Abstract
Purpose
To examine the fertilization and developmental potential of sibling mature oocytes collected from different follicle sizes on day of retrieval in in vitro maturation (IVM) cycles.
Methods
Two hundred thirty eight hCG-primed IVM cycles were performed in 213 patients with polycystic ovaries. If sibling mature oocytes were retrieved on day of collection, they were divided into two groups, Group 1 (n = 78): M-II oocytes obtained from follicles size 10–14 mm; Group 2 (n = 192): M-II oocytes obtained from follicles size <10 mm.
Results
Of the 238 cycles, 63 cycles had more than one M-II oocytes retrieved (total M-II oocytes = 270) both from Groups 1 and 2. There were no significant differences between the two groups for oocyte diameter (117.2 mm vs. 116.9 mm), fertilization (79.5% vs. 72.4%) and good quality embryo on day 3 (66.1% vs. 56.8%).
Conclusions
The M-II oocytes retrieved from the cohort of follicles (<10 mm diameter) can produce the same quality of embryos as that from large follicles, likely contributing to improve the clinical outcome.
Keywords: Embryo development, Follicle diameter, IVM, In vivo matured oocytes
Introduction
There are a number of disadvantages associated with gonadotropin stimulation in conventional in vitro fertilization (IVF) cycles. There include the risk of ovarian hyper-stimulation syndrome (OHSS), high drug costs, the need for daily injections and frequent monitoring. In vitro maturation (IVM) of immature oocytes retrieved from unstimulated ovaries has the potential to avoid those problems. Although recent studies have shown an improvement in pregnancy rates per embryo transfer in IVM cycles, the efficiency of current IVM techniques is still suboptimal compared to IVF cycles in terms of number of mature oocytes, embryo developmental competence and endometrial preparation [1].
Several authors [2–6] have reported good pregnancy rates for PCO(S) patients with the use of hCG priming (10,000 IU) prior to egg collection in IVM cycles. In hCG-primed IVM cycles, the maturation and the developmental stage of collected oocytes can be classified according to the appearance of their cumulus cells (CC) [7]. Oocytes with dispersed CC are usually only found at the time of oocyte retrieval in hCG-primed IVM cycles [7, 8]. Immature oocytes with dispersed CC have been shown to have superior rates of in vitro maturation and higher embryo developmental potential than immature oocytes with compacted or sparse CC [4, 8]. In vivo matured oocytes can only be obtained from oocytes having dispersed CC at the time of retrieval [4, 9]. These in vivo matured oocytes produce better quality cleavage- [4] and blastocyst-stage [10] embryos than in vitro matured oocytes.
In hCG-primed IVM cycles, in vivo matured oocytes can be collected from both large (≥ 10 mm) and small sized follicles (<10 mm) [4, 6, 7, 9]. However, to date, there is no report on the developmental potential of the in vivo matured oocytes retrieved from small follicles. The purpose of this study is to compare the fertilization and developmental potential of sibling in vivo matured oocytes collected from various follicle sizes on day of retrieval in hCG-primed IVM cycles.
Materials and methods
The initial IVM study was approved by the Institutional Review Board of the Hospital. The IVM treatment was explained to the patients, and informed consent was obtained.
Patients
Our study included 60 patients (63 cycles) out of a total of 213 patients with polycystic ovaries (238 cycles) who underwent IVM treatment between June 2005 and December 2008. Patient selection was based on more than one oocyte matured in vivo on the day of collection and the presence of oocytes matured in vivo from both 10–14 mm and <10 mm follicle diameter on the day of collection. The polycystic ovary was defined as containing at least 12 follicles between 2 and 8 mm in diameter on trans-vaginal ultrasound performed between day 2 and day 4 of a spontaneous or induced menstrual cycle. The patients who had a follicle >14 mm at the time of collection were excluded based on a previous study [6]. Subjects were also excluded if they had less than 2 M-II oocytes collected. A baseline ultrasound was obtained for all women between days 2 and 5 of menstrual bleeding to ensure that no ovarian cysts were present and to measure the antral follicle count (AFC) [11]. Transvaginal ultrasound scans were repeated on day 8 of the cycle or on the day of hCG administration.
