Abstract
Mussel adhesion is mediated by foot proteins (mfp) rich in a catecholic amino acid, 3, 4-dihydroxyphenylalanine (dopa), capable of forming strong bidentate interactions with a variety of surfaces. A facile tendency toward auto-oxidation, however, often renders dopa unreliable for adhesion. Mussels limit dopa oxidation during adhesive plaque formation by imposing an acidic, reducing regime based on thiol-rich mfp-6, which restores dopa by coupling the oxidation of thiols to dopaquinone reduction.
Moisture is the nemesis of strong polymer adhesion to polar surfaces. Despite this, marine mussels achieve durable underwater adhesion using a suite of proteins that are peculiar in having high levels of dopa1, 2. The recent demonstration that a single tethered dopa residue adsorbed to wet titania requires a breaking force of nearly 1 nN and is completely reversible3 has spawned the design of many synthetic polymers with dopa-like side chains for diverse applications.4 Despite its virtues as a sticky side chain, dopa has a troubling tendency that presents significant challenges to its use, i.e. coupled to the reduction of dissolved O2, it is readily oxidized to dopaquinone.5 Dopaquinone formation diminishes adhesion to titania3 and mica6 by at least 80% and is very prone to further chemical modification including cross-linking reactions. 7–8
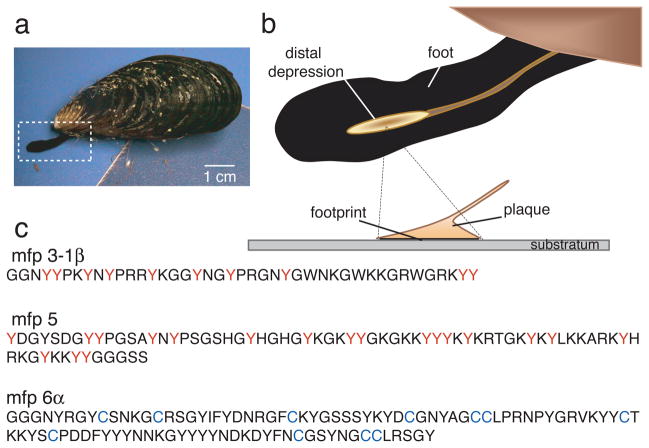
The byssus of a California mussel (Mytilus californianus Conrad) resembles a bundle of threads each of which is distally tipped by a flared adhesive plaque (Fig. 1a–b). About ten plaque proteins are known; half of these are unique to the plaque whereas the others occur elsewhere in the byssus.9, 10 A mussel assembles each new plaque in a few minutes from proteins stockpiled in its foot. The first proteins the foot squirts onto the surface of a rock are mfp-3 and mfp-5 with dopa contents of 20 and 30mole%, respectively 1, 2 (Fig. 1c). With the surrounding seawater at pH ~8 and saturating levels of dissolved oxygen, the 2-electron oxidation of dopa to dopaquinone is highly favorable. When oxidation occurs in isolated mfp-3, adhesion is significantly compromised.6 Here we describe how mussels overcome the adverse effects of mfp-3 dopa oxidation on mfp-3 adhesion to mica surfaces.
Figure 1. Byssal adhesion in the California mussel Mytilus californianus.
(a) An adult mussel attached to a mica sheet by a byssus containing 3 threads. Extended foot is making a new plaque and thread; (b) Schematic zoom of a foot viewed from the underside showing the distal depression lifting off from a completed plaque. The footprint denotes the distalmost part of each plaque in intimate contact with the surface; (c) Primary sequence of selected footprint protein variants of mfp-3 (>25 known variants), mfp-5 (2 known variants), and mfp-6 (5 known variants); the high proportion of dopa (red Y) and cysteine (blue C) in mefp-3, mfp-5 and mfp-6, respectively, is as shown.
