rate
Introduction
Advanced maternal age affects natural fecundity and compromises oocyte number and quality in in-vitro fertilization (IVF) treatments [1, 2]. In fact, aging of ovarian function is recognized as the worst prognosis element in assisted reproduction technology (ART) [3]. However, a large inter-individual variability of the incidence of this factor can be observed at any age [4, 5], as suggested by the fact that impaired ovarian reserve may concern also young women undergoing ovarian stimulation [6, 7].
Because age alone is not always sufficient to predict accurately ovarian response to gonadotrophin stimulation, there is a need for a more comprehensive and informative evaluation of women requiring ART, in order to gain information on ovarian function and determine an appropriate treatment. Serum follicle stimulation hormone (FSH) concentration [8, 9], ovarian volume determined by ultrasound [10], antral follicle count (AFC) [11, 12], and clomiphene citrate challenge test have been proposed as indicators to estimate ovarian functional reserve. Conflicting results on the effectiveness of these methods have been reported [13]. Anti-Müllerian hormone (AMH) has attracted considerable attention as a marker of ovarian function. AMH is an ovarian glycoprotein produced by granulosa cells of primary, pre-antral and early antral follicles [14] but not larger or atretic follicles [14, 15]. AMH action is believed to be critical for the regulation of follicular growth [16–18]. The circulating levels of this factor have been suggested to depend on the number and activity of growing ovarian follicles [19], decreasing throughout life and becoming not detectable after menopause [20, 21]. Hence, AMH has been proposed as an indicator of ovarian reserve [22–24]. It also appears that the age-associated variation in AMH production offers an opportunity for an earlier prediction of perimenopause change in ovarian function, in comparison to FSH and inhibin-B [19]. Furthermore, AMH measurements have been found valuable for the investigation of ovarian dysfunction [21]. Several studies have tested the ability of AMH to support the clinical management and predict the outcome of ART treatment [21, 23–29].
In the last few years, several questions have been raised on disadvantages and benefits of different approaches to ovarian hyperstimulation [30]. Alternative reproductive strategies have been proposed to reduce risks and costs of in-vitro technology or merely simplify the clinical procedure. In-vitro maturation (IVM) of oocytes has been adopted in some centres to avoid ovarian stimulation in women at high risk of ovarian hyperstimulation syndrome (OHSS), such as polycystic ovary (PCO) or polycystic ovary syndrome (PCOS) patients [31–38]. In other cases, IVM has been also suggested to represent an alternative for the treatment of women with normal ovarian physiology, but suffering from tubal pathologies or with an infertile partner [39–41]. In view of the crucial importance to define an appropriate clinical treatment for each category of patients, we performed a study in order to assess the potential of different factors, in particular AMH, to identify normally ovulating women suitable for an IVM procedure on the basis of the number of retrieved oocytes. In fact, it has been suggested that the chances to achieve a pregnancy in IVM cycles is positively associated to the recovery of at least five oocytes [39–41].
Materials and methods
Patient selection
This study was conducted from January to December 2008 at Biogenesi, Centre of Reproductive Medicine - Istituti Clinici Zucchi -, Monza, Italy, in women with an indication for IVM treatment. It was approved by the local Institutional Review Board (IRB). The inclusion criteria were: age <39 years, regular menstrual cycles (25–34 days), morphologically and endocrinologically normal ovaries, body mass index ≤32. PCOS, PCO, and women with other ovarian and endocrinology abnormalities were excluded. PCOS women were considered those with chronic anovulation or other clinical evidence of hyperandrogenic ovaries. PCO women were identified on the basis of more than 15 follicles in one plane showing the features of multicystic ovaries at ultrasound scan without other symptoms of polycystic syndrome.
A written informed consent was obtained from all participating couples.
In vitro maturation
On day 3 of a spontaneous menstrual cycle, women underwent a baseline transvaginal ultrasound assessment to determine ovarian morphology, AFC, and endometrial thickness. Meanwhile, a venous blood test was also performed to measure the basal concentration of FSH, 17-β estradiol, and blood AMH levels.