Oocyte collection
Oocyte retrieval was performed between days 8 and 21 of the menstrual cycle depending upon the length of the patient’s cycle and follicular development. 10,000 IU hCG was administered subcutaneously when endometrial thickness reached at least 6 mm and a lead follicle of 10–12 mm in mean diameter was present [4, 5]. Follicle size and endometrial thickness were measured once again by ultrasound scan at the time of oocyte retrieval. Follicle size was measured as a mean of two perpendicular measurements in a two-dimensional plane. The thickest endometrial segment (between the two interfaces of the endometrial-myometrial junction) was measured transvaginally on a “frozen” midplane, longitudinal section of the uterus, by two-dimensional ultrasonography. Oocyte retrieval was performed 35–38 h after hCG priming. Transvaginal ultrasound–guided collection of oocytes was performed using a 19-gauge aspiration needle (K-OPS-7035-RWH-ET, Cook, Australia) with an aspiration pressure of 7.5 kPa. The largest diameter follicle (10–14 mm) was the first follicle aspirated at the beginning of the procedure, the needle was flushed completely with heparinized saline and oocyte maturity was assessed. Collection of the remaining oocytes with follicles of <10 mm was performed according to accessibility of the follicles after which the maturity of each oocyte was identified. The IVM cycles which had a follicle larger than 14 mm mean diameter at collection were excluded from this study because sibling oocytes could be negatively affected by the size of the follicle [6]. To avoid the possibility of missing oocytes with a small amount of CC, the remaining follicular aspirates were filtered using a mesh with 70-μm holes (Falcon, Becton Dickinson & Company, NJ, USA), washed three times with oocyte wash medium (Cooper Surgical, CT, USA) that contained HEPES buffer supplemented with recombinant human serum albumin, and the oocytes isolated under a stereomicroscope.
In vitro maturation
The nuclear maturity of the collected oocytes was assessed using the spreading method [1]. Briefly, follicular aspirates in the Petri dish were first removed with the remaining small amounts of fluid. The cumulus-oocyte complexes (COC) identified were allowed to spread along the Petri dish. The oocyte cytoplasm was then examined under the dissecting microscope. If no Germinal Vesicle (GV) was observed within the oocyte cytoplasm, the cumulus masses were removed with hyaluronidase and mechanical pipetting, 1 h after the oocyte collection. At this point, oocyte maturity was reassessed. When more than one in vivo matured oocyte was retrieved from different size follicles in the same patient (63 cycles), the sibling mature oocytes were divided into two groups, group 1(n = 78): M-II oocytes were obtained from the follicle sizes 10–14 mm; group 2 (n = 192): M-II oocytes were obtained from the follicle sizes <10 mm. The diameter of sibling M-II oocytes collected from both groups was measured with a micrometer after saving an image of each oocyte in our data base software (Hamilton Thorn, MA, USA).
Oocytes that were mature on the collection day (Day 0: 0–6 h) were inseminated on the same day, while the immature oocytes (GV- or Germinal vesicle breakdown (GVBD)-stage) were cultured in IVM medium (Cooper Surgical, CT, USA) supplemented with 75 mIU/ml FSH and LH until day 2.
IVF, in vitro development and embryo transfer
The M-II oocytes were inseminated by intracytoplasmic sperm injection (ICSI) using the partner’s spermatozoa. ICSI was performed at least 1 h after observing the first polar body (PB) extrusion as suggested by Hyun et al [12]. Fertilization was assessed 17–19 h after insemination by the appearance of two distinct pronuclei and two polar bodies. The zygotes were cultured in Embryo Maintenance Medium (Cooper Surgical, CT, USA). Embryonic development was assessed on day 3 (65–67 h) after insemination according to the regularity of blastomeres, the percentage and pattern of anucleate fragments, and all dysmorphic characteristics of the embryos. For the purpose of this study, we defined embryos as good quality, if on day 3, they had 6-cells to 8-cells, contained <20% anucleate fragments and exhibited no apparent morphological abnormalities [4]. Embryos showing blastomere multi-nucleation, poor cell adhesion, uneven cell division and/or cytoplasmic abnormalities were defined as low quality. The best quality embryos produced from oocytes in vivo or in vitro were transferred on Day 2 or Day 3 after ICSI.
