Abstract
Objective
Bariatric surgery is emerging as an effective method to alleviate a multitude of medical conditions associated with morbid obesity and type 2 diabetes. However, little is known about the effects and mechanisms of bariatric surgery on visceral fat inflammation and endothelial dysfunction in type 2 diabetes. We hypothesize that bariatric surgery ameliorates interferon-gamma (IFNγ-mediated adipose tissue inflammation/oxidative stress and improves endothelial function in type 2 diabetic mice.
Methods and Results
Control mice (m Leprdb) and diabetic mice (Leprdb) were treated with either sham surgery or Improved Gastric Bypass Surgery (IGBS) and then evaluated at 5, 10, 20, and 30 days to assess post-surgical effects. Surgery reduced body weight, abdominal adiposity, blood glucose level, and food intake in Leprdb. The surgery-induced decrease in visceral adiposity was accompanied by amelioration of T-lymphocytes and macrophage infiltration, as well as reduction in the expression of IFNγ and other inflammatory cytokines in the mesenteric adipose tissue (MAT) of Leprdb mice. Furthermore, surgery improved endothelium-dependent, but not endothelium-independent vasorelaxation in small mesenteric arteries (SMA) of Leprdb mice. The improvement in endothelial function was largely attenuated by nitric oxide synthase inhibitor (L-NAME) incubation. IFNγ treatment increased the mRNA expression of tumor necrosis factor-alpha (TNFα) in the MAT of control mice, and incubation of SMA of control mice with TNFα caused impairment of endothelial function. Superoxide production in MAT/SMA and nitrotyrosine protein level in SMA were elevated in diabetic mice. Surgery reduced MAT/SMA oxidative stress in Leprdb mice.
Conclusions
The amelioration of adipose tissue inflammation and the improvement of endothelial function may represent important mechanisms that result in cardiovascular benefits following bariatric surgery.
Keywords: Diabetes, Adipose, Inflammation, Endothelial Function, Interferon-gamma
Obesity and diabetes are becoming pandemic and pose a major risk for a number of comorbidities including cardiovascular diseases.1 Morbid obesity remains largely refractory to diet, exercise and medication, but generally responds well to bariatric surgery.2-7 Bariatric surgery demonstrates the most encouraging results in the treatment of patients with morbid obesity and type 2 diabetes by effectively reducing body weight and profoundly improving insulin sensitivity.8-14 Moreover, a substantial majority of obese patients with diabetes, hypertension, and other cardiovascular complications experience complete resolution or improvement.3 Importantly, endothelium-dependent vasodilatory function was enhanced after gastric bypass surgery in morbidly obese patients with type 2 diabetes,11, 15 but the mechanism by which bariatric surgery improves endothelial function in morbidly obese and diabetic patients has yet to be clearly elucidated.
Macrophage infiltration and chemoattractant gene expression were reduced in white adipose tissue of morbidly obese subjects after gastric bypass surgery-induced weight loss.16 Among various cytokines produced by activated macrophages, tumor necrosis factor-alpha (TNFα) is a key proinflammatory cytokine involved in the pathogenesis and progression of cardiovascular dysfunction17 by stimulating vascular oxidative stress,18 enhancing endothelial permeability,19 promoting inflammation,18 and potentiating vasoconstriction.20 As a hallmark cytokine of T-lymphocyte, interferon-gamma (IFNγ) plays a critical role in the regulation of adipose tissue inflammation and enhances the production of various inflammatory cytokines, including TNFα, in cultured adipose tissue.21 Within this context, the purpose of this study was to examine the effects of bariatric surgery on IFNγ-induced visceral adipose tissue inflammation/oxidative stress and endothelial dysfunction in type 2 diabetic mice.
Methods
Animals
The procedures followed were in accordance with approved guidelines set by the Animal Care Committee at the University of Missouri. Heterozygote control mice (m Leprdb) (Background Strain: C57BLKS/J), and homozygote type 2 diabetic mice (Leprdb) (Background Strain: C57BLKS/J) were purchased from Jackson Laboratory and maintained on a normal rodent chow diet. Male, 20-35g m Leprdb, 40-60 g Leprdb mice were used in this study. m Leprdb was treated with murine recombinant IFNγ (R&D, 330 μg/kg/day, i.p. injection, 5 days) at the age of 12 to 16 weeks.22
Improved Gastric Bypass Surgery
Improved gastric bypass surgery (IGBS) was performed using a modified surgical method that mimics the traditional Roux-en-Y gastric bypass surgery23 (Supplemental Figure I). Mice were anesthetized with sodium pentobarbital (50 mg/kg i.p. injection). The stomach and small intestine were exposed from the abdominal cavity, 15 cm away from Treitz ligament, and prepared for anastomosis. The small intestine and large curve of the stomach were anastomosed with 6-0 silk suture side to side. The pylorus was separated and the two parts of the pylorus were dissected and closed. In the sham surgery, the abdominal cavity was opened, but no further surgical procedures were performed. At age 12 weeks, m Leprdb and Leprdb mice were treated with either sham surgery or IGBS. Leprdb mice were assessed 5, 10, 20, and 30 days post IGBS (P5, P10, P20, and P30), or 20 days after sham surgery. m Leprdb mice were assessed 20 days after either sham surgery or IGBS.
