Abstract
Proteomics allows characterization of protein structure and function, protein-protein interactions, and peptide modifications. It has given us insight into the perturbations of signaling pathways within tumor cells and has improved the discovery of new therapeutic targets and possible indicators of response to and duration of therapy. The discovery, verification, and validation of novel biomarkers are critical in streamlining clinical development of targeted compounds, and directing rational treatments for patients whose tumors are dependent upon select signaling pathways. Studies are now underway in many diseases to examine the immune or inflammatory proteome, vascular proteome, cancer or disease proteome, and other subsets of the specific pathology microenvironment. Successful assay verification and biological validation of such biomarkers will speed development of potential agents to targetable dominant pathways and lead to selection of individuals most likely to benefit. Reconsideration of analytical and clinical trials methods for acquisition, examination, and translation of proteomics data must occur before we march further into future of drug development.
Keywords: proteomics, biomarkers, clinical trial, drug development, cancer, targeted therapy
Introduction
Advances in biotechnology and improved understanding of cancer and disease biology have shifted the treatment paradigm to targeted therapy. We have enhanced our ability to guide application of new and existing treatments with development, assay verification, biological validation and application of biomarkers. However, to be successful, we need a thorough understanding of the relationship between putative biomarkers and treatment effects. We must consider new clinical trial designs that may consist of randomized cohorts, prospectively planned endpoints, and/or post-hoc analyses. These strategies will succeed if reliable, adequately powered, biologically validated biomarkers are identified and appropriately applied for prospective patient selection via clinical trials. Continued inclusion of preplanned biological correlates will allow ongoing optimization of targeted therapy. These events will guide future directions of proteomics, affecting how we integrate proteomic information into the selection of therapy for advanced and recurrent cancers, and other diseases. For the purposes of this discussion, most examples will emanate from the oncology literature, where these issues and advances are at the forefront of current controversy.
Definitions of a biomarker and proteomics
A biomarker is defined by Atkinson et al in the US Food and Drug Administration (FDA) Biomarker Definitions Working Group as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”1. Those characteristics that are informative for clinical outcome can be categorized broadly as prognostic or predictive biomarkers. Prognostic biomarkers classify patients into subgroups with distinct expected clinical outcomes, such as progression or death, but they do not inform the choice of therapy. Conversely, predictive biomarkers should identify subgroups of patients whose tumors are likely to have therapeutic sensitivity or resistance based upon marker status 2, 3.
For example, breast cancers with either HER2 amplification, or triple negative status (negative for estrogen receptor, progesterone receptor, and HER2 amplification), are recognized as poor-risk subgroups and those designations are thereby negative prognostic biomarkers 4, 5. HER2 amplification also functions as a positive predictive biomarker. HER2 amplification defines a subgroup of breast cancer patients for whom trastuzumab and other anti-HER2 interventions have high likelihood of providing benefit, positively predicting outcome to agent(s) 6. Conversely, excision repair cross-complementation group 1 (ERCC1) is both a positive prognostic and a negative predictive marker in non-small cell lung cancer (NSCLC)7. The International Adjuvant Lung Cancer Trial showed that high ERCC1 protein expression was associated with improved survival in patients who did not receive chemotherapy. But, the benefit of adjuvant cisplatin-based chemotherapy was more profound in patients with low ERCC1 expression due to reduced platinum-DNA adduct repair8,9,10. Lastly, in advanced colorectal cancer, the benefit of the anti-EGFR monoclonal antibody, cetuximab, appears limited to patients with tumors with wild-type KRAS genotype 11. This indicates that KRAS wild type status could and should be used to select patients for cetuximab therapy. Thus, knowledge of molecular and protein events will enlighten clinical decision making from different points of view.
Proteomics is a tool with which to characterize protein structure, function, protein-protein interactions, and associated protein modifications. These protein characteristics collaborate to form complex signaling networks mediating the active cellular proteome. Proteomics output, patterns or individual endpoints, also can be evaluated as biomarkers. Understanding the active proteome is critical for development of effective predictive and prognostic biomarkers. Once identified, key potential events in the proteome can be exploited for the development of targeted cancer treatments. This reinforces the need for application of high throughput, accurate, precise, sensitive, and specific tests for discovery and endpoints validation, followed by rapid translation for patient stratification.
Proteomics technologies
Technologies exploring the proteome for biomarker discovery range from classical immunohistochemistry and immunoassays for single or small sets of proteins, to mass spectrometry (MS) and other high throughput approaches to examine millions of peptides. Early MS use evaluated differential patterns of protein and peptide expression in patient serum and other biospecimens12, 13. While preliminary data using the pattern approach was initially promising, the field has moved forward to sequence peptides and proteins, with secondary individual entity validation as the diagnostic discriminant. Sequence information permits both development of biomarkers and/or therapeutic targets, and application of proteomic knowledge to advance understanding of underlying pathophysiology. Studies are now underway in many diseases to examine selectively the immune or inflammatory proteome, vascular proteome, cancer proteome, and other subsets of the disease-specific microenvironment.
Mass spectrometry techniques such as nanoflow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) along with other techniques such as immunocapture platforms have enabled high-throughput analysis of a proteome or functional subsets of the proteome. Reverse phase protein assays (RPPA) have been applied more recently as biased examinations of tissue proteins in formats wherein protein lysates are arrayed on a solid surface. Arrayed proteins are interrogated with standardized antibodies specific for total and activated protein targets allowing for investigation of pre-selected functional signaling outputs14, 15.