Statistical analysis
Statistical analyses were performed using the χ2, Fisher’s exact, or t-test as appropriate. All P-values quoted are two-sided, and values <0.05 indicate statistical significance. Analyses were performed using the SPSS statistical package (SPSS, Inc., Chicago)
Results
Among the IVM cycles (n = 238), 63 had more than one in vivo M-II oocytes retrieved (total M-II oocytes = 270) from both larger (10–14 mm) (Group 1 = 78) and smaller (<10 mm) (Group 2 = 192) follicles in the same patient (Table 1). Figure 1 illustrates the COC appearances of in vivo matured oocytes collected according to follicle size at the time of retrieval, in hCG-primed IVM cycles. Generally, oocytes with more expanded corona radiata were collected from larger follicles in the same patient. Table 1 summarizes the clinical outcomes in those cycles that had in vivo matured oocytes collected from both larger and smaller follicles. High clinical pregnancy rates (52.4%) and reasonable implantation rates (19.1%) were demonstrated in those hCG-primed IVM cycles.
Table 1.
Clinical outcomes in IVM cycles collected in vivo matured oocytes both bigger and small follicles in the same patients
| Variables | |
|---|---|
| No. of Patients (mean ± S.D.) | 60 (32.1 ± 3.3) |
| No. of cycles | 63 |
| Endometrial thickness at oocyte retrieval | 8.5 ± 1.9 |
| Dominant follicle size at the time of hCG | 11.1 ± 1.8 |
| Dominant follicle size at the time of oocyte retrieval | 12.6 ± 1.8 |
| No. of oocytes retrieved (mean ± S.D.) | 1114 (17.7 ± 7.8) |
| No. of oocytes matured on collection day (%) | 270 (24.2) |
| Mean ± S.D. (range) | 4.3 ± 3.1 (2–18) |
| No. of oocytes cultured in vitro | 844 |
| No. of MII oocytes matured in vitro (%) | 575 (68.1) |
| No. of total MII oocytes (%) | 845 (75.9) |
| No. of zygotes (%) | 613 (72.5) |
| No. of embryos cleaved (%) | 554 (90.4) |
| No. of transferred embryos (mean ± S.D.) | 230 (3.7 ± 0.5) |
| No. of clinical pregnancies (%) | 33 (52.4) |
| No. of implantations (%) | 44 (19.1) |
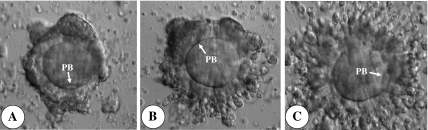
Fig. 1.
Cumulus cell expansion of metaphase II (M-II) stage oocytes retrieved from hCG-primed IVM cycles. A M-II stage oocyte with clumped corona radiata retrieved from 8 mm follicle. B M-II stage oocyte with Moderately expanded corona radiata retrieved from 10 mm follicle. C M-II stage oocyte with completely expanded corona radiata retrieved from 12 mm follicle. Original magnification x200. PB = 1st polar body
To explore possible contributions to the high pregnancy rates observed, embryological aspects were analyzed (Tables 2 and 3). Table 2 details the rates of maturation, fertilization, cleavage and embryo development according to maturation time for the study group. The good quality embryo rate, defined as the number of good quality embryos divided by the number of normal fertilized oocytes, was significantly higher in the embryos derived from the in vivo matured oocytes (59.7%) than from oocytes matured in vitro on Day 1 (45.2%) (P < 0.01) (Table 2). The rates of cleavage and good quality embryos in oocytes matured in vitro on Day 2 were significantly lower than those in oocytes matured in vitro on Day 1, as well as in vivo matured oocytes (P < 0.01).