Women with an FSH ≤10 mIU/ml, 17-β estradiol level ≤200 pg/ml, an AFC ≥5 were admitted, whereas those with ovarian cysts ≥12 mm were excluded.
Women were scheduled for oocyte pick up (OPU) when endometrium thickness was ≥5 mm and the leading follicle diameter was between 9 and 12 mm.
Oocytes were collected and matured in vitro following the procedure previously described [40]. In particular, oocyte aspiration was performed by single lumen needle (code 4551- E2, Gauge 017, 35 cm in length; Gynetics, Belgium) connected to a vacuum pump (pressure 80–100 mmHg; Craft Pump-Rocket UK). During the oocyte collection all women received sedation with Propofol (Astra-Zeneca, Italy). Oocyte recoveries were carried out by only two clinicians, whose equivalence in oocyte collection ability had been previously validated in our IVM program. Follicular aspirates, containing cumulus–oocyte complexes (COC) were collected in a single bottle (tissue culture flask, 50 ml, Becton-Dickinson, USA) containing 15 ml pre-warmed Flushing Medium with heparin (Medicult, Jyllinge, Denmark). After collection, the follicular aspirates were filtered through a 70 μm cell strainer (Becton-Dickinson, USA) and washed twice with Flushing Medium containing heparin. Then, COC were searched under a stereomicroscope and washed once in flushing medium (Medicult, Jyllinge, Denmark). COC were examined and classified. Oocytes with signs of mechanical damage or atresia were discarded. Immature oocytes were placed in a single well Petri dish (Becton-Dickinson, USA) containing 0.5 ml pre-equilibrated Lag Medium (vial 1 of IVM system medium, Medicult, Jyllinge, Denmark) and incubated at 37°C and 5% CO2 in a humidified atmosphere for 3 h. Oocytes were transferred to a four-well culture dish with 0.5 ml IVM medium (vial 2 of IVM system medium, Medicult, Jyllinge, Denmark) supplemented with 0.075 IU/ml recombinant FSH (Serono, Italy) and 0.1 IU/ml HCG (Serono, Italy). Culture of immature oocytes was prolonged for 26 h. Following this period the COC were treated with 80 IU/ml hyaluronidase solution (Sage Media, USA) or cumulase (Medicult, Jyllinge, Denmark) to remove cumulus cells. Semen samples were prepared using discontinuous gradients (47.5% and 90%) of Sil-Select (Ferti-Pro, Belgium). In compliance with the Italian Law regulating assisted reproduction during the period of study, a maximum of three selected oocytes were inseminated by intracytoplasmic sperm injection (ICSI) to achieve fertilization. In all cases, ICSI was adopted to minimise the chances of fertilization failure. Fertilization was assessed 16–18 h after injection and confirmed by the presence of two pronuclei and two polar bodies. All the resulting pre-zygotes were individually cultured in 4-well Petri dishes containing 0.5 ml of IVF medium or ISM1 (Medicult, Jyllinge, Denmark). Embryos were cultured for 2 or 3 days; Embryo quality was evaluated daily by observing the relative proportion of anucleated cellular fragments and the number of blastomeres. As established by law, all resulting embryos were transferred.
All women received oral 17-β estradiol supplementation (6 mg/day of 17-β estradiol (Novo-Nordisk, Bagsværd, Denmark), starting on the day of oocyte retrieval. Luteal support was provided by intravaginal progesterone supplementation (Prometrium, Rottapharm, Monza, Italy) 600 mg/day starting 1 day later. Embryo transfer was carried out using a Gynetics soft catheter (Semtrac 5–2000 SET- Gynetics Belgium). All embryo transfers were performed 48–72 h after ICSI. Pregnancy was tested 12–13 days following embryo transfer by quantitative definition of serum β- human chorionic gonadotropin (β-hCG). In case of positive test, oestrogen and progesterone supplementations were continued until the 12th week of gestation. Clinical pregnancies were defined by the presence of a gestational sac with fetal heart beat at transvaginal ultrasound examination 2 weeks after β-hCG testing.
AMH measurement
Quantitative measurement of AMH in serum was performed by an enzymatic two-site immunoassay (MIS/AMH Enzyme-Linked Immunosorbent (ELISA) kit ® DSL-10-14400 Active, Diagnostic System Laboratories, Inc./Beckman-Coulter) following the manufacturer’s instructions.