These techniques have been applied to oncology tissue samples, leading to the discovery of potential pathway biomarkers to detect disease and monitor established cancer through the course of treatment16. Carey et al examined 80 validated proteins from signaling pathways in advanced-stage, high-grade serous ovarian carcinoma cases via RPPA to identify expressed proteins associated with response to primary chemotherapy17. Normalization of CA125, an established biomarker, by the 3rd cycle of platinum-based chemotherapy was chosen as the primary outcome measure of response; TGF-β pathway signaling correlated strongly with chemoresistance in this study. RPPA were also used in head and neck squamous cell carcinoma (HNSCC) to examine 60 protein endpoints in matched tumor and nonmalignant biopsy specimens from 23 patients. This paired analysis approach identified 18 differentially elevated proteins including PKCι 18. PKCι is overexpressed in 70% of squamous cell NSCLC with amplification of 3q26, which harbors PKCι and PIK3CA, the p110 α catalytic phosphoinositide-3-kinase (PI3K) subunit gene19. This is an example of a discovery-based RPPA application that also has yielded information that can be considered for future clinical benefit.
Components of validation for biomarkers in drug development
The FDA began the Critical Path Initiative in 2004, aiming to improve discovery, validation, and production of current therapeutics by focusing on selected areas. It defined 6 focused topics in 2006, identifying biomarker development as one of the two most important necessary advances. This key area addresses creation of improved tools, such as biomarkers, for evaluation of clinical therapies. The recommendation for co-development of a drug and a selective biomarker was first described in a draft FDA guidance in 2005. Co-development implies generation of processes and guidelines describing analytical (technical) test validation (also known as verification), clinical test validation (demonstration of biological validation), and clinical utility (Table 1)20. This marked the FDA’s first step toward integrating rapidly evolving biology of cancer and other diseases into existing regulatory processes; it is anticipated that this mandate will be applied to targeted drug development for many medical needs. Currently, the FDA recommends application of a verified and validated biomarker for the identification of the target clinical subpopulation when employing the use of a drug for which subpopulation targeting is identified as part of drug registration. For example, the Table of Valid Genomic Biomarkers in the Context of Approved Drug Labels, available on the FDA website (http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm) details trastuzumab and the required indications for treating patients with HER-2 amplified tumor. The initial test identifying the HER-2 amplified subpopulation was required for FDA approval before trastuzumab received approval for adjuvant treatment of women with lymph node-positive, HER-2 over-expressing breast cancer21. Incorporation of predictive biomarkers into clinical trials during the drug development process will translate into greater accuracy in selecting target patient subgroups.
Table 1.
Components of validation for biomarkers in clinical trials for drug development.
| Analytical validation (also known as verification) |
Clinical validation | Clinical utility |
|---|---|---|
| Accuracy | Sensitivity: true positive designation | Evaluation of risk and benefit of diagnosis and consequent treatment resulting from the test |
| Precision | ||
| Reproducibility | Specificity: true negative designation | |
| Intrinsic measurements of error | Behavior of biomarker in a population as a function of biological variability | |
| Positive and negative predictive value | ||
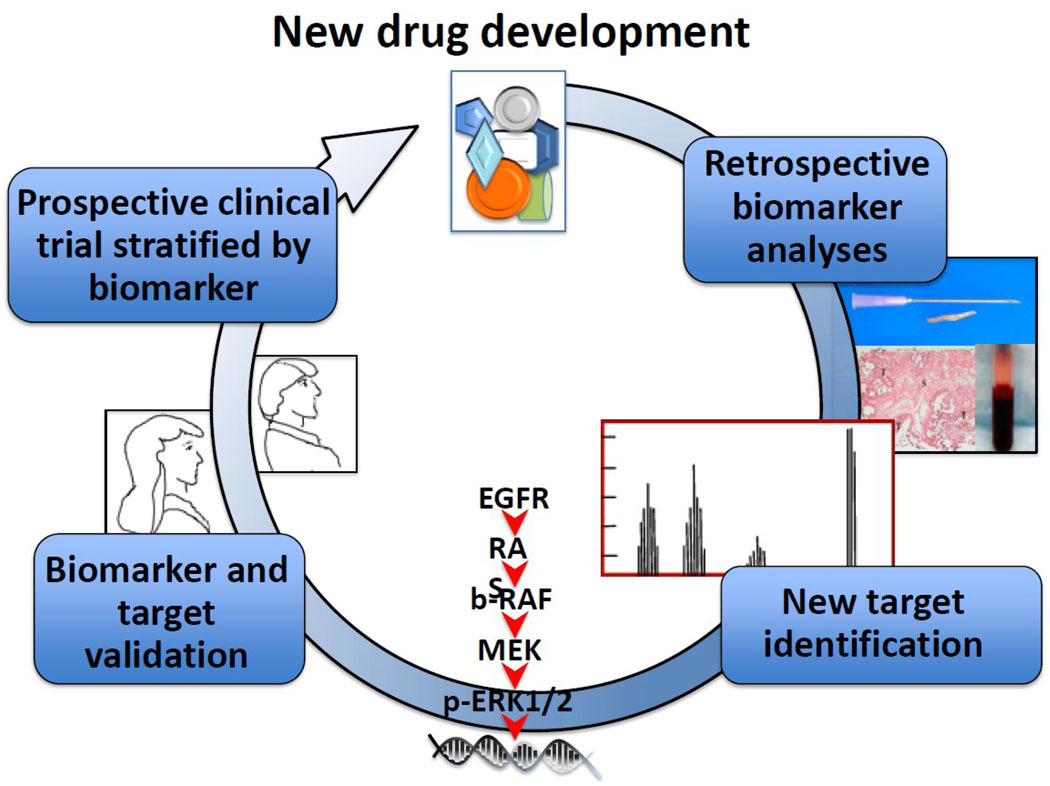
Both verification and validation of biomarkers is necessary for their appropriate application for targeted therapeutics selection and their use as drug response marker(s) in clinical practice. The interpretation of validation is broad and most often has been described as the process of linking a biomarker to clinical or behavioral endpoints. The broad concept of validation includes “efforts to confirm the accuracy, precision, and effectiveness of results and can be defined as analytical and clinical validation”22. Analytical (technical) validation is now called verification. Analytic method verification is the process of confirming the assay, its performance characteristics, and the required optimal conditions to generate reproducibility and accuracy of the assay22–24. Clinical or biological validation is related to how a certain marker behaves in a population and between populations, depending on biological variability within the population22. Both clinical and analytical validation need to be incorporated into clinical correlative studies for biomarker and drug development25 although ultimately they may fall to different review and registration paradigms. To date, most putative biomarkers have fallen short of adequate biological validation even where verification has been confirmed through FDA review. The integration of verified and validated proteomic biomarkers into clinical drug development programs will expedite pipeline decision-making process by adding critical information about the pharmacologic and/or pharmacodynamic mechanism(s) and efficacy of a potential therapeutic agent as shown in the cartoon in (Figure 1).
