Abstract
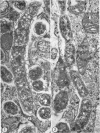
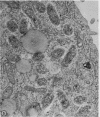
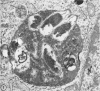
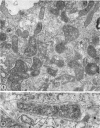
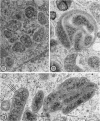
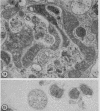
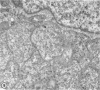
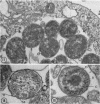
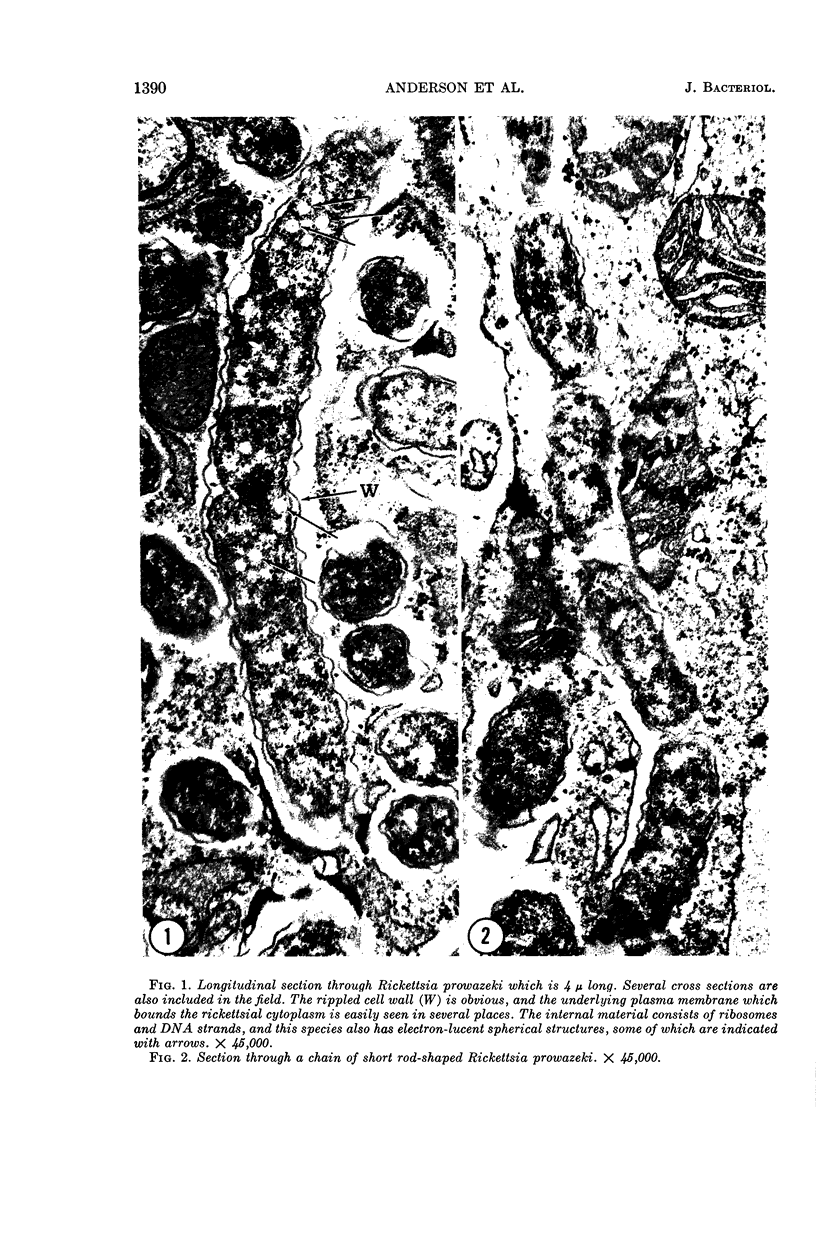
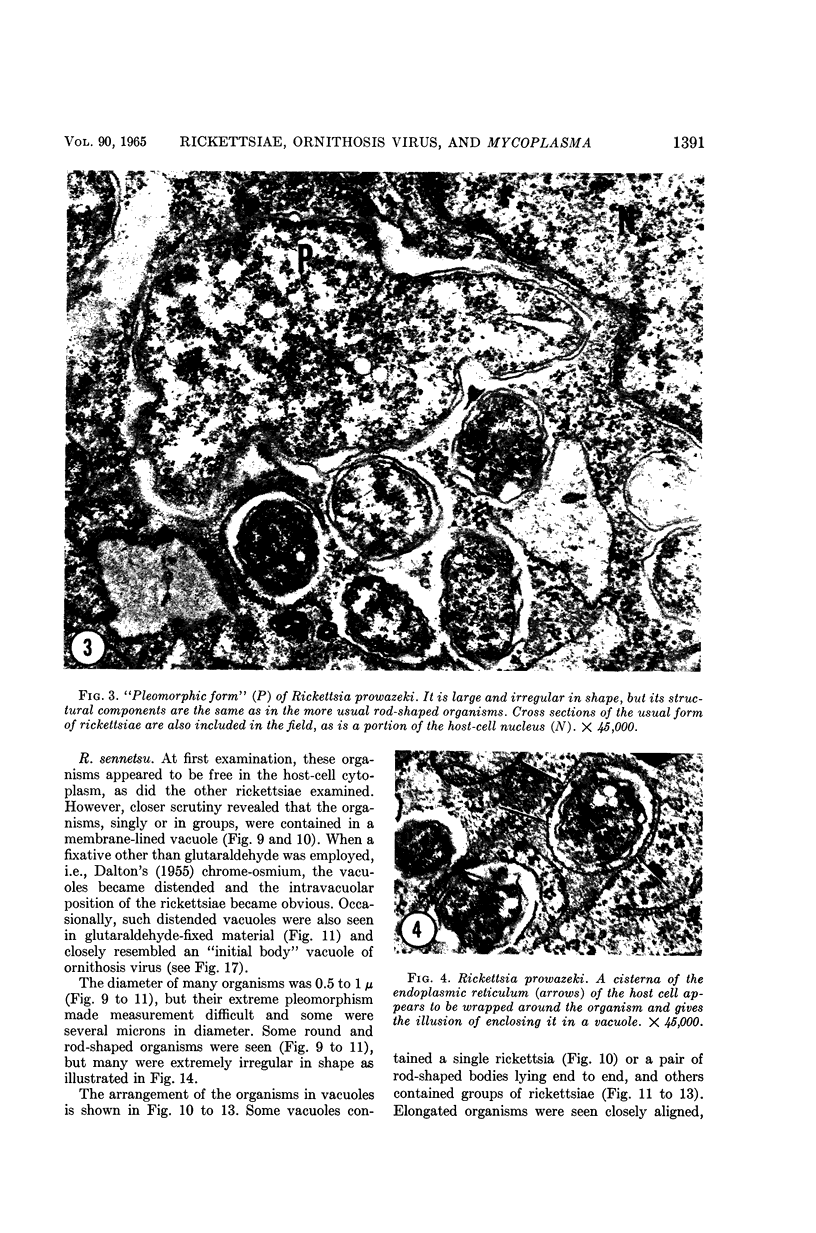
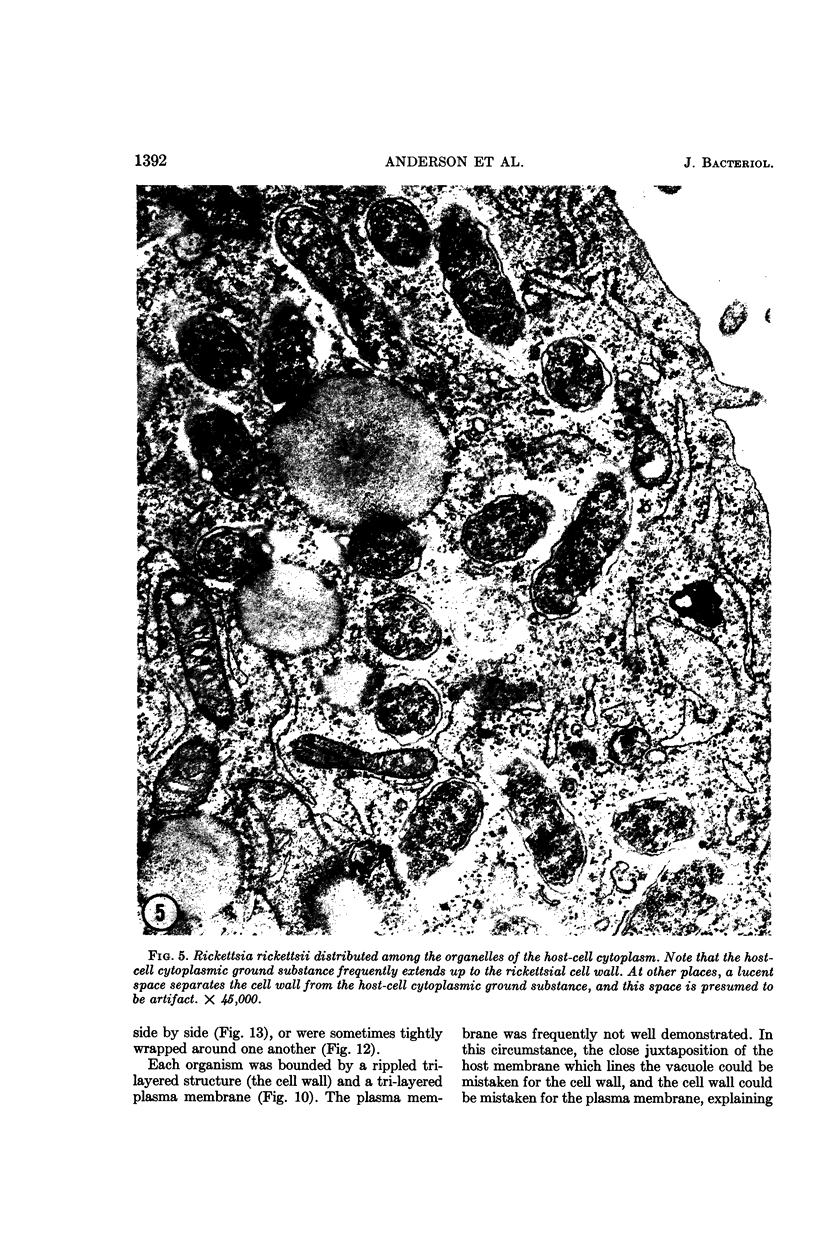
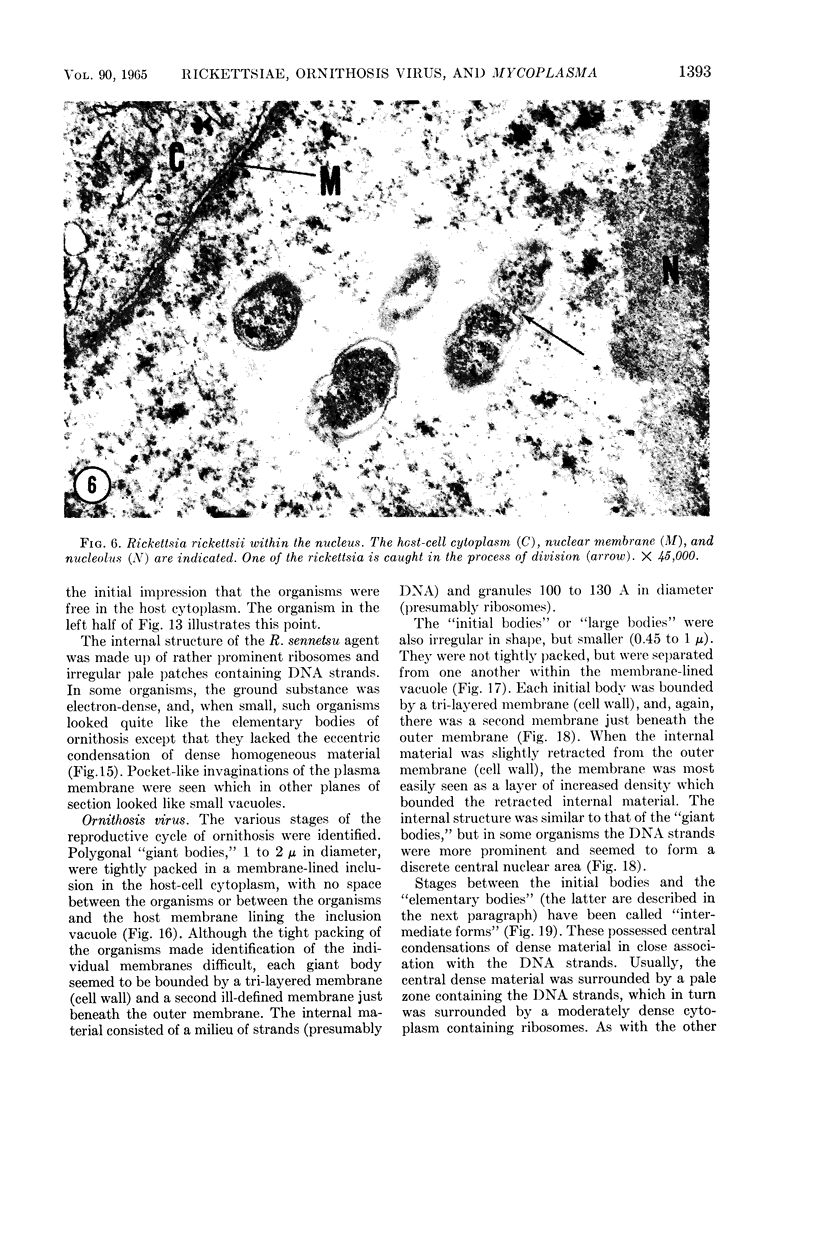