Standards, controls and samples were assayed manually in duplicates using an automatic microplate washer and reader by two operators in three different runs. The intra- and inter-assay coefficients of variation (CV%) were <6.4% and <9.8% respectively. Results were expressed in ng/ml.
Statistical analysis
Mean, standard deviation and range were used to describe results for continuous variables while absolute and percentage frequencies were used for dichotomous variables. The comparison between groups was analyzed by the t-Student test and Fisher exact test. We analysed with univariate linear regression analysis the association between number of oocytes and age, basal FSH, estradiol level, AFC and AMH. The same parameters were included in a multivariate regression model to identify independent variables able to predict the number of oocytes retrieved. Finally, as we verified that in pregnant cycles the number of oocyte collected was 7.2 ± 3.3, we assumed as an outcome a collection of at least 5 oocytes and ROC analysis was used to quantify cut-off AMH values able to predict this outcome. Stata software 9.0 (Stata Corporation, 1999, Texas U.S.A.) was used for performing the statistical analysis. A level of p < 0.05 was adopted for significance.
Results
One hundred seventy-seven women, selected for an IVM procedure according to the criteria described above, were included in the study. Their mean age, BMI, day 3 FSH, 17-β estradiol, AMH, and AFC are described in Table 1.
Table 1.
Characteristics of 177 patients selected for IVM and included in the study
| Variable | Mean±St. Dev. | Range |
|---|---|---|
| Age | 33.3 ± 2.89 | 22.9–37.9 |
| BMI | 21.9 ± 2.98 | 16.2–30.9 |
| FSH | 5.9 ± 1.76 | 1–10 |
| 17−β oestradiol | 40.51 ± 15.47 | 10–85 |
| AFC | 9.14 ± 3.39 | 5–21 |
| AMH | 3.32 ± 2.34 | 0.07–13.5 |
A total of 991 oocytes were recovered (Table 2), with a mean of 5.6 oocytes per cycle (± SD 3.2, range 0–15). In 2.3% (4/177) of cycles no oocytes were retrieved. Immature oocytes were cultured for 30 h, excluding 25 of them (2.5%) which degenerated. Following in vitro maturation, 484 metaphase II (MII) oocytes were obtained, corresponding to a maturation rate of 50.1%.
Table 2.
Laboratory and clinical outcome of 177 IVM cycles included in the study. An AMH threshold value of AMH > 1.28 g/ml identified cycles in which a higher number of oocytes were collected
| Total | AMH ≤ 1.28 g/ml | AMH > 1.28 g/ml | $t-Student test | |
|---|---|---|---|---|
| (n = 177) | (n = 32) | (n = 145) | *Fisher exact test | |
| Collected oocytes | 991 | 96 | 895 | |
| Mean no. of collected oocytes | 5.6 ± 3.2 | 3.2 ± 2.0 | 6.2 ± 3.1 | P < 0.0001$ |
| Mature oocytes after 30 h (%) | 484/966 (50.1%) | 56/91 (61.5%) | 428/875 (48.9%) | P = 0.014* |
| Mean no. of mature oocytes after 30 h | 2.7 ± 1.9 | 1.8 ± 1.5 | 3.0 ± 1.9 | P = 0.0005$ |
| Fertilized oocytes (%) | 276/380 (72.6%) | 31/49 (63.3%) | 245/331 (74.0%) | P = 0.082* |
| Total transferred embryos | 276 | 31 | 245 | |
| Beta-hCG positive tests | 28 | 2 | 26 | |
| Total clinical pregnancies | 25 | 2 | 23 | |
| Miscarriages | 5 (20.0%) | 0 | 5 | |
| Clinical pregnancies/OPU (%) | 25/177 (14.1%) | 2/32 (6.3%) | 23/145 (15.9%) | P = 0.125* |
| Clinical pregnancies/transfer (%) | 25/151 (16.6%) | 2/21 (9.5%) | 23/130 (17.7%) | P = 0.281* |
| Single pregnancies | 20/25 (80.0%) | 1/2 (50.0%) | 19/23 (82.6%) | |
| Twin pregnancies | 5/25 (20.0%) | 1/2 (50.0%) | 4/23 (17.4%) | |
| Implantations (%) | 30/276 (10.9%) | 3/31 (9.7%) | 27/245 (11.0%) | P = 0.557* |
Mature oocytes were microinjected achieving a fertilization rate of 72.6% and obtaining 276 embryos. After assisted hatching, all embryos were transferred in 151 ultrasound-assisted embryo transfers, with an average number of 1.6 (± SD 1.0, range 1–3) embryos replaced per transfer.