Table 2.
Details in embryology of hCG-primed IVM cycles (n = 63)
| Day of maturity and ICSI | Retrieval Day | Day 1 | Day 2 |
|---|---|---|---|
| No. of M-II oocytes | 270 | 385 | 190 |
| No. of zygotes (%) | 201 (79.5) | 283 (72.4) | 129 (67.9) |
| No. of embryos cleaved (%) | 195 (97.0) | 267 (94.3) | 92 (71.3)c |
| No. of good quality embryos (%d) | 120 (59.7)a | 128 (45.2)a,b | 19 (14.7)b |
Values with the same superscript letter are significantly different: aP <0.01, bP <0.01, cP <0.01.
dThe number of good quality embryos divided by number of zygotes.
Table 3.
Comparison of diameter and embryo developmental competence between oocytes matured in vivo collected from different follicular size of sibling follicles derived from the same patients in hCG-primed IVM cycles (n = 63)
| Variables | Group 1 | Group 2 | P value |
|---|---|---|---|
| No. of M-II oocytes retrieved | 78 | 192 | |
| Diameter of oocytes (mean ± S.D.) | 117.2± 1.5 mm | 116.9± 1.7 mm | NS |
| No. of zygotes (%) | 62 (79.5) | 139 (72.4) | NS |
| No. of embryos cleaved (%) | 60 (96.8) | 135 (97.1) | NS |
| No. of good quality embryos on Day 3a (%b) |
41 (66.1) | 79 (56.8) | NS |
Group 1: In vivo mature oocytes retrieved from between 10–14 mm diameter of follicles
Group 2: In vivo mature oocytes retrieved from <10 mm diameter of follicles
a6–8 cells contained <20% anucleate fragments and exhibited no apparent morphological abnormalities.
bThe number of good quality embryos divided by number of zygotes.
NS non significant.
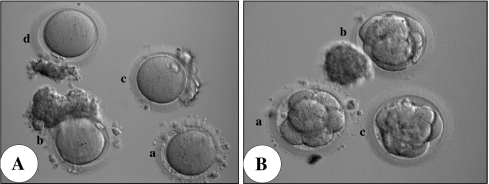
For the in vivo matured oocytes, there was no significant difference between diameters of the oocytes retrieved from Group 1 (117.2± 1.5 mm) and Group 2 (116.9± 1.7 mm) (Table 3). In addition, there were no significant differences between the two groups in terms of fertilization rates (62/78, 79.5% vs. 139/192, 72.4%), embryo cleavage rates (96.8% vs. 97.1%), or the proportion of good quality embryos on day 3 (41/62, 66.1% vs. 79/139, 56.8%) (Table 3). The morphology of sibling in vivo matured oocytes collected from various size follicles in a single patient and the resultant Day 3 embryos are shown in Fig. 2. There was no difference in embryo quality on day 3 among embryos (Fig. 2b). All three embryos had started compaction already (Fig. 2b).
Fig. 2.
AIn vivo matured oocytes retrieved from different follicle diameter in a patient B Embryos produced from the oocytes collected from different follicle sizes. a: from 12 mm follicle diameter. b, c and d: from <10 mm diameter of follicles. Original magnification x100
Discussion
The collection of immature oocytes from unstimulated ovaries, followed by in vitro maturation and subsequent insemination, is particularly useful for patients with high antral follicle counts. When stimulated with gonadotropins, these patients are at increased risk of developing ovarian hyperstimulation syndrome [7]. There have been conflicting reports on the pregnancy outcomes of cycles where hCG-priming is given before IVM collection [2, 13]. The advantage of hCG-priming in IVM cycles relates to the possibility of retrieving mature oocytes [9, 14, 15].