Experimental Design
IFNγ, MCP-1 and nitrotyrosine protein expression were determined by western blotting. mRNA expression of CD3, CD68, IFNγ, MCP-1 etc. was examined by quantitative RT-PCR. Immunohistochemistry was used to examine mesenteric adipose tissue (MAT) accumulation of CD3 positive T-lymphocytes, Mac-3 or F4/80 positive macrophages. EPR (Electron Paramagnetic Resonance) spectroscopy was used to determine the superoxide production in both MAT and small mesenteric arteries (SMA). Isolated SMA responses were studied using wire Myograph. The expanded Methods section in the Online Data Supplement can be found at http://atvb.ahajournals.org.
Data Analysis
All data were presented as mean±SEM except as specifically stated. Statistical comparisons were performed with 2-way ANOVA for vasomotor responses under various treatments, and with one-way ANOVA for other data. Intergroup differences were tested with LSD inequality. Significance was accepted at P < 0.05.
Results
Bariatric surgery reduced body weight, adiposity, and improved glycemic control
The effects of bariatric surgery on weight loss and glycemic control were examined. We note that 5, 10, 20, and 30 days post surgery in mice are equivalent to 0.5, 1, 2, and 3 years after surgery in human beings. Our results revealed rapid weight loss and decrease in body fat mass by day 5 after surgery, and the body weight and body fat mass continued to decrease at day 10, 20, and 30 following surgery (Supplemental Table I). Surgery also reduced abdominal adiposity by decreasing abdominal girth, mesenteric bed weight and MAT adipocyte size in diabetic mice (Supplemental Table II). The food intake decreased by 15%-25% in diabetic mice following surgery (Supplemental Table I). Adiponectin level was lower in the serum of Leprdb mice, and surgery increased serum adiponectin levels (Supplemental Figure II).
Bariatric surgery also exerted profound effects on glycemic control and metabolism. IGBS significantly decreased blood glucose level as early as 5 days post surgery, and the blood glucose level continued to decrease at 10 and 20 days post surgery. Within 20 days following surgery, glucose had a parallel evolution to weight, abdominal girth and fat mass although at 30 days after surgery, we noted a slight, but non-significant increase in glucose level (Supplemental Table I).
The Effects of Bariatric Surgery on Adipose Tissue Inflammatory Cell Infiltration and Inflammatory Cytokine Expression
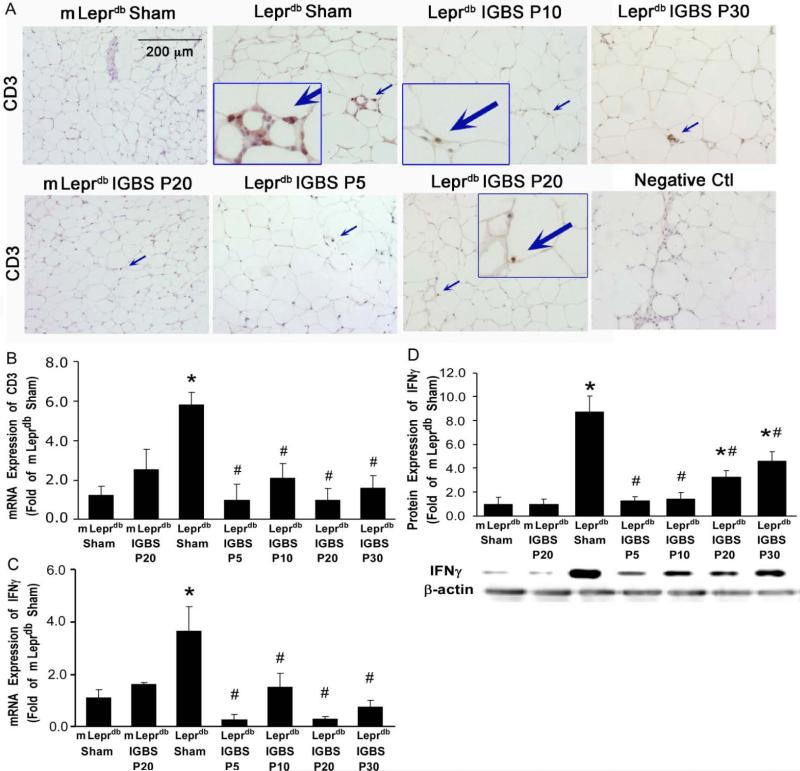
CD3 is the marker of T-lymphocytes. The CD3 positive T-lymphocyte infiltration was increased in the MAT of diabetic mice. The mRNA expression of CD3 was also higher in the MAT of diabetic mice. Bariatric surgery reduced MAT CD3 positive T-lymphocytes infiltration as well as CD3 mRNA expression (Figure 1A and 1B). IFNγ is the hallmark cytokine of T-lympohcytes. The mRNA and protein expression of IFNγ were elevated in the MAT of diabetic mice. Bariatric surgery reduced MAT expression of IFNγ in diabetic mice but not in control mice (Figure 1C and 1D).
Figure 1.