Twenty-eight β-hCG positive tests were reported, 25 of which developed into clinical pregnancies involving 30 gestational sacs with fetal heart beat.
The clinical pregnancy rates were 14.1% per oocyte retrieval and 16.6% per embryo transfer. An implantation rate of 10.9% and a miscarriage rate of 20.0% (5/25) were reported.
Univariate linear regression analysis (Table 3) showed that the number of oocytes recovered negatively correlated with age, basal FSH and 17-β estradiol. On the contrary oocyte number was positively associated with AFC and AMH. We found a significant positive correlation between AMH and AFC (p < 0.0001), a negative significant correlation between AMH and FSH (p < 0.0001) and AMH and 17-β estradiol (p = 0.008) while no correlation was found between AMH and age.
Table 3.
Univariate and multiple linear regression analyses examining the relative and independent correlations of AMH, FSH, 17-β estradiol, AFC and age with respect to the number of oocytes harvested
| Univariate linear regression analysis | Multivariate linear regression model | |||||
|---|---|---|---|---|---|---|
| Coef. | 95% IC | p-value | Coef. | 95% IC | p-value | |
| Age | -0.17 | -0.33–-0.01 | 0.038 | -0.11 | -0.28–0.06 | 0.196 |
| FSH | -0.36 | -0.63–-0.10 | 0.008 | -0.06 | -0.34–0.22 | 0.690 |
| 17−β oestradiol | -0.03 | -0.07–-0.00 | 0.044 | -0.01 | -0.04–0.02 | 0.602 |
| AFC | 0.36 | 0.23–0.49 | < 0.0001 | 0.13 | -0.02–0.28 | 0.092 |
| AMH | 0.66 | 0.48–0.84 | < 0.0001 | 0.54 | 0.30–0.77 | < 0.0001 |
A multiple linear regression analysis, examining AMH, FSH, 17-β estradiol, AFC and age (Table 3) was also performed in order to evaluate the independent predictive value of each factor with reference to the number of oocytes harvested. The analysis showed a significant positive correlation only for AMH (p < 0.0001) whilst other markers did not predict independently the number of oocytes recovered. Linear regression analyses were carried out also by using a stricter inclusion criterion of AFC ≥8. Under this condition, the univariate test revealed that the number of retrieved oocytes was positively correlated with AFC (p = 0.044) and above all AMH (p < 0.0001), whereas AMH was the only factor positively associated (p = 0.004) with the number of collected oocytes in the multivariate analysis.
No correlation between either AMH or AFC and clinical pregnancy rate was observed. In pregnant cycles the number of oocyte collected (7.2 ± 3.3 oocytes) was significantly higher (p = 0.003) in comparison to non-pregnant cycles (5.3 ± 3.1 oocytes). A ROC curve was calculated, considering as a criterion of analysis the retrieval of at least 5 immature oocytes (Figure 1). In fact, this factor has been suggested to be positively associated to a higher chance to achieve a pregnancy in IVM cycles [39–41]. On this basis, a threshold AMH value of 1.28 ng/ml as an indicator of sufficient yield of retrieved oocytes was identified (Table 4). Using this threshold, the sensitivity, namely the ability to predict the retrieval of ≥5 oocytes, reached 93.4% while the specificity, or the ability to predict the recovery of less than 5 immature oocytes, was 33.8%. This corresponded to a negative predictive value (NPV) of 75.0% and a positive predictive value (PPV) of 67.6%. On the basis of this prediction, 24 out of 32 cycles with AMH ≤1.28 ng/ml should have been cancelled before oocyte collection. The comparison between the outcomes of the AMH <1.28 ng/ml and AMH >1.28 ng/ml groups are reported in Table 2. Pregnancy rates per OPU and transfer and implantation rates were higher in the AMH >1.28 ng/ml group but these differences were not statistically significant.