In conventional IVF cycles, an increasing rate of in vivo matured oocytes has been observed as follicle diameter increase [16–20]. However, a significant number of M-II stage oocytes (about 40% of oocytes retrieved) were collected from follicles <10 mm diameter [19, 20]. One possible explanation put forth by these authors is that the prolonged exposure to exogenous FSH might induce an increase in LH receptors gene expression in granulosa cells of small follicles, not expected to demonstrate LH receptors. Therefore, at the time of hCG administration, the smaller follicles (<10 mm) would have already achieved the ability to undergo meiotic maturation. In this study, however, all patients who underwent IVM cycles were stimulated with 10,000 hCG alone without any FSH stimulation prior to oocyte collection. Nevertheless, some in vivo matured oocytes were collected from <10 mm follicles. It remains unclear at what stage of folliculogenesis LH receptors appears. They may appear on small follicles without FSH stimulation at the time of hCG-priming, causing in vivo matured oocytes. In fact, Yang et al [8] reported finding LH receptor transcripts in dispersed cumulus cells (CC) collected in hCG-primed IVM cycles.
The dominant follicle (DF) can be distinguished from other follicles in the cohort when it reaches at least 10 mm in diameter [21, 22]. To investigate the reason for high pregnancy rates (52.4%) in women who had retrieved in vivo matured oocytes from both large and small follicles, therefore, we analyzed embryological aspects such as the rates of fertilization, cleavage and embryo development between siblings in vivo matured oocytes collected from follicles 10–14 mm and <10 mm in diameter, from the same patient.
In conventional IVF cycles, the definition of small follicles varies from <10 mm to <16 mm diameter [16–20]. Several reports have been published on the effect of fertilization and embryonic developmental competence from in vivo matured oocytes collected from different follicle diameters in COH cycles [16–20]. A positive correlation was observed between follicular diameter and fertilization rates as well as rates of good quality embryos when oocytes were fertilized by ICSI [18–20]. On the other hand, Bergh [17] showed similar fertilization, cleavage and implantation rates, regardless of the size of the follicles from which oocytes were aspirated (small at ≤ 16 mm, or large at >16 mm) in ICSI cycles. Those authors suggested that in vivo matured oocytes from small follicles may favor increased number of good quality and transferrable embryos. In the present study, it is interesting that we did not find an association between follicular diameter and embryo quality in the in vivo matured oocytes. This suggests that in hCG-primed IVM cycles, in vivo matured oocytes collected from small follicles (<10 mm) have the same potential as those from larger follicles (10–14 mm).
This discrepancy with the results published for COH cycles could be due to the nature of the oocytes. It is possible that in vivo matured oocytes collected from small follicles in COH cycles may be inherently of lower quality since they failed to respond in a similar fashion as the lager follicles to supra-physiologic gonadotropin stimulation. These smaller follicles in gonadotropin stimulated cycles might have fewer FSH receptors or a lower capability to respond to FSH stimulation. In contrast, in IVM cycles without exogenous FSH stimulation, the follicle diameter is less likely to represent follicular capacity to respond to gonadotropins since the ovarian levels of FSH are likely to be significantly lower than those in the exogenously stimulated cycles.
Contrary to COH cycles, there is no consensus on the optimal timing of oocyte collection in IVM cycles. Generally, it is thought that the oocytes and their embryonic development potential may be affected by endocrine changes that occur in the remaining cohort after selection of a dominant follicle (DF). Therefore, the most common view shared by investigators for the optimal timing of oocyte collection in IVM cycles is based on the presence of a DF. There is conflicting evidence regarding the importance of a DF on the day of aspiration in IVM cycles [1]. Our previous data demonstrated that when a DF of over 14 mm was present at the time of collection in the hCG-primed IVM cycles, clinical outcomes depended on the presence of an in vivo matured oocytes collected from the DF [6]. We therefore suggested that hCG should be given when the largest follicle reached 10–12 mm. This would take into consideration the fact that the DF tend to grow more between hCG priming and oocyte retrieval. Some of the oocytes in early atretic follicles still possess the competence to support embryonic development [23]. In this study, therefore, oocytes retrieved from small follicles (<10 mm) might have been retrieved just after induction of atresia but before prolonged exposure to potentially detrimental endocrine and paracrine effects of the DF (≤14 mm at collection).