Improved Gastric Bypass Surgery (IGBS) reduced T-lymphocyte infiltration and IFNγ expression in mesenteric adipose tissue (MAT) of diabetic mice. A, Immunohistochemical staining was performed in control (m Leprdb) and diabetic (Leprdb) mice treated with either sham surgery or IGBS. Leprdb mice were assessed 5, 10, 20, and 30 days post IGBS (P5, P10, P20, and P30), or 20 days after sham surgery. m Leprdb mice were assessed 20 days after either sham surgery or IGBS. The results show that CD3 positive T-lymphocyte infiltration in MAT was higher in Leprdb+Sham versus IGBS. Data shown are representative of 4 separate experiments. B, mRNA expression of CD3 increased in MAT of Leprdb+Sham. IGBS significantly reduced CD3 mRNA levels in MAT. The mRNA (C) and protein (D) expression of T-lymphocyte hallmark cytokine, IFNγ, increased in MAT of Leprdb+Sham. IGBS decreased the mRNA and protein expression of IFNγ. Data represent mean±SEM, n=4-12 mice. *P<0.05 compared with m Leprdb+Sham surgery; #P<0.05 compared with Leprdb+Sham surgery.
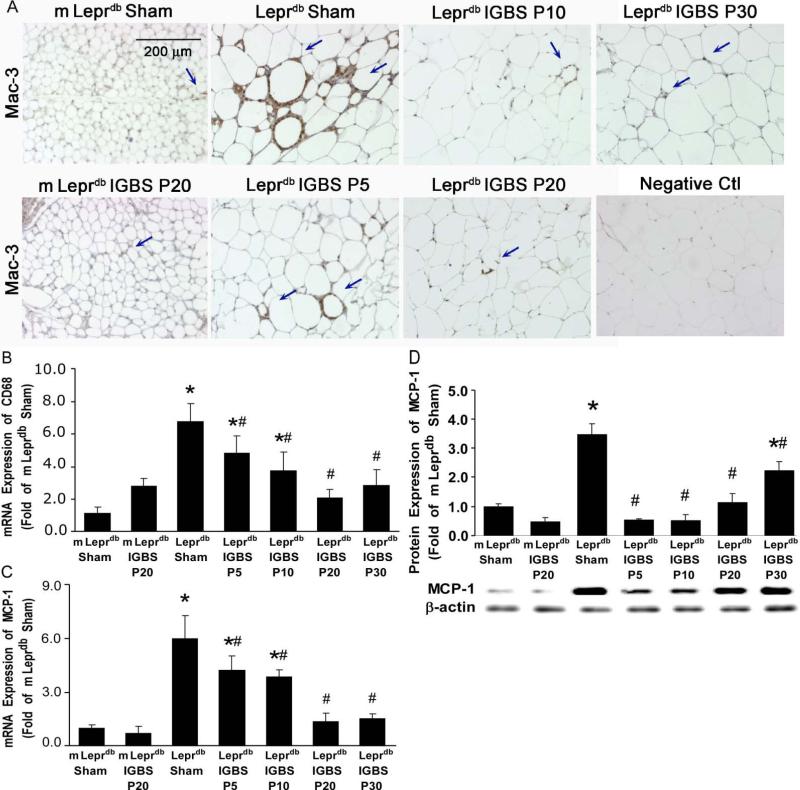
Mac-3, CD68 and F4/80 are the markers of macrophages. The Mac-3 or F4/80 positive macrophage infiltration and CD68 mRNA expression were higher in MAT of diabetic mice vs. control mice. Bariatric surgery reduced macrophage accumulation in MAT of diabetic mice (Figure 2A and 2B, and Supplemental Figure III). MCP-1 is mainly produced by macrophages. The mRNA and protein expression of MCP-1 were higher in diabetic mice vs. control mice. Bariatric surgery ameliorated the mRNA and protein expression of MCP-1 in MAT of diabetic mice (Figure 2C and 2D). Additionally, the mRNA expression of other macrophage-derived inflammatory cytokines, such as TNFα, macrophage inflammatory protein-1-alpha (MIP-1α) and MIP-1β, were also increased in diabetic mice. Bariatric surgery inhibited MAT TNFα, MIP-1α and MIP-1β mRNA expression (Supplemental Figure IV).
Figure 2.
IGBS reduced macrophage infiltration and MCP-1 expression in MAT of diabetic mice. A, Immunohistochemical staining shows that Mac-3 positive macrophage infiltration in MAT was higher in Leprdb+Sham versus IGBS. Data shown are representative of 4 separate experiments. B, mRNA expression of CD68 was increased in MAT of Leprdb+Sham. IGBS significantly reduced CD68 mRNA levels in MAT. The mRNA (C) and protein (D) expression of MCP-1 were increased in MAT of Leprdb+Sham, and were reduced by IGBS. Data represent mean±SEM, n=4-12 mice. *P<0.05 compared with m Leprdb+Sham surgery; # P<0.05 compared with Leprdb+Sham surgery.