Figure 1. The decision-making process of drug development incorporating proteomics.
Proteomic profiling has been applied for identification of predictive markers and therapeutic targets through retrospective analyses. The discovery and validation of biomarkers would lead to development of new potential drugs via prospective clinical trials stratified by biomarker for more effective targeted therapy in recurrent cancers.
Approaches for the identification of potential therapeutic targets and biomarkers
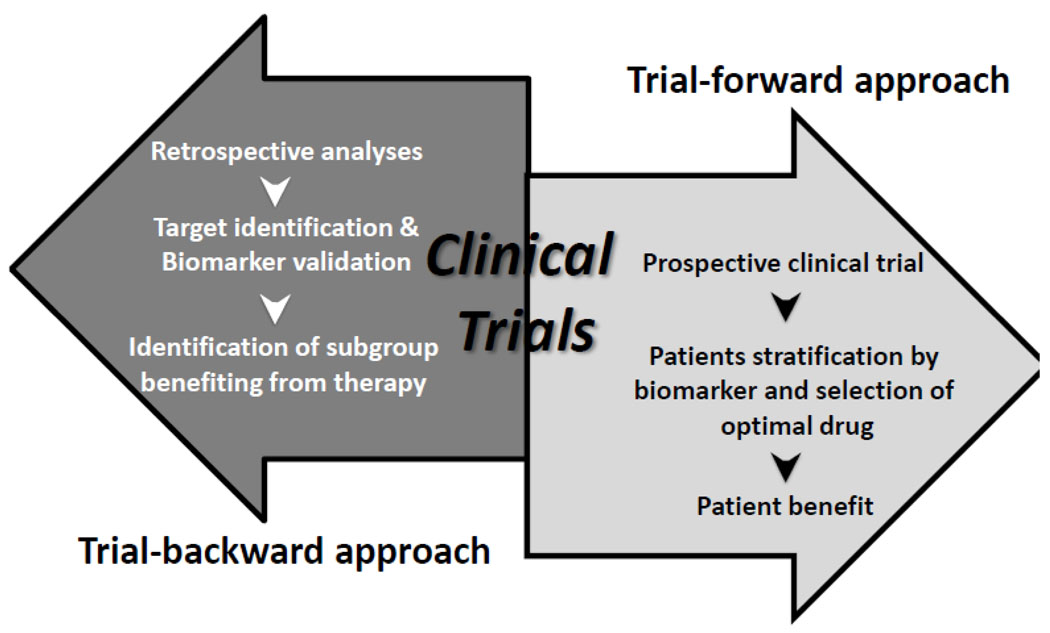
What is the best approach with which to identify and characterize a biomarker for drug selection? One is a biomarker-forward, focused, target-associated view specifically addressing biomarkers emanating from the drug development hypothesis to select patients for therapy prospectively. Alternatively, an agnostic, trial-backwards, view would apply biased selection retrospectively with biological validation of translational endpoints against patient outcome in targeted agents’ trials (Figure 2).
Figure 2. Prospective and retrospective approaches for identification of potential biomarkers.
A biomarker-forward, focused, target-associated prospective approach specifically address biomarkers to select patients for therapy. Alternatively, an agnostic, trial-backwards, retrospective approach would apply biased selection with biological validation of translational endpoints against patient outcome in targeted agents’ trials.
The first approach requires definition, verification, and validation of biomarkers selected for their defined target modulation, and is an immediate and logical extension of the drug development process. This approach would lead rapidly to patient stratification prior to therapy if clinically successful; this requires the discriminant to be “spot-on”. The recent success of selective b-Raf inhibitors for V600E BRAF mutant melanoma is an example of a biomarker-forward selection where prior knowledge of BRAF mutations was required 26. This is illustrated also in treatment of HER-2 overexpressing breast, gastric, and other cancers, where the requirement for >2 copies of HER2 by FISH or a 3+ IHC stain are required and have been shown to predict clinical potential for trastuzumab and other HER2-directed treatments27. The biomarker-forward approach is limited in that it leaves little room for unanticipated on-target effects and may miss important off-target outcomes.
The trial-backwards approach allows investigation, identification, and examination of potential biomarkers in the context of molecular therapeutic clinical investigation. This identification approach includes evaluation of initially unanticipated events and has the capacity to uncover important off-target events. Examination of prospectively collected biospecimens either within the trial design or post-hoc analysis may yield broader results than the narrow trial-forward approach. The stratification of benefit to cetuximab as a function of EGFR mutation, overexpression, and downstream pathway activation, was studied retrospectively in advanced colorectal cancer. Here, mutational activation of KRAS was shown to be a negative predictive biomarker for outcome11. This was unexpected, but scientifically logical when considered in the context of the biochemical pathways involved.