Two methods could be considered to increase the number of oocytes matured in vivo; a time-dependent and dose-dependent response to hCG priming. Gulekli et al showed no dose response 36 h after hCG priming in the rates maturation and cleavage between 10,000 IU and 20,000 IU hCG [24]. However, a positive correlation was observed between delayed collection time after hCG priming and the number of oocytes matured in vivo in hCG-primed IVM cycles [6]. Therefore, prolonging the interval between hCG administration and oocyte collection in IVM cycles might be a valuable method to collect more oocytes matured in vivo for PCOS [6].
In conclusion, in vivo matured oocytes in hCG-primed IVM cycles produced more viable embryos than that of in vitro matured oocytes, resulting in higher pregnancy rates. The in vivo matured oocytes retrieved from a cohort of small follicles generated embryos with similar developmental potential to oocytes derived from larger follicles and contributed to increased pregnancy rates.
Footnotes
Capsule The M-II oocytes retrieved from the cohort of follicles (< 10 mm diameter) can produce the same quality of embryos as that from large follicles, likely contributing to improve the clinical outcome.
References
- 1.Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Human Reprod Update. 2010;16:675–689. doi: 10.1093/humupd/dmq014. [DOI] [PubMed] [Google Scholar]
- 2.Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15:165–170. doi: 10.1093/humrep/15.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Lin YH, Hwang JL, Huang LW, Mu SC, Seow KM, Chung J, et al. Combination of FSH priming and hCG priming for in-vitro maturation of human oocytes. Hum Reprod. 2003;18:1632–1636. doi: 10.1093/humrep/deg335. [DOI] [PubMed] [Google Scholar]
- 4.Son WY, Chung JT, Demirtas E, Holzer H, Sylvestre C, Buckett W, et al. Comparison of cycles programmed for IVM with and without in vivo matured oocytes retrieved. Reprod Biomed Online. 2008;17:59–67. doi: 10.1016/S1472-6483(10)60294-5. [DOI] [PubMed] [Google Scholar]
- 5.Son WY, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, et al. A 38-hour interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23:2010–2016. doi: 10.1093/humrep/den210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son WY, Chung JT, Herrero B, Dean N, Demirtas E, Holzer H, et al. Selection of the optimal day for oocyte retrieval based on the diameter of the dominant follicle in hCG-primed in vitro maturation cycles. Hum Reprod. 2008;23:2680–2685. doi: 10.1093/humrep/den332. [DOI] [PubMed] [Google Scholar]
- 7.Son WY, Yoon SH, Lim JH. Effect of gonadotrophin priming on in-vitro maturation of oocytes collected from women at risk of OHSS. Reprod Biomed Online. 2006;13:340–348. doi: 10.1016/S1472-6483(10)61438-1. [DOI] [PubMed] [Google Scholar]
- 8.Yang SH, Son WY, Yoon SH, Ko Y, Lim JH. Correlation between in vitro maturation and expression of LH receptor in cumulus cells of the oocytes collected from PCOS patients in HCG-primed IVM cycles. Hum Reprod. 2005;20:2097–2103. doi: 10.1093/humrep/dei045. [DOI] [PubMed] [Google Scholar]
- 9.Son WY, Yoon SH, Lee SW, Ko Y, Yoon HG, Lim JH. Blastocyst development and pregnancies after IVF of mature oocytes retrieved from unstimulated patients with PCOS after in-vivo HCG priming. Hum Reprod. 2002;17:134–136. doi: 10.1093/humrep/17.1.134. [DOI] [PubMed] [Google Scholar]
- 10.Son WY, Lee SY, Lim JH. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Hum Reprod. 2005;20:3204–3207. doi: 10.1093/humrep/dei195. [DOI] [PubMed] [Google Scholar]