The Effects of Bariatric Surgery on SMA Endothelial Function
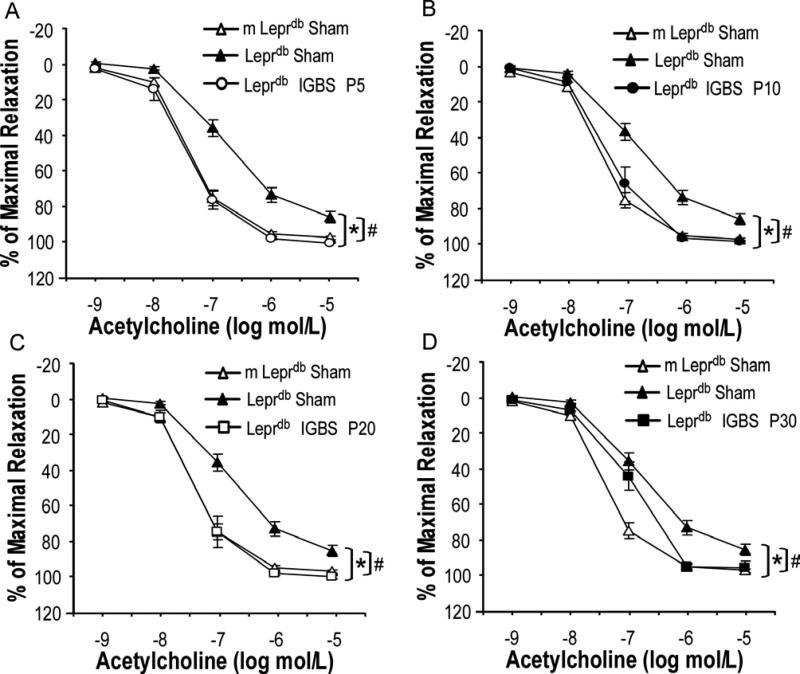
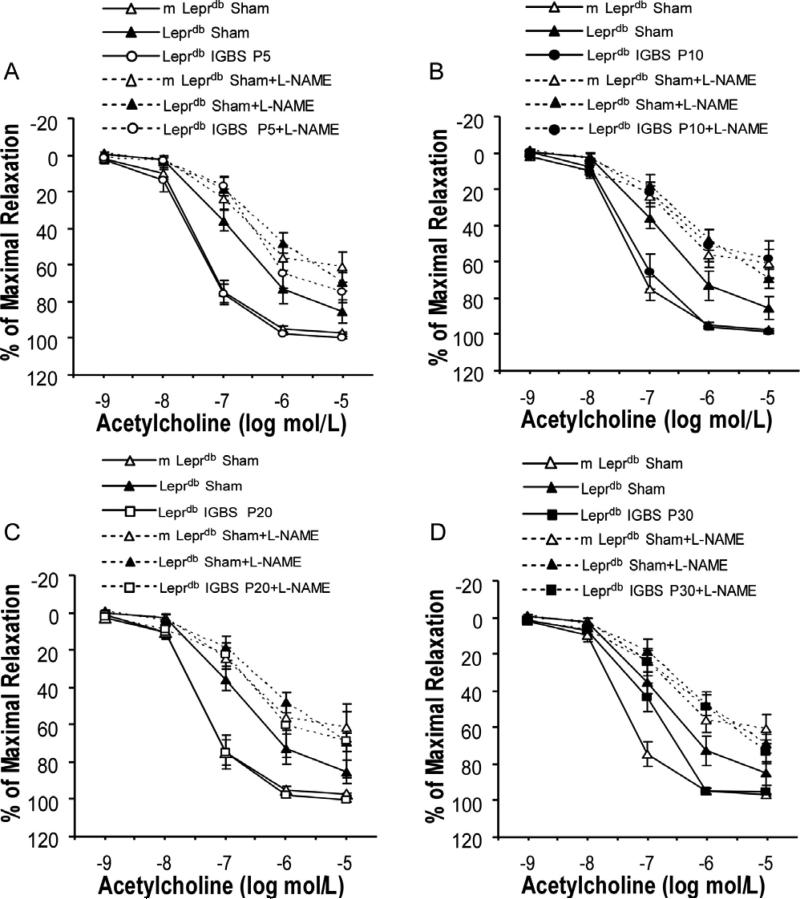
Acetylcholine (ACh)-induced endothelium-dependent vasorelaxation was impaired in SMA of diabetic mice vs. control mice. Bariatric surgery improved endothelial function of diabetic mice (Figure 3). Sodium nitroprusside (SNP)-induced endothelium-independent vasorelaxation and phenylephrine (PE)-induced vasoconstriction were comparable among groups (Supplemental Figure V and Supplemental Figure VI). Nitric oxide synthase inhibitor (L-NAME) incubation largely attenuated the surgery-induced improvement of endothelial function in diabetic mice (Figure 4). Despite the profound effects of bariatric surgery on improving endothelial function of diabetic mice, bariatric surgery affected neither the endothelium-dependent nor the endothelium-independent vasorelaxation in non-diabetic control mice (Supplemental Figure VII).
Figure 3.
IGBS improved endothelium-dependent vasorelaxation to acetylcholine (ACh) in small mesentery arteries (SMA) of Leprdb mice. Data represent mean±SEM. n=6-31 rings from 4-18 mice (1 or 2 rings per mouse). *P<0.05 compared with m Leprdb+Sham surgery; # P<0.05 compared with Leprdb+Sham surgery.
Figure 4.
Incubation with nitric oxide synthase inhibitor, L-NAME, largely attenuated the improvement of SMA endothelial function in surgery-treated diabetic mice. Data represent mean±SEM. n=6-31 rings from 4-18 mice (1 or 2 rings per mouse).