Fig. 1.
ROC curve representing the relationship between the retrieval of ≥5 oocytes and AMH, corresponding to reference and classification variables, respectively
Table 4.
ROC analysis results
| AMH ≤1.28 ng/ml | ||
|---|---|---|
| % | No. | |
| Sensitivity (True positive) | 93.4 | 98/106 |
| Specificity (True negative) | 33.8 | 24/71 |
| Correctly Classified | 69.4 | 122/177 |
| Positive Predictive Value (PPV) | 67.6 | 98/145 |
| Negative Predictive Value (NPV) | 75.0 | 24/32 |
Discussion
The chances to achieve a full term healthy pregnancy in women requiring ART treatment are closely related to their ability to produce an adequate number of good quality oocytes [13]. The number of antral follicles that may be observed during the initial phase of the ovarian cycle seems to represent a good indicator of the endowment of primordial follicles forming the ovarian functional reserve. Hence, the significance to assess the ovarian functional reserve is to determine the woman’s ovarian potential and, on this basis, select the more appropriate treatment. However, the ovarian response observed during an ovarian stimulation with gonadotrophins may reveal an otherwise unrecognized incompetent ovarian function. Women with a reduced response to gonadotrophin stimulation, referred to as poor responders, are detected only during ultrasound monitoring [42]. It is arduous to estimate the percentage of women, still in their thirties, who fall within the definition of poor responders. In our experience up to 20% of women undergoing ART present with some problems associated to oocyte number and quality (unpublished data).
During an IVF procedure involving gonadotrophin stimulation, an impaired ovarian response may be recognized before oocyte collection. Vice versa, in unstimulated natural cycles, poor ovarian response may be not discernible until egg recovery. Therefore, the determination of ovarian potential becomes more crucial in IVF procedures not requiring ovarian stimulation, such as IVM.
It has been reported by various authors [31, 32, 40, 43, 44] that in IVM cycles the number of the available oocytes is well correlated with the outcome of the procedure. For that reason, women with polycystic ovaries, who have several antral follicles by definition, are the best candidates for an IVM treatment [31–38].
Likewise, in normally ovulating women the single most important factor that may affect the IVM outcome is the number of available oocytes which, if inadequately small, can deny the possibility to select embryos from a larger cohort [45, 46].
In the present study, the number of retrieved oocytes was significantly higher in pregnant women, suggesting that the prediction of this parameter should be considered an essential criterion for patient selection. In one of our previous studies [40] we focused on the criteria for an appropriate selection of patients with morphological and functional normal ovaries to treat by an IVM approach. We proposed strict criteria based on age, BMI, FSH and estradiol blood concentrations, and AFC. We suggested that only those cases which meet specific requirements should be eventually selected for an IVM procedure, although such recommendations sometimes fail to identify women with poor functional ovaries. That study showed that FSH and 17-β estradiol levels are negatively correlated to the number of oocytes retrieved, in agreement with the results of other authors [31, 40, 43].
The number of antral follicles in early follicular phase was reported to be one of the most important predictors of ovarian response in women treated with stimulated IVF cycles [47]. In IVM cycles, AFC is also significantly associated to the number of retrieved oocytes and pregnancy rate [32, 43, 44]. In the present study, AFC was confirmed to be highly associated to the number of retrieved oocytes. The same conclusions were reported in one of our previous investigations in which, by statistical regression analysis, we showed that AFC is the best independent predictive factor for the number of the oocytes harvested and pregnancy rate [40].
In the present analysis, AFC, FSH, and 17-β estradiol showed a linear significant correlation with AMH concentration. In addition, the multiple linear regression analysis, carried out to assess the independent prognostic value of each of these parameters for the prediction of the number of retrieved oocytes, confirmed exclusively AMH as a highly predictive factor. Conversely, AMH did not show a correlation with age, a fact that may be explained by the limited age range and sample size of the study.