- 11.Tan SL, Child T, Gulekli B. In vitro maturation and fertilization of oocytes from unstimulated ovaries: predicting the number of immature oocytes retrieved by early follicular phase ultrasonography. Am J Obstet Gynecol. 2002;186:684–689. doi: 10.1067/mob.2002.122146. [DOI] [PubMed] [Google Scholar]
- 12.Hyun CS, Cha JH, Son WY, Yoon SH, Kim KA, Lim JH. Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum Reprod. 2007;22:1991–1995. doi: 10.1093/humrep/dem124. [DOI] [PubMed] [Google Scholar]
- 13.Söderström-Anttila V, Mäkinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20:1534–1540. doi: 10.1093/humrep/deh768. [DOI] [PubMed] [Google Scholar]
- 14.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19:343–351. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 15.Lim JH, Yang SH, Xu Y, Yoon SH, Chian RC. Selection of patients for natural cycle in vitro fertilization combined with in vitro maturation of immature oocytes. Fertil Steril. 2009;91:1050–1055. doi: 10.1016/j.fertnstert.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 16.Ectors FJ, Vanderzwalmen P, Hoeck JV, Nijs M, Verhaegen G, Delvigne A, et al. Relationship of human follicular diameter with oocyte fertilization and development after in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1997;12:2002–2005. doi: 10.1093/humrep/12.9.2002. [DOI] [PubMed] [Google Scholar]
- 17.Bergh C, Broden H, Lundin K, Hamberger L. Comparison of fertilization, cleavage and pregnancy rates of oocytes from large and small follicles. Hum Reprod. 1998;13:1912–1915. doi: 10.1093/humrep/13.7.1912. [DOI] [PubMed] [Google Scholar]
- 18.Sereepapong W, Ahnonkitpanit V, Chompurat D, Pansatha J, Suwajanakorn S, Pruksananonda K, et al. Correlation between human follicular diameter and oocyte recovery, metaphase II oocytes and fertilization rate in intracytoplasmic sperm injection programs. J Med Assoc Thai. 2001;84(suppl 1):367–370. [PubMed] [Google Scholar]
- 19.Triwitayakorn A, Suwajanakorn S, Pruksananonda K, Sereepapong W, Ahnonkitpanit V. Correlation between human follicular diameter and oocyte outcomes in an ICSI program. J Assist Reprod Genet. 2003;20:143–147. doi: 10.1023/A:1022977002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogueira D, Friedler S, Schachter M, Raziel A, Ron-El R, Smitz J. Oocyte maturity and preimplantation development in relation to follicle diameter in gonadotropin-releasing hormone agonist or antagonist treatments. Fertil Steril. 2006;85:578–583. doi: 10.1016/j.fertnstert.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Pache TD, Wladimiroff JW, Jong FH, Hop WC, Fauser BC. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertil Steril. 1990;54:638–642. doi: 10.1016/s0015-0282(16)53821-7. [DOI] [PubMed] [Google Scholar]
- 22.Fauser BC, Heusden AM. Manipulation of Human Ovarian Function: Physiological Concepts and Clinical Consequences. Endocr Rev. 1997;18:71–106. doi: 10.1210/er.18.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Barnes FL, Sirard MA. Oocyte maturation. Semin Reprod Med. 2000;18:123–131. doi: 10.1055/s-2000-12551. [DOI] [PubMed] [Google Scholar]
- 24.Gulekli B, Buckett W, Chian RC, Child T, Abdul-Jalil AK, Tan SL. Randomized, controlled trial of priming with 10, 000 IU versus 20, 000 IU of human chorionic gonadotropin in women with polycystic ovary syndrome who are undergoing in vitro maturation. Fertil Steril. 2004;82:1458–1459. doi: 10.1016/j.fertnstert.2004.04.043. [DOI] [PubMed] [Google Scholar]