We investigated the adhesive strategy of M. californianus by 1) determining the protein and solution conditions in the foot during plaque formation, and 2) using the surface forces apparatus (SFA) 6, 10,12 to measure the adhesive interactions between purified mfps and mica under different pH and redox conditions. Each adhesive plaque is injection molded by the mussel into a small dimple known as the distal depression located near the tip of the foot (Fig. 1b); the depression is placed like an inverted cup over a selected surface and a minute or so later, proteins are exuded from pores in the depression ceiling.13 We mimicked plaque protein secretion by injecting KCl into the pedal nerve (Supplementary Fig. 1a) shown by previous reports to be indistinguishable from natural secretion.13, 14 A recording microelectrode inserted into the distal depression (1–2 mm diameter in adult M. californianus) provides a time course of pH after the KCl injection (Supplementary Fig. 1a–d). Within 2 min of injection, the pH of the distal depression dropped by an average of 1.5 pH units (SD ± 0.3 n=15) from a resting pH of pH 7.3 to 5.8. Given microelectrode fouling by protein, the pH decrease of 1.5 units should be considered as conservative. Conductivity tests of the induced byssal secretions of five mussels indicated an average ionic strength of 80 mM (SD ± 23 mM) compared with ionic strengths of 215 mM and 700 mM for cytoplasmic fluid and seawater, respectively.15
To confirm that the products of KCl-induced and natural plaque secretion are indistinguishable, we sampled the distal depression of a mussel foot for proteins at preinjection and at 1, 2, 5 and 10 min post-injection by MALDI TOF mass spectrometry (Supplementary Fig. 2a–e). Mfp-3 variants, specifically mfp-3-1α (5.3 kDa), mfp-3-1β (5.4 kDa), mfp3-2 (5.5 kDa) and mfp-3-3α (6.6 kDa)1, are secreted first, followed closely by mfp-6 (~11.6 kDa). The cumulative pattern of induced secretion matches the distribution of proteins in a natural plaque footprint (Supplementary Fig. 2f).
Although mfp-3 and mfp-6 variants are both secreted during initial plaque formation1, 2, mfp-6 composition differs from mfp-3 variants by its low dopa and high cysteine content.1 Of the eleven cysteines in freshly isolated mfp-6, two are coupled as a disulfide leaving nine presumably as thiols.1 These thiols are not readily accessible to modification: at best 1–2 thiols can be carboxymethylated with iodoacetamide at pH 7, whereas 4–5 react with Ellman’s reagent (Supplementary Table 1). With respect to adhesion, mfp-6 adsorbed to one or both mica surfaces exhibited only weak adhesion compared with mfp-3 (Supplementary Fig. 3 & 4).
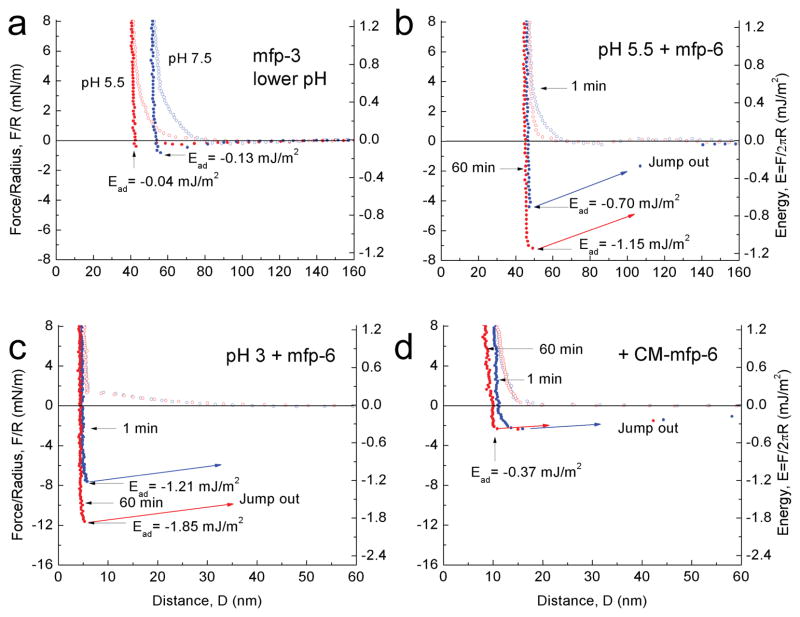
Given mfp-6’s poor adhesion, we investigated whether mfp-6 bestowed any benefits on mfp-3 adhesion. Accordingly, the adhesion energy between two mfp-3 films (−2.08 mJ/m2 at pH3) was diminished by stepwise elevation of pH (Supplementary Fig. 4). Titration with periodate at pH 3 confirmed the correlation between quinones and adhesion6 (Supplementary Fig. 5a–c). Returning the gap buffer to pH 5.5 did not restore adhesion (Fig. 2a), suggesting that pH alone is insufficient to reverse the auto-oxidation of dopa. About 100 pmoles of mfp-6 were then introduced into the gap at pH 5.5. The films were brought into brief contact and upon separation exhibited an adhesive energy of about −0.70 ± 0.05 mJ/m2 (s.d. n=6), a 130 % recovery of adhesion; following a 60-min contact, there was a 200% recovery of initial adhesion (Fig. 2b). At pH 3, mfp-6 further increased adhesion of two mfp-3 films to −1.21± 0.05 mJ/m2 and −1.85 mJ/m2 after ~1 and 60 min contacts, respectively (Fig. 2c). These results resemble but are more potent than the ascorbate rescue of mfp-3 adhesion (Supplementary Fig. 5d) and implicate mfp-6 as a plaque antioxidant.