A recent and comprehensive meta-analysis [48] was conducted in order to evaluate the efficacy of AMH levels and AFC to predict IVF outcome. With regard to the ability to identify poor responder women, the analysis demonstrated the AMH is at least as efficient as AFC to predict a high ovarian response [49, 50]. Various reports suggest a correlation between low AMH levels and poor ovarian response [28, 50–52]. Recently, on the basis of a large prospective cohort study, Nelson et al. [53] have proposed to use the AMH value as a reliable tool to design individualized IVF approaches and maximize the clinical outcome.
In our study, relevant to an IVM programme in natural cycles, the AMH measurement was instrumental for the recognition of women with reduced ovarian function. A ROC curve was elaborated to determine an AMH cut-off value able to select women suitable for an IVM treatment. One of the major criticisms to IVM, especially if applied to regularly cycling women, is the low clinical outcome caused mainly from a small number of retrieved oocytes. Therefore, the identification of cycles able to produce numerous oocytes would reduce the efficiency gap between IVM and conventional IVF.
Our overall experience in IVM suggests that the chances to achieve a pregnancy are critically dependent on the recovery of at least five immature oocytes. Hence, five immature oocytes were considered as a criterion for the generation of the ROC curve.
An interesting study [54] involving standard IVF cycles reported an AMH lower value of 1.26 ng/ml by which 88% of poor responders (women with less than 5 oocytes retrieved after gonadotrophin stimulation) were identified. In such a case, the AMH cut off was proposed to be functional for the modulation of appropriate doses of gonadotrophins in a stimulated cycle. In our study, a similar cut off was intended as a guidance to decide the appropriateness of IVM as a treatment. In particular, in an IVM scenario our study suggests how the application of a threshold AMH value of 1.28 ng/ml decreases the number of recruited cycles in which less than five oocytes are retrieved. These cycles could benefit from a standard ovarian stimulation approach in order to achieve a higher number of retrieved oocytes. Adopting the 1.28 ng/ml cut-off, 24 out of 32 cycles (NPV 75%) could have been appropriately cancelled before oocyte collection, leading to a good identification of true negative cases. Overall, the accuracy (i.e. correctly classified) of the ELISA method applied in the study would have been 69.5%.
Some authors have reported a positive correlation between high AMH levels and oocyte and embryo quality [55, 56]. Other authors [22, 53, 57, 58] found a correlation between AMH levels and pregnancy rate. We were unable to confirm an association between AMH and pregnancy rate, probably as a consequence of the limited size of our study. We did not consider the achievement of a pregnancy as a major endpoint because in that respect other factors may play an important role and therefore their possible influence should be analyzed separately. However, we hypothesize that, by increasing the number of cycles, it might be possible to establish the ability of AMH to predict a pregnancy event also because in IVM cycles the clinical outcome is not influenced by gonadotrophin administration.
In line with several other authors, in conclusion we suggest that AMH can contribute to determine an appropriate approach for each patient. In stimulated IVF cycles, measurement of circulating AMH during the course of treatment may be utilized as a criterion to predict ovarian response and adjust down regulation and dose of gonadotrophins [51, 54]. In IVM, it can be used to select patients with appropriate characteristics. Vice versa, the identification of women whose traits are less suitable for IVM can offer an opportunity to re-design the treatment and convert it into standard ovarian stimulation, thereby preventing an insufficient oocyte collection.
Footnotes
Capsule In oocyte in-vitro maturation (IVM) treatments, the chances to achieve a pregnancy are critically dependent on the retrieval of a suitable number of oocytes. AMH level appears able to identify women candidate for an IVM treatment from whom a suitable number of oocytes may be retrieved.