Retrospective analysis of candidate biomarkers in cancer clinical trials has been the most common early biomarker approach used to date. Unfortunately, many reports are exploratory and correlative and only a very small number of putative biomarkers move forward to full verification and validation. This is the basis for arguments for prospectively planned proteomic and genetic biomarker profiling pre- and post/during treatment, with tissue acquisition following clear standard operating procedures, and powered for definitive analysis. Applying biomarker-backwards approaches for discovery followed by powered prospective biomarker-forward studies for biological validation and confirmation may reduce the primary problem of indiscriminate application of targeted agents in many diseases expediting an expensive and time consuming drug development process.
Challenges and opportunities for biomarkers for targeted therapies
Recent high-throughput molecular proteomic technologies have yielded potential in both identification and development of biomarkers and therapeutic targets. Treatment of patients with advanced NSCLC with EGFR tyrosine kinase inhibitors (TKIs) is established for first-line, maintenance, and subsequent treatment in patients with EGFR mutations28,29–31. Although EGFR mutation is a validated predictive marker for first-line therapy in patients with advanced NSCLC, only 70% to 75% of patients will respond and all the patients will eventually develop resistance to the therapy. Thus, it is a continuing challenge to optimize predictive biomarkers with which to pre-select patients with EGFR mutations who will not benefit from EGFR TKI treatment. These biomarkers are likely also to provide insight into the biology of the treatment resistance.
The insulin like growth factor receptor (IGFR) pathway interacts with the EGFR pathway, and plays an important role as a resistant mechanism to EGFR TKI treatment. Signaling through the IGF-1R is required for neoplastic transformation by a number of oncogenes, and therefore makes IGF-1R an attractive target for anticancer treatment 32. Figitumumab, a neutralizing IGF-1R antibody, has been examined in a NSCLC phase III study 33. Both tissue- and serum-based IGF-1 pathway-related proteins, including IGF-I and IGF-I binding proteins, are included for study as potential biomarkers for resistance to EGFR TKIs and sensitivity to IGF-1R inhibitors. Matei et al examined b-Raf and ERK in ovarian cancers, and phosphorylated (p)-ERK in peripheral blood lymphocyte (PBL) to evaluate response prediction for sorafenib, a drug targeting c-Raf and VEGFR2. Protein quantities of the logical downstream or related targets, ERK and b-Raf and pretreatment pERK in PBLs, were not associated with tumor response or survival. But, high on-treatment pERK levels on RPPA were associated with better tumor response and lower risk of tumor progression in treated patients. This is exploratory evidence that these could be applied as surrogate markers of sorafenib activity 34. Our group independently demonstrated that sorafenib reduced pERK quantity in tumor tissue from solid tumor patients taking sorafenib. We showed this was associated with improved clinical benefit; other descriptive biomarkers related to the activity of sorafenib were identified (Azad and Kohn35, and manuscript in preparation). Thus, examination of the biology of the drug resistance would offer the opportunity of new insights of the biology of treatment resistance for future clinical benefit.
Identification of biomarkers as therapeutic targets
Identification of biomarkers and potential drug targets has been approached via both an unbiased high throughput and selective protein analyses. High throughput MS with serum and tissues yielded high sensitivity and specificity in identification of multiple diagnostic protein signatures 36. Bateman et al analyzed microdissected cancer epithelial cells derived from 25 breast cancer patients using liquid chromatography (LC)-/MS. Comparative analysis of stage 0 and stage III patients revealed 113 proteins that significantly differentiated between these groups. Known factors associated with disease pathogenesis, such as CDH1 and CTNNB1, as well as those previously implicated in breast cancer, such as TSP-1, were identified 37. These data uncovered new protein candidates indicative of disease stage and recurrence in breast cancer warranting further investigation for diagnostic utility and as potential targets. An example where this progression of events has occurred is the development of mesothelin as a screening diagnostic, a biomarker, and as a molecular target for successful drug development. Mesothelin is overexpressed in mesothelioma, and pancreatic, lung, and ovarian cancers38. Although mesothelin was initially identified through global gene profiling, all subsequent studies were done in selective proteome-based methods, as an immunohistochemical marker in tumors, and with serum mesothelin measured in ELISA or bead based immunoassay39. It has been demonstrated to be positively interactive with CA125 in the diagnostics of ovarian cancer 40, and shown recently to be a biological binding partner of CA125 41. Mesothelin was subsequently examined as a potential druggable target, resulting in the successful development of an anti-mesothelin immunotoxin, SS1P 42, and a neutralizing monoclonal antibody, amatuximab (MORAb009) 43. Therapeutic benefit has been reported for both as treatment for patients with mesothelin-expressing tumors.
Introduction of targeted therapies, also called biological therapies or biologics, into cancer therapy and therapy of other diseases, such as psoriasis and rheumatoid arthritis (RA), are the result of focused drug development derived from new understanding of the underlying pathophysiology. Agents used in biological therapy include biological products that regulate the immune system, e.g. vaccines are now termed immunotherapies. In many situations, it has meant a markedly different approach to treatment, displacing traditional cytotoxic chemotherapy in cancer, and steroids, anti-malarials, and gold in RA. However, early application of these biologics has not been promising in all clinical venues, including in treatment of some solid tumors. This may be due to indiscriminant application of biologics to all patients with a general cancer subtype diagnosis. We now know, as with the many types of arthritis, that there are phenotypically similar cancers belong to different molecular subsets of disease. The cancers in the different subsets may have different driving molecular events. This could lead to an under-appreciation of potentially beneficial agents when the agents are applied indiscriminately across all subtypes. Further development of biomarker application in trial design may improve clinical outcome, require fewer patients for analysis, and perhaps yield stronger results.