Figure 2. Adhesion of mfp-3 at different pH before and after mfp-6 addition.
In each experiment, the adhesion of symmetric mfp-3 films was determined at pH 3 before raising the pH to 7.5. (a) Simply decreasing the pH from 7.5 to 5.5 did not recover significant adhesion (see Supplementary Fig. 3) (b) After injecting mfp-6 into the gap solution at pH 5.5 (following a), an increased adhesion energy was measured after keeping the two surfaces in contact for 1 min (blue); the energy increased even more after a 60 min contact time (red). (c) As in (a), dropping the pH from 7.5 to 3 did not recover mfp-3 adhesion (not shown), however, injecting mfp-6 at pH 3 increased the adhesion energy to its highest levels at 1 min (blue) and 60 min (red); (d) Injecting S-carboxymethylated mfp-6 at pH 3 failed to recover the lost adhesion of mfp-3 cycled from pH 3 to 7.5, and back to pH 3. The experiments of (a) and (b) were repetitive, whereas (c) and (d) were de novo.
A mechanism of anti-oxidant action by mfp-6 is suggested by its rich thiol content. We surmised that thiols in mfp-6 provide reducing equivalents for dopaquinone formed in mfp-3. To test this, accessible cysteines in mfp-6 were S-carboxymethylated with iodoacetate at pH 7.5 (Supplementary Table 1), and the modified protein was then added to films of oxidized mfp-3 at pH 3. Carboxymethylated mfp-6 rescue of mfp-3 adhesion was at best feeble after a short contact (Fig. 2d) and did not improve after a 60 min contact. The slight adhesive rescue suggests additional antioxidant functionalities, or that SH groups were not fully blocked. The failure of carboxymethylated mfp-6 to rescue the adhesion of mfp-3 supports the premise that cysteines in mfp-6 provide a limited pool of reducing thiols to regenerate dopa from losses to dopaquinone. As thiolates not thiols are the more strongly reducing, the persistence of reducing activity even to pH 3.0 suggests that mfp-6 thiols have unusually low pKas. The pH-dependence of the turnover rate kobs for mfp-6 thiol oxidation by Ellman’s reagent approximates thiol pKa. At pH 4–5, thiols are 25% as reactive as they are at pH 7, which greatly exceeds reactivity expected for a typical thiol pKa of 8–9, i.e. <1% (Supplementary Fig. 6). To directly test whether these thiols perform as antioxidants at pH 5, mfp-6 was added to a standard antioxidant assay15 based on the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical (molar ratio 1:5) (Supplementary Fig. 7). Native mfp-6 completely reduced DPPH, whereas partially carboxymethylated mfp-6 reduced DPPH to between 40–50%.
A mussel imposes a specific chemical microenvironment on the distal depression of the foot during the initial stages of plaque formation. Conditions include an acidic pH ~5.5, ionic strength of ~0.1M, and both mfp-3 and mfp-6. The low pH is consistent with recent SFA studies showing that mfp-3 adhesion to mica increases with decreasing pH, 6 decreases with dopa oxidation, 6 and is counteracted by antioxidants that reduce dopaquinone to dopa.
Using the SFA to reenact initial molecular events in plaque formation, we found that mfp-6 restored adhesion in oxidized mfp-3, presumably by imposing a potent reducing regime. The mechanism of mfp-6 action is only partially understood. Mfp-6 carboxymethylation blocked mfp-6 rescue of mfp-3 adhesion suggesting a thiolate-mediated action (Supplementary Table 1). Typically thiolates, not thiols, are the operative antioxidant groups.17 Thiol pKas in mfp-6 are substantially lower than typical cysteine pKas at 8–9. The lowest reported thiol pKa of 3.5 with Cys-30 in the sequence CxxC in the redox protein DsbA from E. coli 18. Lowering the thiol pKas gives mfp-6 more reducing power for dopa rescue from dopaquinone at the pH of plaque formation.