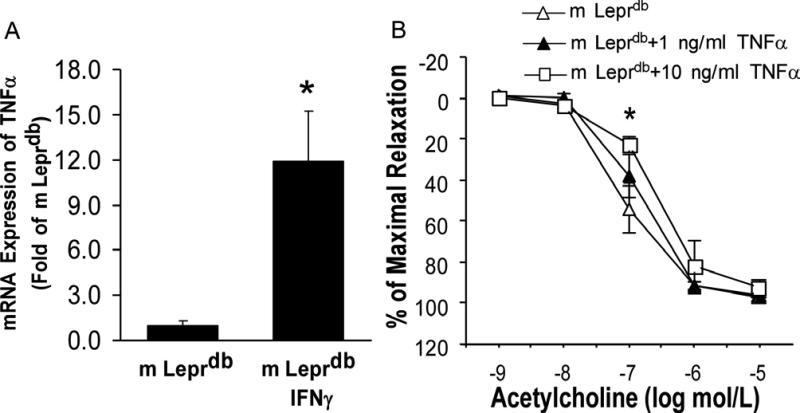
m Leprdb mice treated with recombinant IFNγ showed significantly increased TNFα mRNA expression in MAT (Figure 5A). Incubation of SMA with 10 ng/ml of recombinant TNFα impaired endothelial function of SMA in m Leprdb mice (Figure 5B).
Figure 5.
IFNγ stimulated the expression of proinflammatory cytokine TNFα, which impaired endothelial function of SMA. A. mRNA expression of TNFα increased in the MAT of m Leprdb mice treated with IFNγ. Data represent mean±SEM, n=6-8 mice. *P<0.05 compared with m Leprdb. B, 1 ng/ml recombinant TNFα incubation (90 minutes) only slightly impaired endothelial function of m Leprdb mice. 10 ng/ml TNFα incubation significantly impaired endothelial function. n=4-5 rings from 4-5 mice (1 ring per mouse). *P<0.05 compared with m Leprdb.
The Effects of Bariatric Surgery on MAT/SMA oxidative stress
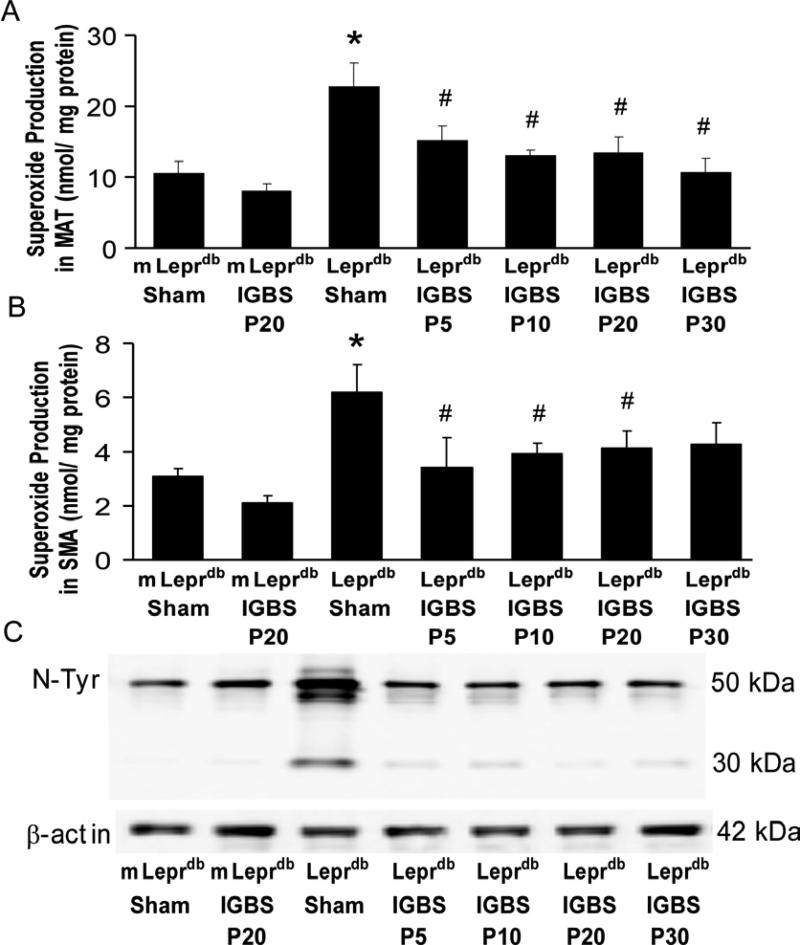
The superoxide level was elevated in both MAT and SMA of diabetic mice. Bariatric surgery reduced superoxide production in diabetic mice without affecting that in control mice (Figure 6A and 6B). Furthermore, the nitrotyrosine protein expression in SMA was higher in diabetic mice vs. control mice. Bariatric surgery decreased SMA nitrotyrosine protein expression (Figure 6C).
Figure 6.
IGBS ameliorated MAT/SMA oxidative stress. A and B, IGBS reduced superoxide level in MAT and SMA of Leprdb mice. Data represent mean±SEM. n=6-8 mice. *P<0.05 compared with m Leprdb+Sham surgery; # P<0.05 compared with Leprdb+Sham surgery. C, IGBS decreased protein expression of nitrotyrosine in SMA of diabetic mice. Data shown are representative of 3 separate experiments.
Discussion
Bariatric surgical procedures have increased exponentially in the United States24 and animal models are increasingly being used in the study of bariatric surgery in order to examine the underlying mechanisms of the therapeutic effects.25 However, no studies to date have examined the effects of bariatric surgery in the type 2 diabetic murine model. We modified the work of Troy et al.23 to establish the Improved Gastric Bypass Surgery (IGBS) method in murine model of type 2 diabetes; this allows the study of mechanisms responsible for the therapeutic effects of bariatric surgery in morbid obesity and type 2 diabetes. The major findings in this study are: 1) Bariatric surgery leads to rapid weight loss, reduces whole body and abdominal adiposity, and improves glycemic control; 2) Bariatric surgery serves as an effective anti-inflammatory strategy by ameliorating IFNγ-mediated adipose tissue inflammation; and 3) Bariatric surgery reverses endothelial dysfunction by improving NO availability and inhibiting vascular oxidative stress.