Criteria for development of validated therapeutic targets in clinical trials
Discovery, verification, and validation of reliable biomarkers are necessary in this time of ever more complex disease in order to optimally qualify drugs and their targets. Four criteria should be realized in clinical credentialing of a target (Table 2) in order to result in successful targeted therapy where the biological drive is known. Simply, these include demonstration of target against which the drug is focused, documentation that the target is active, that the agent affects its target, and that this translates into clinical benefit. For example, if a pro-inflammatory driving event is defined to due to activation of the TNFα protein signaling network, neutralizing antibodies to the TNFα axis should be successful 44, 45. In clinic, infliximab and other monoclonal antibodies targeting TNFα have shown efficacy in inducing and maintaining remission in patients with Crohn's disease 44. This example shows where the application of proteomics aided in the elucidation of disease of mechanism can be translated into clinical application. Incorporation of mechanism criteria into development and design will maintain focus on identification of clinically useful targets and biomarkers and should improve lead agent selection and clinical advancement.
Table 2.
Four criteria and examples for credentialing therapeutic targets.
| Criteria | Examples |
|---|---|
| The target was present. | Rheumatoid arthritis70 |
| TNFα overexpression was present and was etiologic in driving local inflammation and tissue destruction | |
| The target was activated. | Crohn’s disease44 |
| TNFα overexpression was a driving event. | |
| The target was altered by the intervention. | Ovarian cancer34 |
| Ras/Raf/ERK pathway was altered by sorafenib, a c-RAF kinase inhibitor. | |
| The target alteration was associated with the clinical outcome. | Breast cancer 6 |
| HER2 amplification was associated with improved survival by trastuzumab, an anti-HER2 neutralizing antibody. | |
Oncology is one of the most active new areas of targeted drug and biomarker development. HER2 is a successful target where all four criteria are satisfied by three FDA-approved agents, trastuzumab, pertuzumab, and lapatinib 6, 46. Here, it is proven that when HER2 is amplified in most cases, it also is activated. The agents inhibit this activation and have been shown to be clinically valuable. However, few trials are being conducted to evaluate selective targets or general proteomic profiling to date. Additional trials are necessary to develop the knowledge required to optimally direct biomarker and therapeutic development. While HER2 is regarded as perhaps the most successful of the molecular targets, there are many biochemical signaling events waiting to be mined in the tumor microenvironment.
There are also examples in oncology for which the criteria of presence and activation of target are fulfilled, but no clinical activity of a therapeutic target was observed. Evaluation of the criteria using proteomics in prospectively collected clinical samples allowed examination of why targeted therapies have not result in clinical benefit. EGFR is overexpressed in approximately 50% of ovarian cancer 47 and some studies showed over-expression of EGFR correlated with poor prognosis in advanced stage ovarian cancer patients 48–50. However, targeted inhibition of the prognostic factor, EGFR, has not resulted in patient benefit. This is an example where prognostic factors may be neither predictive nor sufficient for therapeutic targeting. Posadas et al reported that treatment with the EGFR inhibitor, gefitinib, lacked clinical benefit in ovarian cancer 51. RPPA evaluation of paired biopsies obtained prior to gefitinib treatment and after one month demonstrated drug-associated reduction of phosphorylation of c-kit and EGFR, and these changes correlated with treatment-induced GI toxicity, indicating a pharamcodynamic link 52. A follow-on phase II clinical trial of vandetanib, an inhibitor of both EGFR and VEGFR2 also was negative53. It was designed to examine on-target effects of both EGFR and VEGFR2. RPPA analysis of paired biopsies again showed EGFR activation was inhibited, but inhibition of VEGFR activation was not observed. The clinical pharmacodynamic effects also indicated EGFR inhibition without activity against VEGFR2. These data reinforced the lack of a therapeutically important EGFR drive in ovarian cancer. Further proteomic examination of the tissue could identify other activated signaling events for subsequent targeting.
Angiogenesis is a targetable event of particular interest 54. A dose escalation and proof of mechanism study in patients with solid tumors tested the combination of sorafenib, a c-Raf kinase inhibitor, with the neutralizing anti-VEGF monoclonal antibody, bevacizumab. This phase I study incorporated a novel design for drug exposure and acquisition of tumor samples for proteomic endpoint analysis. Patients were randomized to receive monotherapy for the first month, bracketed by collection of tumor tissue and blood and functional imaging; combination therapy was initiated with the second month and included an additional biopsy and set of imaging. RPPA was used to examine tissue protein endpoints. The results indicated on-target effects for both drugs when used alone: reduction in activated ERK by sorafenib, and angiogenesis inhibition by bevacizumab (Azad and Kohn35,55, and manuscript in preparation). This study showed the molecularly targeted combination of sorafenib and bevacizumab is biologically active in an on-target fashion with promising clinical activity in recurrent epithelial ovarian cancer patients 56. The exploratory imaging endpoints will be examined as predictive biomarkers and the proteomic endpoints confirmed in a second. An understanding of the effects of targeted therapeutics on signaling networks and correlated biomarkers is essential in order to guide subsequent steps and registration trial design. Proteomics can yield a breadth of information from which to assess a given candidate target. A key advance would be the ability to detect presence or stage of disease and/or response to targeted therapy through validated surrogate in blood or tissue biomarkers. The presence of such a bridge can rapidly triage a lengthy list of lead biologic target candidates prior to investing excess amount of time, money and hope of patients on the development of new drugs suitable for use in cancer therapy.
Biomarkers and targeted therapy in clinical trials for drug development
Drug development is typically divided into segments of lead compound identification, preclinical development, and clinical development. The entire process can cost billions of dollars and is a narrow pyramid where few drugs make it to market. Strategies for patient stratification and personalized medicine must be developed, verified, and validated during the preclinical and clinical development phases. Early consideration of the biomarker / outcome / patient stratification relationship may shorten time to the clinic and improve cost and time of drug development by allowing focused application of agent where they are most likely to succeed. Biomarker validation needs to be incorporated into optimal trial design, with appropriate statistical power for those endpoints as well. Retrospective biomarker analyses may be fraught with suboptimal power to discern an effect because of unavailable patient specimens or inconsistent quality control of available biospecimens. Conversely, if marker-based analyses were planned prospectively pre- and during/post-treatment, and tissue specimens were available on all or most patients, adequate statistical power may remain with which to compare the clinical outcome against the biomarker(s). The benefit of this approach is the ability to obtain new knowledge with increasing likelihood of success.