References
- 1.Gynaecologists CoGPoACoOa Age-related fertility decline: a committee opinion. Fertil Steril. 2008;90(3):486–487. doi: 10.1016/j.fertnstert.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. doi: 10.1196/annals.1434.011. [DOI] [PubMed] [Google Scholar]
- 3.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 4.Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 5.Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. Detection and clinical relevance. Hum Reprod. 2003;18(6):1137–1139. doi: 10.1093/humrep/deg245. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaou D, Templeton A. Early ovarian ageing. Eur J Obstet Gynecol Reprod Biol. 2004;113(2):126–133. doi: 10.1016/j.ejogrb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51(4):651–654. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 9.Toner JP, Philput CB, Jones GS, Muasher SJ. Basal follicle-stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril. 1991;55(4):784–791. doi: 10.1016/s0015-0282(16)54249-6. [DOI] [PubMed] [Google Scholar]
- 10.Wallace WH, Kelsey TW. Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod. 2004;19(7):1612–1617. doi: 10.1093/humrep/deh285. [DOI] [PubMed] [Google Scholar]
- 11.Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC. teJong FH, teVelde ER. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003;18(4):700–706. doi: 10.1093/humrep/deg135. [DOI] [PubMed] [Google Scholar]
- 12.Hendriks DJ, Mol BW, Bancsi LF, Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83(2):291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 14.Weenen C, Laven JS, Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 15.Baarends WM, Uilenbroek JT, Kramer P, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136(11):4951–4962. doi: 10.1210/en.136.11.4951. [DOI] [PubMed] [Google Scholar]
- 16.Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891–4899. doi: 10.1210/en.142.11.4891. [DOI] [PubMed] [Google Scholar]
- 17.Vet A, Laven JS, Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–362. doi: 10.1016/S0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 19.Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 20.Lee MM, Donahoe PK, Hasegawa T, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81(2):571–576. doi: 10.1210/jc.81.2.571. [DOI] [PubMed] [Google Scholar]
- 21.Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24(9):2264–2275. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- 22.Rooij IA, Broekmans FJ, Velde ER, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 23.Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? Bjog. 2005;112(10):1384–1390. doi: 10.1111/j.1471-0528.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 24.Penarrubia J, Fabregues F, Manau D, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist--gonadotropin treatmen. Hum Reprod. 2005;20(4):915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 25.Marca A, Malmusi S, Giulini S, et al. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19(12):2738–2741. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- 26.Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45(1):20–24. doi: 10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 27.Rooij IA, Broekmans FJ, Hunault CC, et al. Use of ovarian reserve tests for the prediction of ongoing pregnancy in couples with unexplained or mild male infertility. Reprod Biomed Online. 2006;12(2):182–190. doi: 10.1016/S1472-6483(10)60859-0. [DOI] [PubMed] [Google Scholar]
- 28.Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Mullerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14(5):602–610. doi: 10.1016/S1472-6483(10)61053-X. [DOI] [PubMed] [Google Scholar]
- 29.Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Mullerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89(6):1670–1676. doi: 10.1016/j.fertnstert.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 30.Edwards RG. IVF, IVM, natural cycle IVF, minimal stimulation IVF—time for a rethink. Reprod Biomed Online. 2007;15(1):106–119. doi: 10.1016/S1472-6483(10)60699-2. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen AL, Lindenberg S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction. 2001;122(4):587–592. doi: 10.1530/rep.0.1220587. [DOI] [PubMed] [Google Scholar]
- 32.Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76(5):936–942. doi: 10.1016/S0015-0282(01)02853-9. [DOI] [PubMed] [Google Scholar]
- 33.Chian RC. In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod Biomed Online. 2004;8(5):547–552. doi: 10.1016/S1472-6483(10)61101-7. [DOI] [PubMed] [Google Scholar]
- 34.Du A, Kadoch IJ, Bourcigaux N, et al. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod. 2005;20(2):420–424. doi: 10.1093/humrep/deh603. [DOI] [PubMed] [Google Scholar]