Bariatric surgery serves as a successful anti-inflammatory strategy
Obesity-related chronic inflammation is implicated in the pathogenesis of type 2 diabetes.26 Previous studies demonstrated that long-term weight loss after bariatric surgery is accompanied by a decreased proinflammatory state. Bariatric surgery decreased circulating levels of c-reactive protein (CRP),27-30 IL-6,29 serum amyloid A (SAA),31-32 and leptin,8 but increased the circulating level of adiponectin.8 Bariatric surgery also reduced subcutaneous adipose tissue macrophage attraction, and gene expression of inflammatory cytokines, such as TNFα and IL-6.16, 29 Compared with subcutaneous fat, visceral fat showed a higher transcript level of IFNγ and a broader leukocytosis that included macrophages, T cells and natural killer (NK) cells.33 Our murine model of IGBS allowed us to examine the effects of bariatric surgery on the inflammatory status of MAT. Our results showed that bariatric surgery reduced T-lymphocyte and macrophage infiltration, as well as the expression of IFNγ, MCP-1, TNFα, MIP-1α and MIP-1β in MAT of diabetic mice. Thus, surgery-induced weight loss is accompanied by reduced adipose tissue inflammation, and bariatric surgery serves as a successful anti-inflammatory strategy in type 2 diabetes.
The association between adipose tissue inflammation and endothelial dysfunction
Increased adipose tissue inflammation in type 2 diabetes reflects the positive association between cardiovascular diseases and diabetes.34 An abdominal fat pattern, as determined by an increased waist-to-hip ratio and visceral fat diameter, was the sole significant predictor of flow-mediated vasodilation (FMD) in overweight adults,10, 35 suggesting the link between visceral adiposity and vascular dysfunction.36-37 The mechanisms whereby excessive visceral fat depot leads to deterioration of vascular health are complex. Adipose tissue-derived inflammatory cytokines may serve as mechanisms linking adipose tissue inflammation and endothelial dysfunction.34 As an important adipose-derived proinflammatory mediator, TNFα plays a key role in endothelial dysfunction associated with ischemia reperfusion injury,38-39 obesity40 and diabetes.41 In type 2 diabetic mice, increase in TNFα and TNFα receptor 1 (TNFR1) expression induced activation and production of superoxide via NAD(P)H oxidase and/or the mitochondria respiratory chain, leading to endothelial dysfunction in coronary microcirculation and aortas.42-44 Our results suggest that IFNγ treatment significantly increased the mRNA expression of TNFα in the MAT of non-diabetic control mice. Recombinant TNFα incubation impaired the endothelial function of SMA in control mice, suggesting the potential role of the IFNγ-induced MAT pro-inflammatory status in the regulation of SMA endothelial function. Moreover, the superoxide level in the MAT of diabetic mice was significantly higher, but bariatric surgery reduced MAT superoxide production. Thus, visceral obesity-associated alterations of the vasculature are likely a consequence of perturbation of the normal physiological balance of adipose-derived inflammatory cytokines and oxidative stress, and bariatric surgery can reverse the alteration.
Bariatric Surgery Improves Endothelial Function by Inhibiting Oxidative Stress and Increasing NO Availability
In morbidly obese patients, bariatric surgery rapidly improved endothelial function.45-46 The mechanisms of bariatric surgery-induced amelioration of endothelial dysfunction are not clearly elucidated, but some studies suggest that reduction in circulating level of markers of endothelial activation and oxidative stress may serve as mechanisms.47-48 Our study shows that bariatric surgery remarkably improved the endothelium-dependent vasorelaxation of SMA without affecting endothelium-independent vasorelaxation and PE-induced vasoconstriction (Figure 3, Supplemental Figure V and Figure VI). The superoxide level and nitrotyrosine protein expression in the SMA were elevated in diabetic mice, but reversed by bariatric surgery (Figure 6). Although bariatric surgery improved endothelium-dependent vasorelaxation of SMA in diabetic mice, the improvement was largely attenuated by incubating the vessels with nitric oxide synthase inhibitor, L-NAME, suggesting that bariatric surgery improves endothelial function by improving NO availability (Figure 4).
Although we observed that the SMA endothelial function of Leprdb at 5, 10, and 20 days after surgery was completely restored to the level of non-diabetic control mice, In Leprdb at 30 days, this procedure only partially improved endothelial function (Figure 3). Moreover, the protein expression of IFNγ and MCP-1 in diabetic mice at 30 days post surgery slightly returned towards the level observed in the Leprdb+Sham surgery group even though there was no significant body weight regain or hyperglycemia. We postulate that an early indicator of post-surgery relapse may be characterized by the partial restoration of adipose tissue inflammation and endothelial dysfunction that precedes a regain of body weight and increased incidence of hyperglycemia over the long term after surgery in type 2 diabetic mice. Thus, weight is likely not the determinant of endothelial dysfunction.49-50 The inflammatory milieu that was rapidly corrected by surgery is linked to endothelial dysfunction in diabetes.