Current clinical trials using proteomics for biomarker discovery and/or evaluation are ongoing in many types of cancers and other diseases. Recent technologies employing protein microarrays such as RPPA has allowed for quantification of multiple endpoints in a high-throughput fashion. These endpoints include expression levels of key proteins and their activated forms that compose critical signaling nodes involved in proliferation, survival, and angiogenesis. For example, the PI3K pathway has been shown to be a driving pathway in subsets of serous ovarian cancer due to a common somatic gain-of-function mutation on chromosome 3p in PI3KCA, described above for lung cancer and occurring commonly in a variety of solid tumors. Molecular activation of PI3K drives the Akt pathway, yielding a strong survival signal 57. Agents against PI3K itself and its protein effectors Akt and mTOR are now in clinical investigation in ovarian and other cancers58, 59. Proteomic assays are being applied to evaluate modulation of PI3K/AKT/mTOR pathway 60–62.
There are other pathways downstream of receptor tyrosine kinase (RTK)s, in addition to the PI3K/AKT pathway, for which therapeutics have been developed and proteomic endpoints can be analyzed. Most are downstream signals from RTKs and integrins via the Src/Ras/Raf/MEK pathway. The mitogen-activated protein kinase, MEK, and its effector, ERK, is one such RTK downstream target. Knowledge of these downstream pathway protein targets also facilitates agent selection. Application of pathway dissection has led to an ongoing phase II collaborative study of the MEK inhibitor, AZD6244, in multiple myeloma (NCT00551070). Annunziata et al demonstrated MAF oncogene upregulation in 30% of multiple myeloma cases occurring through MEK-ERK regulation of MAF transcription. Subsequent MEK inhibition induced apoptosis selectively in MAF-expressing myelomas 63. This study provided the proof of concept of MEK/ERK/MAF as a target for therapeutic intervention in multiple myeloma 64 leading to the present trial. Genomic analysis of MAF may not be necessary if activation of MEK and ERK is demonstrated to be predictive for response. These studies hint at a promise that proteomic markers can be identified and used to guide selecting and developing drugs in cancer.
Linking proteomics and genomics data for drug development
Several validated molecular tests performed in tumor tissue or assessing the patient’s genome are now part of standard therapeutic decision making in breast, colorectal, and lung cancers 6, 11, 28. Another successful example of biomarkers by genomics is the development of imatinib mesylate. Imatinib inhibits the BCR-ABL fusion protein translocation, c-KIT mutation, and platelet derived growth factor receptor (PDGFR). This molecular-targeted drug is highly efficacious in chronic myeloid leukemia 65,66 and gastrointestinal stromal tumor 67. These genomic changes provided both a predictive biomarker and a therapeutic target for this rationally designed small molecule. Many genetic events translate into definable proteomic changes; linking genomic and proteomic data for biomarker and a therapeutic target at the protein level is ongoing in many fields. These proteomics analyses will provide predictive molecular marker(s) for the targeted drug to apply to therapeutic decision-making. Clinical trials are underway at multiple institutions analyzing the value of proteomics data in clinical decisions 68.
A recently presented phase II study 69, BATTLE (biomarker-integrated approaches of targeted therapy for lung cancer elimination; NCT00409968), demonstrated that biomarker tailored targeted lung cancer treatments may improve patient outcome. This study identified subgroups of patients with advanced NSCLC who were more likely to benefit from a specific agent(s) based on biomarker analyses done using fresh tissue biopsies. Overall, it did not yield a dramatic clinical benefit, 46% of patients had stable disease or partial response after 8 weeks of treatment with a median overall survival 9 months compared with 30% on traditional chemotherapy for advanced NSCLC. However, this example demonstrates progress has been made in stratifying patients based on biomarkers who will benefit from the biomarker-defined drugs. Thus, comprehensive proteomic and genomic profiling for advanced cancers shows promise in addressing the goals of predictive biomarkers for effective and targeted patient and therapy selections.
Future direction of biomarker, proteomics for drug development
Incorporating proteomics and biomarkers into clinical trials remains an important direction for drug development. Assay verification and biological validation remain critical for confidence in application of biomarkers and new target identification. Rigorous peer reviewed data and approval should be required for best patient safety and benefit, and cost/benefit, minimizing general application of assays. Verification and validation of tests will be a key step in maximizing the clinical and commercial success of a biomarker. Such validated biomarkers may yield clean selection criteria for defined subsets of patients susceptible to specific targeted therapies. In the future, proteomics can be applied to identify optimally targeted agent(s) and biologically effective dose(s) for each patient's disease, allowing for the monitoring of response and relapse, and engineering of new drugs and strategies to circumvent resistance mechanisms. Comprehensive systems biology proteomic approaches applied to cancers will both help the patient and tumor community. This also will create a comprehensive database of information pairing genomic change with proteomic expression with agents that may successfully target that proteomic drive. Organized studies are needed in order to initiate this direction.
Such is the biomarker strategy-based prospective clinical trial design conducted by Mills and colleagues. The “T9” (Ten Thousand Tumors Ten Thousand Tests Ten Thousand Therapies) project will test with genomic and proteomic technologies the tumors of 10,000 patients with relapsed refractory cancer for whom are at high risk for recurrence with no therapeutic standard of care. These results will be applied towards identification of best treatment considerations. This trial was designed prospectively to develop an atlas of mutation and co-mutations in patients entering clinical trials and to develop cohorts of patients with rare gene aberrations. It will help associate these mutations and co-mutations with clinical outcomes and allow evaluation of molecular evolution in metastases and due to treatment. Unlike other foundation or commercial approaches that are available, this is designed as a prospective clinical trial, presenting the investigative component and optimizing informed consent. This example project is positioned on the backbone of genomic change; the findings to be applied clinically are those that alter the protein target and the protein signaling pathway and are organized to evaluate the reliability of individualized treatment decision making.