- 35.Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of embryos from in vitro-matured primary human oocytes. Fertil Steril. 1996;65(6):1151–1156. doi: 10.1016/s0015-0282(16)58330-7. [DOI] [PubMed] [Google Scholar]
- 36.Trounson A, Anderiesz C, Jones GM, Kausche A, Lolatgis N, Wood C. Oocyte maturation. Hum Reprod. 1998;13(Suppl 3):52–62. doi: 10.1093/humrep/13.suppl_3.52. [DOI] [PubMed] [Google Scholar]
- 37.Nargund G, Waterstone J, Bland J, Philips Z, Parsons J, Campbell S. Cumulative conception and live birth rates in natural (unstimulated) IVF cycles. Hum Reprod. 2001;16(2):259–262. doi: 10.1093/humrep/16.2.259. [DOI] [PubMed] [Google Scholar]
- 38.Cha KY, Chung HM, Lee DR, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization-embryo transfer. Fertil Steril. 2005;83(5):1461–1465. doi: 10.1016/j.fertnstert.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 39.Dal Canto MB, Mignini Renzini M, Brambillasca F, et al. IVM--the first choice for IVF in Italy. Reprod Biomed Online. 2006;13(2):159–165. doi: 10.1016/S1472-6483(10)60610-4. [DOI] [PubMed] [Google Scholar]
- 40.Fadini R, Dal Canto MB, Renzini MM, et al. Predictive factors in in-vitro maturation in unstimulated women with normal ovaries. Reprod Biomed Online. 2009;18(2):251–261. doi: 10.1016/S1472-6483(10)60263-5. [DOI] [PubMed] [Google Scholar]
- 41.Fadini R, Dal Canto MB, Mignini Renzini M, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19(3):343–351. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 42.Farhi J, Homburg R, Ferber A, Orvieto R. Ben Rafael Z. Non-response to ovarian stimulation in normogonadotrophic, normogonadal women: a clinical sign of impending onset of ovarian failure pre-empting the rise in basal follicle stimulating hormone levels. Hum Reprod. 1997;12(2):241–243. doi: 10.1093/humrep/12.2.241. [DOI] [PubMed] [Google Scholar]
- 43.Child TJ, Sylvestre C, Pirwany I, Tan SL. Basal serum levels of FSH and estradiol in ovulatory and anovulatory women undergoing treatment by in-vitro maturation of immature oocytes. Hum Reprod. 2002;17(8):1997–2002. doi: 10.1093/humrep/17.8.1997. [DOI] [PubMed] [Google Scholar]
- 44.Tan SL, Child TJ, Gulekli B. In vitro maturation and fertilization of oocytes from unstimulated ovaries: predicting the number of immature oocytes retrieved by early follicular phase ultrasonography. Am J Obstet Gynecol. 2002;186(4):684–689. doi: 10.1067/mob.2002.122146. [DOI] [PubMed] [Google Scholar]
- 45.Soderstrom-Anttila V, Makinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20(6):1534–1540. doi: 10.1093/humrep/deh768. [DOI] [PubMed] [Google Scholar]
- 46.Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod Biomed Online. 2004;8(2):148–166. doi: 10.1016/S1472-6483(10)60511-1. [DOI] [PubMed] [Google Scholar]
- 47.Chang MY, Chiang CH, Chiu TH, Hsieh TT, Soong YK. The antral follicle count predicts the outcome of pregnancy in a controlled ovarian hyperstimulation/intrauterine insemination program. J Assist Reprod Genet. 1998;15(1):12–17. doi: 10.1023/A:1022518103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705–714. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Lee TH, Liu CH, Huang CC, Hsieh KC, Lin PM, Lee MS. Impact of female age and male infertility on ovarian reserve markers to predict outcome of assisted reproduction technology cycles. Reprod Biol Endocrinol. 2009;7:100. doi: 10.1186/1477-7827-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nardo LG, Gelbaya TA, Wilkinson H, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 51.Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles–implications for individualization of therapy. Hum Reprod. 2007;22(9):2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 52.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril 2008. [DOI] [PubMed]
- 53.Nelson SM, Yates RW, Lyall H, et al. Anti-Mullerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24(4):867–875. doi: 10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- 54.Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23(6):1359–1365. doi: 10.1093/humrep/den108. [DOI] [PubMed] [Google Scholar]
- 55.Silberstein T, MacLaughlin DT, Shai I, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159–163. doi: 10.1093/humrep/dei270. [DOI] [PubMed] [Google Scholar]
- 56.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 57.Ficicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85(3):592–596. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 58.Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Thomas CM, Braat DD. Antimullerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87(1):223–226. doi: 10.1016/j.fertnstert.2006.06.019. [DOI] [PubMed] [Google Scholar]