One caveat to this study is that the mice were fairly young (3 month old) when subjected to the surgery procedure. However, since the lifespan of Leprdb mice is up to 10 month, our protocol will potentially allow the observation of long-term effects by bariatric surgical procedures. We found that the endothelial function of Leprdb at 90 days after surgery was slightly impaired compared with Leprdb at 30 days after surgery (although still better than Leprdb+Sham surgery), with a slight increase in body weight and blood glucose level (unpublished data), which highlights the need to examine the long-term effects of bariatric surgery. Indeed, the long-term follow-up study of patients undergoing bariatric surgery showed that body weight reached the lowest point at approximately 2 years and there was a significant increase in BMI from the nadir to 5 years and from 5 years to 10 years post-surgery.51 Thus, although bariatric surgery is a favorable option in the treatment of diabetic patients with severe obesity, discerning the benefits over time requires further evaluation. Due to the difficulties in conducting long-term follow-up studies in human subjects treated with bariatric surgery over time, our study using type 2 diabetic mice can explore a wider spectrum of interest more quickly and definitely to evaluate and refine the most relevant protocols that may be translatable to clinical studies.
Conclusion
In summary, bariatric surgery reduces body weight, whole body and abdominal adiposity, and improves glycemic control in type 2 diabetic mice. Bariatric surgery ameliorates IFNγ-mediated MAT inflammation/oxidative stress and improves SMA endothelial function in type 2 diabetes. We posit that the vascular benefits of bariatric surgery are chiefly derived from a surgery-induced reduction in adipose tissue inflammation. These data demonstrate that the amelioration of adipose tissue inflammation and the improvement of endothelial function may represent important mechanisms that result in cardiovascular benefits following bariatric surgery.
Supplementary Material
Acknowledgments
N/A
Sources of Funding
This study was supported by grants from NIH grants (RO1-HL077566 and RO1-HL085119, to C.Z., R01-DK085495 to J Ye and C.Z. and RO1-HL073101 to J Sowers and C.Z.) and American Heart Association Predoctoral Fellowship (10PRE4300043 to H.Z.).
Footnotes
Subject Codes:
(95) Endothelium/vascular type/nitric oxide
(130) Animal models of human disease
(190) Type 2 diabetes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: An american heart association scientific statement from the atherosclerosis, hypertension, and obesity in the young committee (council on cardiovascular disease in the young) and the diabetes committee (council on nutrition, physical activity, and metabolism). Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 2.Park Y, Wu J, Zhang H, Wang Y, Zhang C. Vascular dysfunction in type 2 diabetes: Emerging targets for therapy. Expert Rev Cardiovasc Ther. 2009;7:209–213. doi: 10.1586/14779072.7.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: Effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 5.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 6.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 7.Ashrafian H, le Roux CW, Darzi A, Athanasiou T. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–2102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- 8.Garcia de la Torre N, Rubio MA, Bordiu E, Cabrerizo L, Aparicio E, Hernandez C, Sanchez-Pernaute A, Diez-Valladares L, Torres AJ, Puente M, Charro AL. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-a, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–4281. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 9.Konukoglu D, Uzun H, Firtina S, Cigdem Arica P, Kocael A, Taskin M. Plasma adhesion and inflammation markers: Asymmetrical dimethyl-l-arginine and secretory phospholipase a2 concentrations before and after laparoscopic gastric banding in morbidly obese patients. Obes Surg. 2007;17:672–678. doi: 10.1007/s11695-007-9113-3. [DOI] [PubMed] [Google Scholar]
- 10.Sturm W, Tschoner A, Engl J, Kaser S, Laimer M, Ciardi C, Klaus A, Weiss H, Sandhofer A, Patsch JR, Ebenbichler CF. Effect of bariatric surgery on both functional and structural measures of premature atherosclerosis. Eur Heart J. 2009;30:2038–2043. doi: 10.1093/eurheartj/ehp211. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez LA, Pazos F, Berrazueta JR, Fernandez-Escalante C, Garcia-Unzueta MT, Freijanes J, Amado JA. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005;90:316–322. doi: 10.1210/jc.2003-032059. [DOI] [PubMed] [Google Scholar]
- 12.Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31(Suppl 2):S290–296. doi: 10.2337/dc08-s271. [DOI] [PubMed] [Google Scholar]
- 13.Couzin J. Medicine. Bypassing medicine to treat diabetes. Science. 2008;320:438–440. doi: 10.1126/science.320.5875.438. [DOI] [PubMed] [Google Scholar]
- 14.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: A randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 15.Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M, Lipinska I, Keaney JF, Jr., Apovian CM. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 17.Kleinbongard P, Heusch G, Schulz R. Tnfalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: The fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 20.Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, Elter-Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108:344–352. doi: 10.1161/CIRCRESAHA.110.235713. [DOI] [PubMed] [Google Scholar]
- 21.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson SH, Miller GF, Segal BH, Mardiney M, 3rd, Domachowske JB, Gallin JI, Holland SM. Ifn-gamma is effective in reducing infections in the mouse model of chronic granulomatous disease (cgd). J Interferon Cytokine Res. 2001;21:567–573. doi: 10.1089/10799900152547821. [DOI] [PubMed] [Google Scholar]