Conclusions
Emerging proteomic technology is being applied to help select patients who may be more likely to benefit from targeted therapies and will bring to reality the clinical adoption of molecular proteomic stratification. Comprehensive proteomic profiling and trial-focused endpoint profiling will be critical for development of biomarkers and potential drug targets. Proteomics will help dissect these protein signaling pathways to define the preferable targets of molecular therapy. Incorporating translational endpoints of preplanned biologic correlates in a prospective validation study remains an important method to guide future direction of proteomics. The discovery of novel, validated biomarker signatures will broaden our understanding of the disease and will lead to development of new potential drugs for more effective targeted therapy in recurrent cancers. These events will guide future directions of proteomics as a tool of new biomarker and drug discovery in cancer and other diseases.
Acknowledgement
This work was supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkinson AJCW, DeGruttola V, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL. Biomarkers Definitions Working Group: Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994;69:979–985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020–2027. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 7.Allingham-Hawkins D, Lea A, Levine S. ERCC1 Expression Analysis to Guide Therapy in Non-Small Cell Lung Cancer. PLoS Curr. 2010;2:RRN1202. doi: 10.1371/currents.RRN1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 9.Olaussen KA, Mountzios G, Soria JC. ERCC1 as a risk stratifier in platinum-based chemotherapy for nonsmall-cell lung cancer. Curr Opin Pulm Med. 2007;13:284–289. doi: 10.1097/MCP.0b013e32816b5c63. [DOI] [PubMed] [Google Scholar]
- 10.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 11.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 12.Denkert C, Budczies J, Kind T, et al. Mass spectrometry-based metabolic profiling reveals different metabolite patterns in invasive ovarian carcinomas and ovarian borderline tumors. Cancer Res. 2006;66:10795–10804. doi: 10.1158/0008-5472.CAN-06-0755. [DOI] [PubMed] [Google Scholar]
- 13.Lemaire R, Menguellet SA, Stauber J, et al. Specific MALDI imaging and profiling for biomarker hunting and validation: fragment of the 11S proteasome activator complex, Reg alpha fragment, is a new potential ovary cancer biomarker. J Proteome Res. 2007;6:4127–4134. doi: 10.1021/pr0702722. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan KM, Calvert VS, Kay EW, et al. Use of reverse phase protein microarrays and reference standard development for molecular network analysis of metastatic ovarian carcinoma. Mol Cell Proteomics. 2005;4:346–355. doi: 10.1074/mcp.T500003-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Winters M, Dabir B, Yu M, Kohn EC. Constitution and quantity of lysis buffer alters outcome of reverse phase protein microarrays. Proteomics. 2007;7:4066–4068. doi: 10.1002/pmic.200700484. [DOI] [PubMed] [Google Scholar]
- 16.Tchabo NE, Liel MS, Kohn EC. Applying proteomics in clinical trials: assessing the potential and practical limitations in ovarian cancer. Am J Pharmacogenomics. 2005;5:141–148. doi: 10.2165/00129785-200505030-00001. [DOI] [PubMed] [Google Scholar]
- 17.Carey MS, Agarwal R, Gilks B, et al. Functional proteomic analysis of advanced serous ovarian cancer using reverse phase protein array: TGF-beta pathway signaling indicates response to primary chemotherapy. Clin Cancer Res. 2010;16:2852–2860. doi: 10.1158/1078-0432.CCR-09-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frederick MJ, VanMeter AJ, Gadhikar MA, et al. Phosphoproteomic analysis of signaling pathways in head and neck squamous cell carcinoma patient samples. Am J Pathol. 2011;178:548–571. doi: 10.1016/j.ajpath.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh B, Stoffel A, Gogineni S, et al. Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am J Pathol. 2002;161:365–371. doi: 10.1016/S0002-9440(10)64191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrer E, Dieterle F. Promises of biomarkers in drug development--a reality check. Chem Biol Drug Des. 2007;69:381–394. doi: 10.1111/j.1747-0285.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 21.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 22.Brenner DE, Normolle DP. Biomarkers for cancer risk, early detection, and prognosis: the validation conundrum. Cancer Epidemiol Biomarkers Prev. 2007;16:1918–1920. doi: 10.1158/1055-9965.EPI-07-2619. [DOI] [PubMed] [Google Scholar]
- 23.Chau CH, Rixe O, McLeod H, Figg WD. Validation of analytic methods for biomarkers used in drug development. Clin Cancer Res. 2008;14:5967–5976. doi: 10.1158/1078-0432.CCR-07-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers. 2002;18:41–46. doi: 10.1155/2002/929274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 26.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayson D, Richel D, Chia S, Jackisch C, van der Vegt S, Suter T. Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies. Ann Oncol. 2008;19:1530–1539. doi: 10.1093/annonc/mdn292. [DOI] [PubMed] [Google Scholar]
- 28.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 30.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zha J, Lackner MR. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res. 16:2512–2517. doi: 10.1158/1078-0432.CCR-09-2232. [DOI] [PubMed] [Google Scholar]
- 33.Jassem JLC, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:15s. doi: 10.1200/JCO.2013.54.4932. (suppl;abstr7500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matei D, Sill MW, Lankes HA, et al. Activity of Sorafenib in Recurrent Ovarian Cancer and Primary Peritoneal Carcinomatosis: A Gynecologic Oncology Group Trial. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azad NS, MY RH, Davidson B, Figg WD, Calvo K, Venkatasen A, Annunziata C, Meltzer P, Kohn EC. Translational proof of mechanism (PoM) for sorafenib with bevacizumab: Endpoint analysis and clinical activity. J Clin Oncol. 2009;27 abst3574. [Google Scholar]