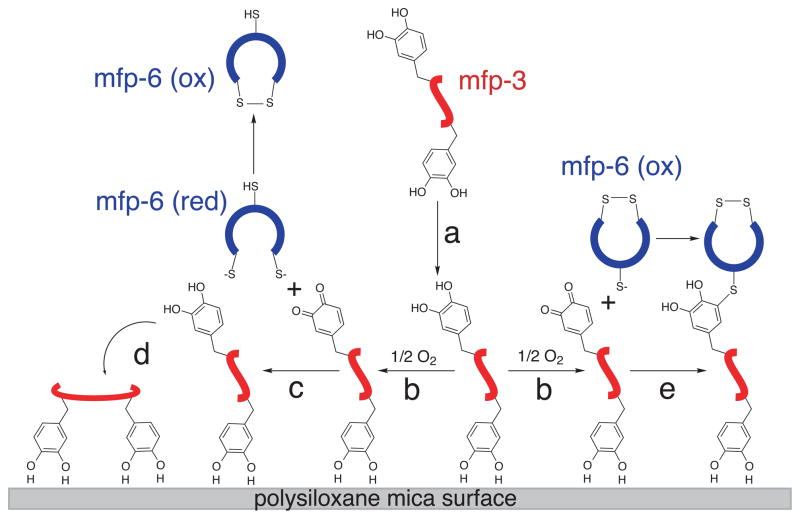
Redox control in mfp-3 must be considered in the context of plaque formation. A small volume (~10–20 μl) of fluid containing mfp-3 variants is secreted into the distal depression (Fig. 3a) at a pH ≤5.5. Each protein binds the mica surface by up to 10 dopa-mediated bidentate H-bonds. 2, 19 Given the small size of mfp-3 (5–6 kDa) and its extended unstructured conformation, adsorption is rapid and irreversible. 6, 19 Dopa H-bonded to mica is probably shielded from oxidation by the matched molecular spacings -0.29 and 0.28 nm - between the dopa o-hydroxyls and the inter-oxygen distances of mica, respectively. 19 As predicted by Bell theory and by analogy to the A-T pairs in DNA, the well oriented bidentate hydrogen bonding (E=~−28 kT) of dopa to mica should have a binding lifetime (τ =τoe−E/kT) that is 106 times as long as the monodentate form (E=~−14 kT) 20. Dopa residues not initially adsorbed are prone to oxidation (Fig. 3b). This loss is transiently repaired by the thiols of mfp-6 (Fig. 3c) enabling fuller adsorption of mfp-3 (Fig. 3d). However, keeping all dopa reduced in the plaque cannot be sustained forever by mfp-6. As the population of available reducing thiols dwindles, the coupled thiol-quinone redox system accumulates S-cysteinyl dopa adducts (Fig. 3e). As shown (Supplementary scheme 1), the quinone reducing action of thiolates consists of two steps: a nucleophilic attack of the quinone by the first thiolate anion to form S-cysteinyl-dopa adducts, followed by the attack of the thioether by the second thiolate anion to form a disulfide and dopa.21 This scheme is consistent with the detection of 0.5 to 1.0 mol% 5-S-cysteinyl-dopa cross-links in acid hydrolyzed plaques. 1
Figure 3. Redox control and the stepwise adsorption and cross-linking of mfp-3.
Mfp-3 variants are secreted into the distal depression (a) and partially adsorbed by dopa mediated H-bonds to the mica surface. The oxidation of unadsorbed dopa to dopa-quinone (b) is counteracted by reducing thiolates (c) in mfp-6, which enables enhanced adsorption (d). Depletion of thiolate pairs in mfp-6 transforms mfp-6 into a cross-linker with mfp-3 (e).
The emerging picture is that mfp-6 may be efficiently adapted to be first an antioxidant and later a cross-linking partner for mfp-3, the latter function being crucial for improving cohesion among the plaque proteins. 1, 22 Other cohesive interactions between plaque proteins are possible: intrinsic protein-protein binding energy between mfp-5 and mfp-2 is independent of quinones and requires up to 1.5 mJ/m2 to break 10; equally important, the Fe3+ mediated bridges between dopa residues in mfp-2 and mfp-1 range from 2 to 5 mJ/m2. 23
Byssal plaque formation is an unprecedented example of redox control beyond the living organism. Perhaps because of the unusual requirements of this adaptation, the mfp-6 sequence has no homology with any known protein. Although mfp-6 is 11 mole% cysteine, the cysteine thiol content of plaque proteins from other mussel species (e.g. Perna) can be as high as 20 mole% 24. There is no evidence so far that the thiols of mfp-6 can be regenerated from disulfides as they are within compartments of living cells.