- 23.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Rao RS, Rao V, Kini S. Animal models in bariatric surgery--a review of the surgical techniques and postsurgical physiology. Obes Surg. 2010;20:1293–1305. doi: 10.1007/s11695-010-0135-x. [DOI] [PubMed] [Google Scholar]
- 26.Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab. 2010;95:2845–2850. doi: 10.1210/jc.2009-2371. [DOI] [PubMed] [Google Scholar]
- 27.Habib P, Scrocco JD, Terek M, Vanek V, Mikolich JR. Effects of bariatric surgery on inflammatory, functional and structural markers of coronary atherosclerosis. Am J Cardiol. 2009;104:1251–1255. doi: 10.1016/j.amjcard.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Sledzinski T, Sledzinski M, Smolenski RT, Swierczynski J. Increased serum nitric oxide concentration after bariatric surgery--a potential mechanism for cardiovascular benefit. Obes Surg. 2010;20:204–210. doi: 10.1007/s11695-009-0041-2. [DOI] [PubMed] [Google Scholar]
- 29.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 30.Laimer M, Ebenbichler CF, Kaser S, Sandhofer A, Weiss H, Nehoda H, Aigner F, Patsch JR. Markers of chronic inflammation and obesity: A prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes Relat Metab Disord. 2002;26:659–662. doi: 10.1038/sj.ijo.0801970. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Ambrosi J, Salvador J, Rotellar F, Silva C, Catalan V, Rodriguez A, Jesus Gil M, Fruhbeck G. Increased serum amyloid a concentrations in morbid obesity decrease after gastric bypass. Obes Surg. 2006;16:262–269. doi: 10.1381/096089206776116525. [DOI] [PubMed] [Google Scholar]
- 32.Catalan V, Gomez-Ambrosi J, Ramirez B, Rotellar F, Pastor C, Silva C, Rodriguez A, Gil MJ, Cienfuegos JA, Fruhbeck G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–1474. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- 33.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, Jobe BA, Roberts CT, Jr., Slifka MK, Marks DL. Depot-specific differences in inflammatory mediators and a role for nk cells and ifn-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Zhang C. Adipose “Talks” To distant organs to regulate insulin sensitivity and vascular function. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 36.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: The paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 37.Perrini S, Leonardini A, Laviola L, Giorgino F. Biological specificity of visceral adipose tissue and therapeutic intervention. Arch Physiol Biochem. 2008;114:277–286. doi: 10.1080/13813450802334752. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Wu J, Xu X, Potter BJ, Gao X. Direct relationship between levels of tnf-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol. 2010;105:453–464. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bramos D, Ikonomidis I, Tsirikos N, Kottis G, Kostopoulou V, Pamboucas C, Papadopoulou E, Venetsanou K, Giatrakos N, Yang GZ, Nihoyannopoulos P, Toumanidis S. The association of coronary flow changes and inflammatory indices to ischaemia-reperfusion microvascular damage and left ventricular remodelling. Basic Res Cardiol. 2008;103:345–355. doi: 10.1007/s00395-008-0720-5. [DOI] [PubMed] [Google Scholar]
- 40.Belin de Chantemele EJ, Ali MI, Mintz J, Stepp DW. Obesity induced-insulin resistance causes endothelial dysfunction without reducing the vascular response to hindlimb ischemia. Basic Res Cardiol. 2009;104:707–717. doi: 10.1007/s00395-009-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ihm SH, Chang K, Kim HY, Baek SH, Youn HJ, Seung KB, Kim JH. Peroxisome proliferator-activated receptor-gamma activation attenuates cardiac fibrosis in type 2 diabetic rats: The effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res Cardiol. 2010;105:399–407. doi: 10.1007/s00395-009-0071-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Park Y, Picchi A, Potter BJ. Maturation-induces endothelial dysfunction via vascular inflammation in diabetic mice. Basic Res Cardiol. 2008;103:407–416. doi: 10.1007/s00395-008-0725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of par2 in regulating tnf-alpha and nad(p)h oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–123. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: Role of tnf{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nerla R, Tarzia P, Sestito A, Di Monaco A, Infusino F, Matera D, Greco F, Tacchino RM, Lanza GA, Crea F. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Lind L, Zethelius B, Sundbom M, Eden Engstrom B, Karlsson FA. Vasoreactivity is rapidly improved in obese subjects after gastric bypass surgery. Int J Obes (Lond) 2009;33:1390–1395. doi: 10.1038/ijo.2009.188. [DOI] [PubMed] [Google Scholar]
- 47.Nijhuis J, van Dielen FM, Fouraschen SM, van den Broek MA, Rensen SS, Buurman WA, Greve JW. Endothelial activation markers and their key regulators after restrictive bariatric surgery. Obesity (Silver Spring) 2007;15:1395–1399. doi: 10.1038/oby.2007.167. [DOI] [PubMed] [Google Scholar]
- 48.Uzun H, Zengin K, Taskin M, Aydin S, Simsek G, Dariyerli N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic swedish adjustable gastric banding. Obes Surg. 2004;14:659–665. doi: 10.1381/096089204323093453. [DOI] [PubMed] [Google Scholar]
- 49.Focardi M, Dick GM, Picchi A, Zhang C, Chilian WM. Restoration of coronary endothelial function in obese zucker rats by a low-carbohydrate diet. Am J Physiol Heart Circ Physiol. 2007;292:H2093–2099. doi: 10.1152/ajpheart.01202.2006. [DOI] [PubMed] [Google Scholar]
- 50.Phillips SA. Effects of low-carbohydrate diet on vascular health: More than just weight loss. Am J Physiol Heart Circ Physiol. 2007;292:H2037–2039. doi: 10.1152/ajpheart.00188.2007. [DOI] [PubMed] [Google Scholar]
- 51.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.