- 36.Hood BL, Stewart NA, Conrads TP. Development of high-throughput mass spectrometry-based approaches for cancer biomarker discovery and implementation. Clin Lab Med. 2009;29:115–138. doi: 10.1016/j.cll.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Bateman NW, Sun M, Bhargava R, et al. Differential Proteomic Analysis of Late-Stage and Recurrent Breast Cancer from Formalin-Fixed Paraffin-Embedded Tissues. J Proteome Res. 2010 doi: 10.1021/pr101073s. [DOI] [PubMed] [Google Scholar]
- 38.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 39.Shah CA, Lowe KA, Paley P, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125. Cancer Epidemiol Biomarkers Prev. 2009;18:1365–1372. doi: 10.1158/1055-9965.EPI-08-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntosh MW, Drescher C, Karlan B, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol Oncol. 2004;95:9–15. doi: 10.1016/j.ygyno.2004.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneko O, Gong L, Zhang J, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 43.Hassan R, Cohen SJ, Phillips M, et al. Phase I Clinical Trial of the Chimeric Anti-Mesothelin Monoclonal Antibody MORAb-009 in Patients with Mesothelin Expressing Cancers. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 45.Maini RN, Elliott MJ, Brennan FM, et al. Monoclonal anti-TNF alpha antibody as a probe of pathogenesis and therapy of rheumatoid disease. Immunol Rev. 1995;144:195–223. doi: 10.1111/j.1600-065x.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 46.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilekis JV, Connor JP, Prins GS, Ferrer K, Niederberger C, Scoccia B. Expression of epidermal growth factor and androgen receptors in ovarian cancer. Gynecol Oncol. 1997;66:250–254. doi: 10.1006/gyno.1997.4764. [DOI] [PubMed] [Google Scholar]
- 48.Psyrri A, Kassar M, Yu Z, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11:8637–8643. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 49.Niikura H, Sasano H, Sato S, Yajima A. Expression of epidermal growth factor-related proteins and epidermal growth factor receptor in common epithelial ovarian tumors. Int J Gynecol Pathol. 1997;16:60–68. doi: 10.1097/00004347-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Sewell JM, Macleod KG, Ritchie A, Smyth JF, Langdon SP. Targeting the EGF receptor in ovarian cancer with the tyrosine kinase inhibitor ZD 1839 ("Iressa") Br J Cancer. 2002;86:456–462. doi: 10.1038/sj.bjc.6600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Posadas EM, Liel MS, Kwitkowski V, et al. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109:1323–1330. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posadas EM, Kwitkowski V, Kotz HL, et al. A prospective analysis of imatinib-induced c-KIT modulation in ovarian cancer: a phase II clinical study with proteomic profiling. Cancer. 2007;110:309–317. doi: 10.1002/cncr.22757. [DOI] [PubMed] [Google Scholar]
- 53.Annunziata CM, Walker AJ, Minasian L, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 16:664–672. doi: 10.1158/1078-0432.CCR-09-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 55.Azad NS, EMP, Kwitkowski VE, Annunziata CM, Barrett T, Premkumar A, Kotz HL, Sarosy GA, Minasian LM, Kohn EC. Increased efficacy and toxicity with combination anti-VEGF therapy using sorafenib and bevacizumab; Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I; 2006. Jun 20, 3004 2006. [Google Scholar]
- 56.Lee JM, Sarosy GA, Annunziata CM, et al. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer. 2010;102:495–499. doi: 10.1038/sj.bjc.6605514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shayesteh L, Lu Y, Kuo WL, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Angulo AM, Hennessy BT, Mills GB. Future of personalized medicine in oncology: a systems biology approach. J Clin Oncol. 2010;28:2777–2783. doi: 10.1200/JCO.2009.27.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–164. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nanjundan M, Byers LA, Carey MS, et al. Proteomic profiling identifies pathways dysregulated in non-small cell lung cancer and an inverse association of AMPK and adhesion pathways with recurrence. J Thorac Oncol. 2010;5:1894–1904. doi: 10.1097/JTO.0b013e3181f2a266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murph MM, Smith DL, Hennessy B, et al. Individualized molecular medicine: linking functional proteomics to select therapeutics targeting the PI3K pathway for specific patients. Adv Exp Med Biol. 2008;622:183–195. doi: 10.1007/978-0-387-68969-2_15. [DOI] [PubMed] [Google Scholar]
- 62.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer research. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333–6338. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 64.Annunziata CM, Davis RE, Hernandez L, et al. A mechanistic rationale for MEK inhibitor therapy in myeloma based on blockade of MAF oncogene expression. Blood. 2010 doi: 10.1182/blood-2010-04-278788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 66.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 67.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 68.Pao W, Kris MG, Iafrate AJ, et al. Integration of molecular profiling into the lung cancer clinic. Clin Cancer Res. 2009;15:5317–5322. doi: 10.1158/1078-0432.CCR-09-0913. [DOI] [PubMed] [Google Scholar]
- 69.Edward S, Kim RSH, J Jack Lee, George R, Blumenschein, Anne Tsao, Christine M, Alden, Ximing Tang, Suyu Liu, David J, Stewart, John V, Heymach, Hai T, Tran, Marshall E, Hicks, Jeremy Erasmus, Jr, Sanjay Gupta, Garth Powis, Scott M, Lippman, Ignacio I, Wistuba, Waun K, Hong The BATTLE trial (Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination): personalizing therapy for lung cancer. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; AACR; Washington DC Philadelphia (PA). 2010. Apr 17–20, 2010 Abstract LB-1 2010. [Google Scholar]
- 70.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]