Supplementary Material
Acknowledgments
We thank the US National Institutes of Health Grant #R01 DE018468 and the National Science Foundation MRSEC Program Grant # DM R05-20415 and the Human Frontiers of Science Program for supporting this research. C. Thorpe and an anonymous reviewer provided helpful insights about thiol chemistry. S. Nicklisch and P. Schmitt introduced us to the DPPH assay.
Footnotes
Author contributions: J. Y. designed and performed the SFA experiments, W. W. purified and modified proteins, E. D. and R. K. A. contributed protein, J. H. W. wrote the manuscript and designed and co-supervised the whole project with J. N. I., who helped analyze the results.
References
- 1.Zhao H, Waite JH. J Biol Chem. 2006;281:26150–26158. doi: 10.1074/jbc.M604357200. [DOI] [PubMed] [Google Scholar]
- 2.Zhao H, Robertson NB, Jewhurst S, Waite JH. J Biol Chem. 2006;281:11090–11096. doi: 10.1074/jbc.M510792200. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Scherer NF, Messersmith PB. Proc Nat Acad Sci USA. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart RJ. Appl Microbiol Biotechnol. 2011;89:27–33. doi: 10.1007/s00253-010-2913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proudfoot GM, Ritchie IM. Aust J Chem. 1983;36:885–894. [Google Scholar]
- 6.Yu J, Wei W, Danner E, Israelachvili JN, Waite JH. Adv Mat adma. 2011:201003580. doi: 10.1002/adma.201003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rzepecki LM, Nagafuchi T, Waite JH. Arch Biochem Biophys. 1991;285:17–26. doi: 10.1016/0003-9861(91)90323-b. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Burdine L, Kodadek T. J Am Chem Soc. 2006;129:12348–12349. doi: 10.1021/ja072904r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman HG, Roberto FF. Mar Biotechnol. 2007;9:661–681. doi: 10.1007/s10126-007-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang DS, et al. J Biol Chem. 2010;285:25850–858. doi: 10.1074/jbc.M110.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waite JH. Int J Adhesion and Adhesives. 1987;7:9–15. [Google Scholar]
- 12.Lin Q, et al. Proc Nat Acad Sci USA. 2007;104:3782–3786. doi: 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamarin A, Lewis P, Askey J. J Morphol. 1976;149:199–222. doi: 10.1002/jmor.1051490205. [DOI] [PubMed] [Google Scholar]
- 14.Sagert J, Waite JH. J Exp Biol. 2009;212:2224–2236. doi: 10.1242/jeb.029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund J, Kalbitzer HR. J Biomolec NMR. 1995;5:321–322. doi: 10.1007/BF00211760. [DOI] [PubMed] [Google Scholar]
- 16.Hunsaker DB, Schenk GH. Talanta. 1983;30:475–480. doi: 10.1016/0039-9140(83)80113-1. [DOI] [PubMed] [Google Scholar]
- 17.Jensen KS, Hansen RE, Winther JR. Antioxid Redox Signal. 2009;11:1047–1058. doi: 10.1089/ars.2008.2297. [DOI] [PubMed] [Google Scholar]
- 18.Brandes N, Schmitt S, Jacob U. Antioxid Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson TH, et al. Advanced Functional Materials. 2010;20:4196–4205. doi: 10.1002/adfm.201000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israelachvili JN. Intermolecular and Surface Forces. 3. Elsevier; London: 2010. [Google Scholar]
- 21.Inaba K. Genes to Cells. 2010;15:935–943. doi: 10.1111/j.1365-2443.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 22.McDowell LM, Burzio LA, Waite JH, Schaefer J. J Biol Chem. 1999;274:20293–20295. doi: 10.1074/jbc.274.29.20293. [DOI] [PubMed] [Google Scholar]
- 23.Zeng H, Hwang DS, Israelachvili JN, Waite JH. Proc Nat Acad Sci USA. 2010;107:12850–12853. doi: 10.1073/pnas.1007416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkawa K, Nagai T, Nishida A, Yamamoto H. J Adhes. 2009;85:770–791. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.