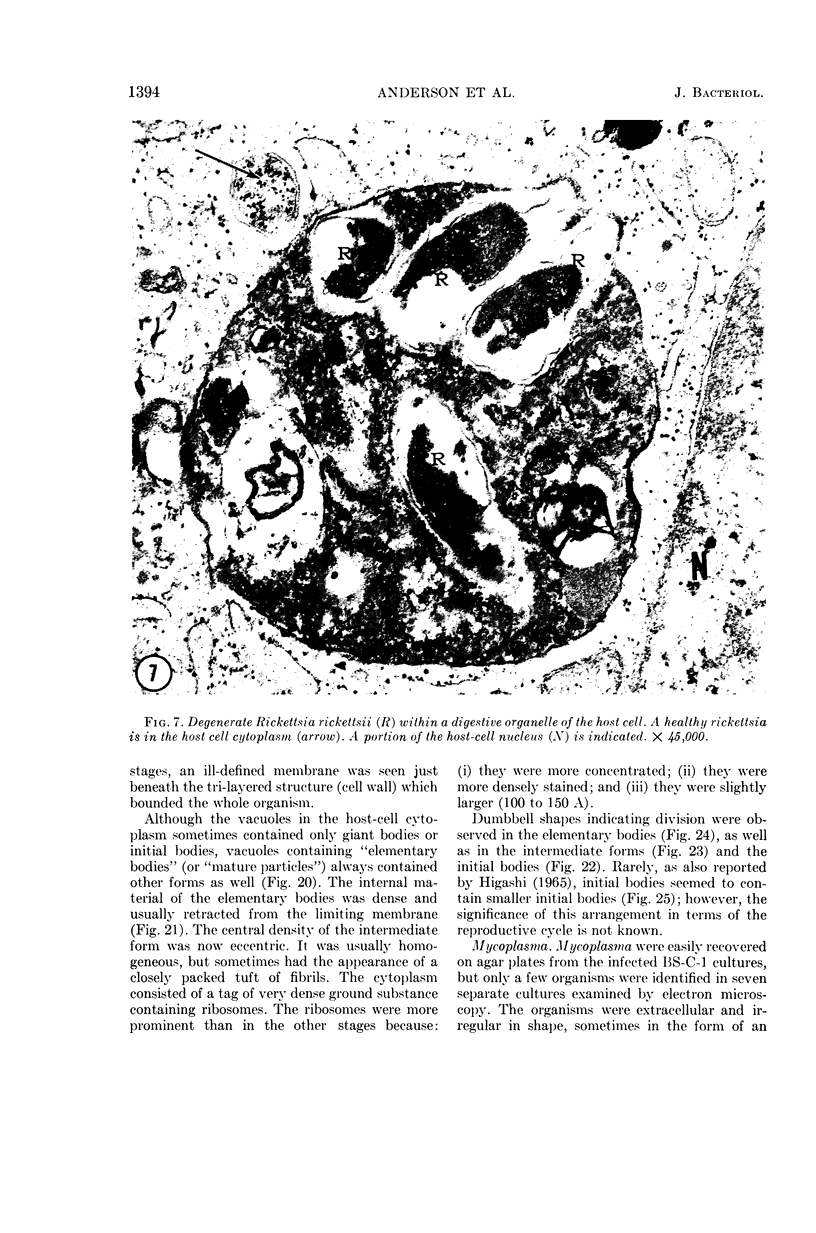
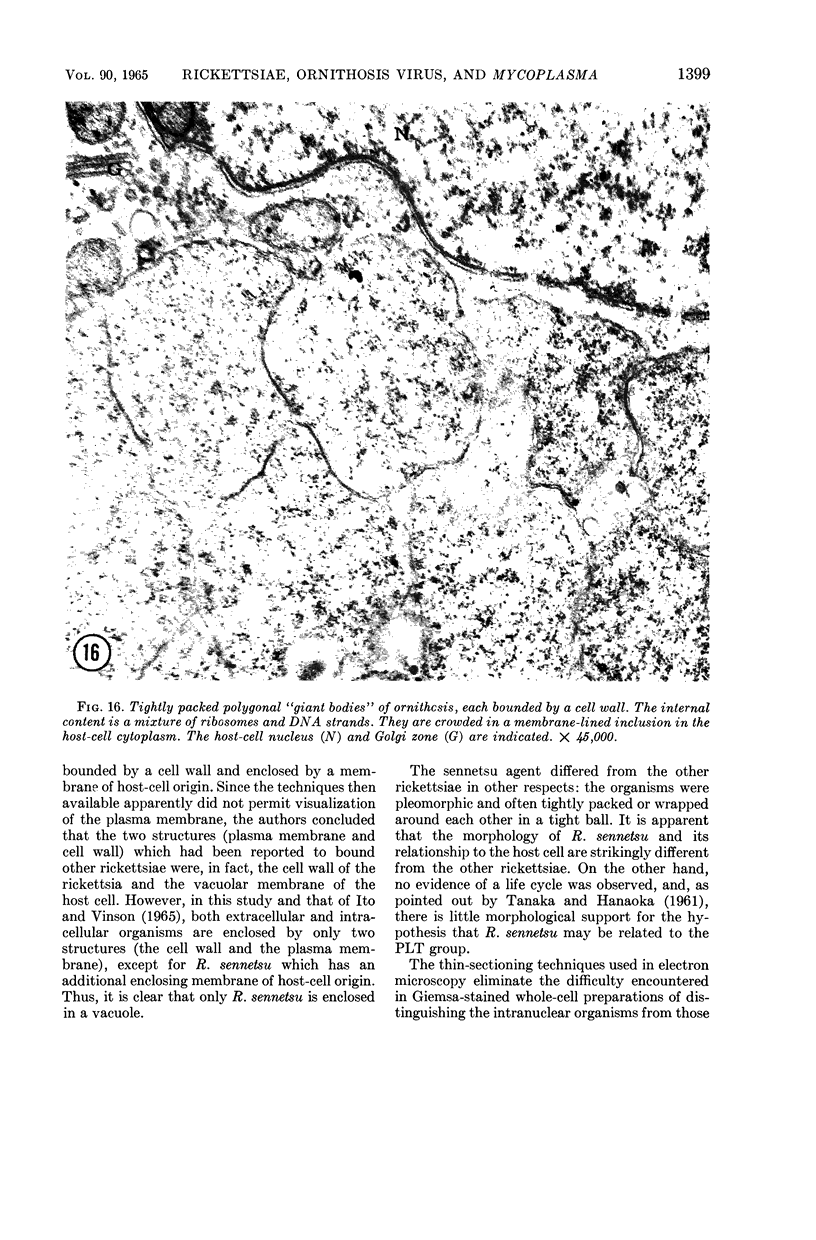
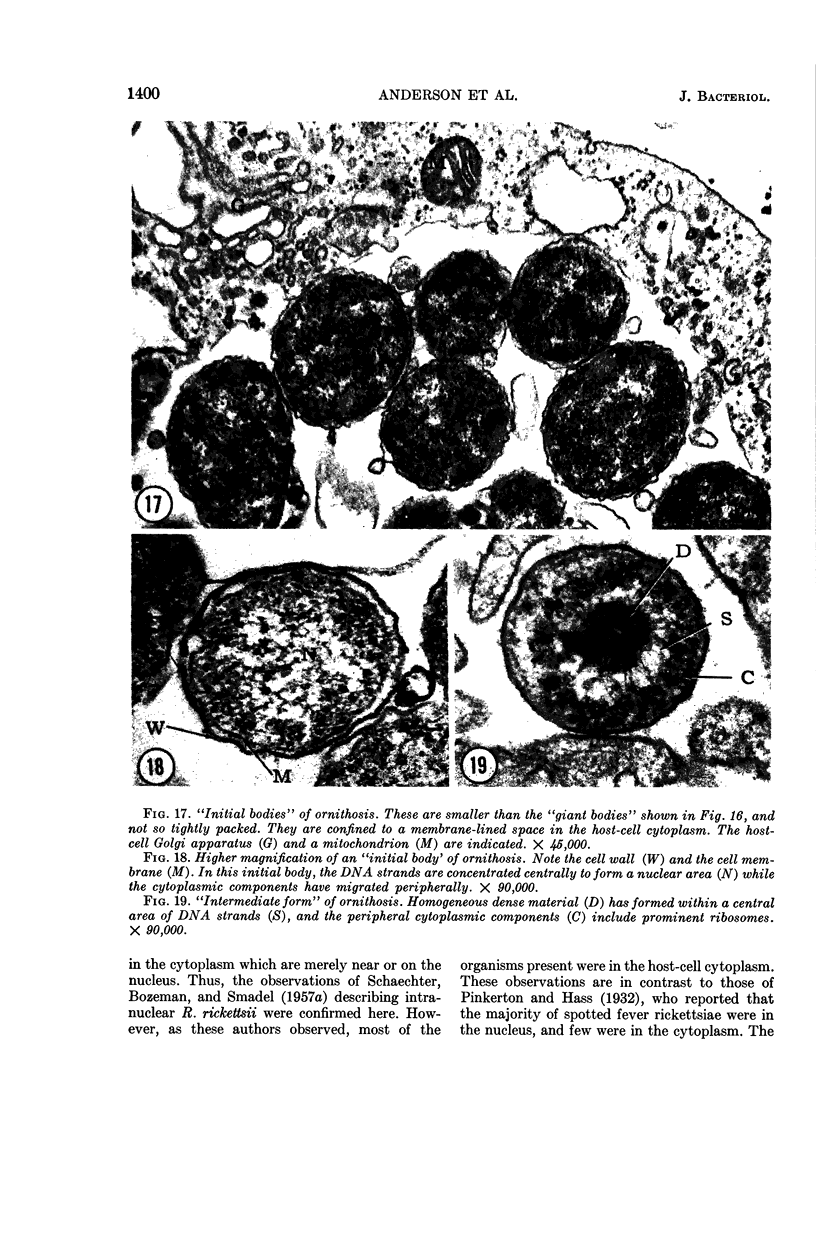
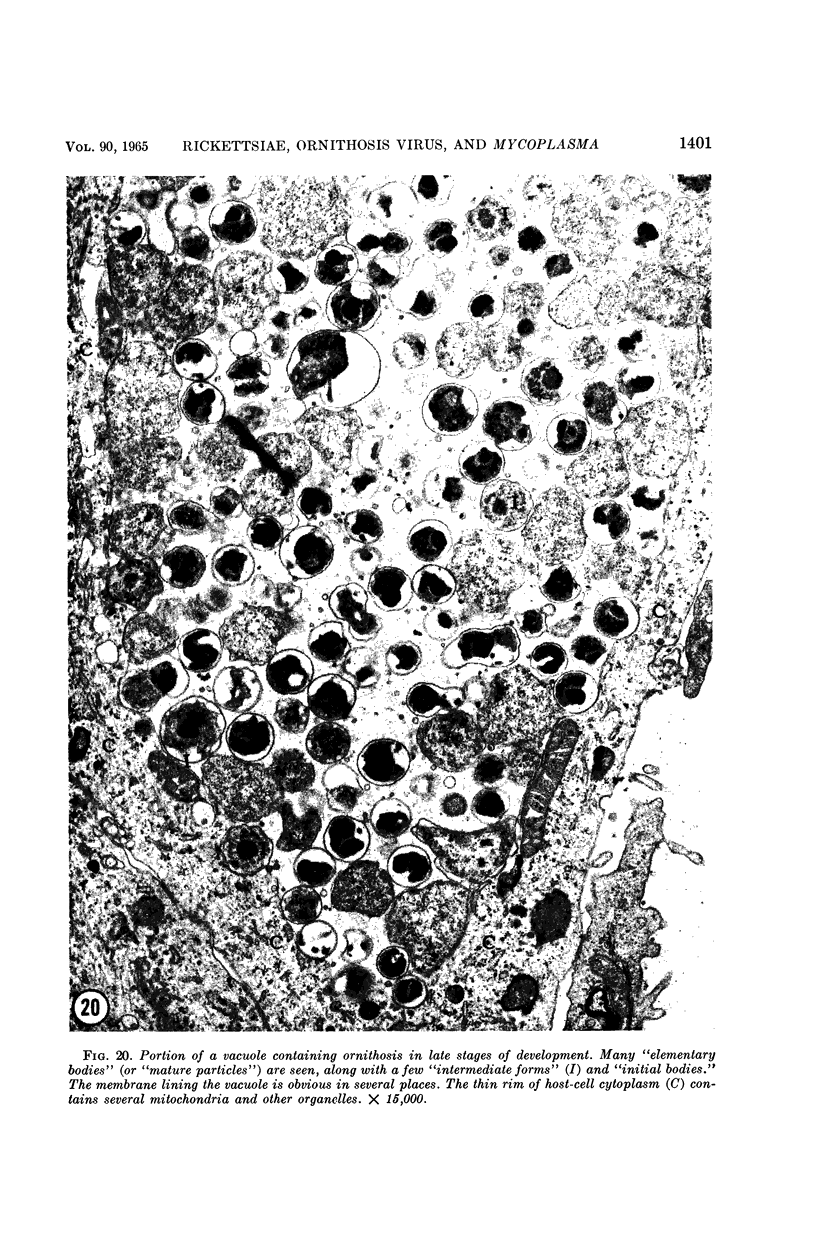
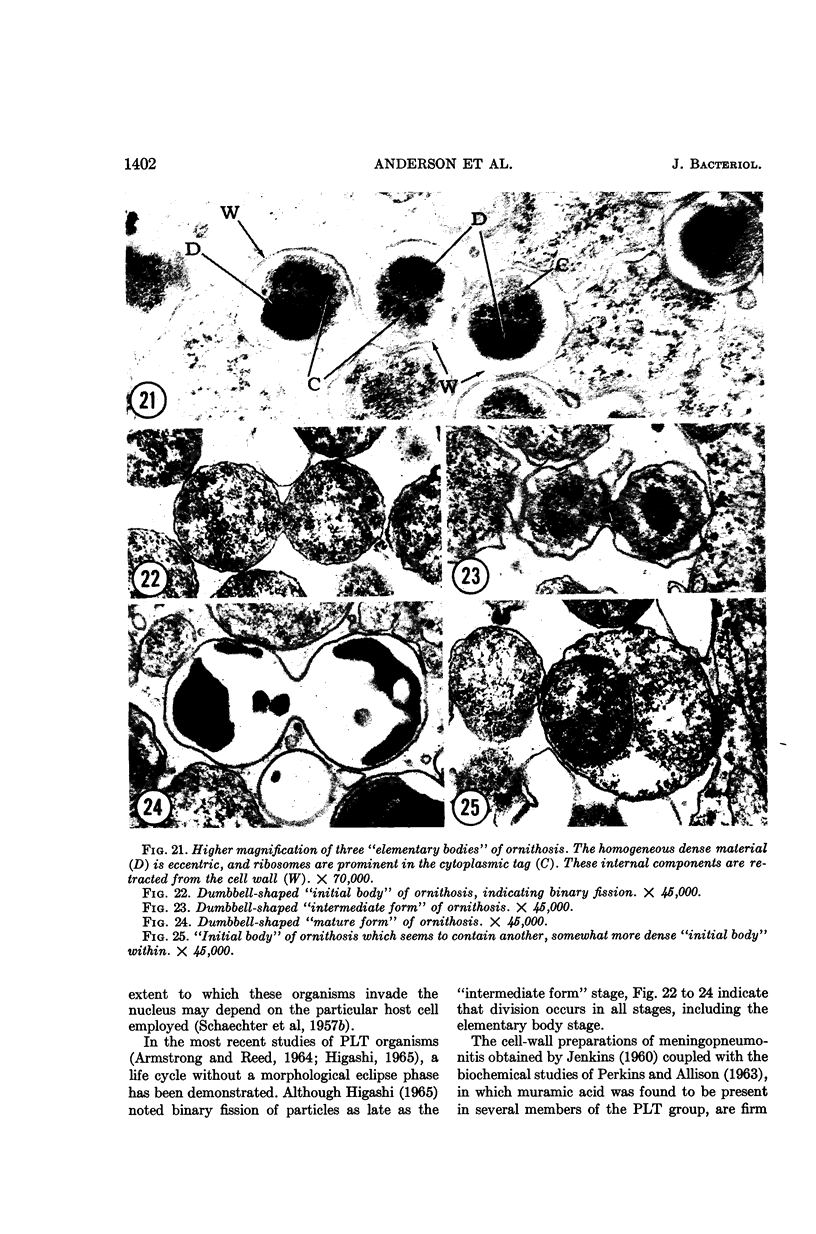
Anderson, Douglas R. (National Cancer Institute, Bethesda, Md.), Hope E. Hopps, Michael F. Barile, and Barbara C. Bernheim. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J. Bacteriol. 90:1387–1404. 1965.—In an effort to make a valid comparison of the ultrastructure of several intracellular parasites, selected agents were propagated under identical conditions in a single type of tissue culture cell; such infected preparations were processed for examination by electron microscopy by use of a standardized procedure for fixation and embedding. The organisms studied were: the Breinl and E strains of epidemic typhus, Rickettsia prowazeki; the Bitterroot strain of R. rickettsii; the Karp strain of R. tsutsugamushi (R. orientalis); R. sennetsu; the P-4 strain of ornithosis virus; and the HEp-2 strain of Mycoplasma hominis type I. Each of the rickettsial species examined had a cell wall and a plasma membrane, and contained ribosomes and deoxyribonucleic acid (DNA) in a ground substance. However, certain differences were noted. Both strains of R. prowazeki contained numerous intracytoplasmic electron-lucent spherical structures (4 to 10 mμ), not previously described. R. sennetsu, unlike the other rickettsiae, was not free in the host cytoplasm but was always enclosed in a vacuole. R. rickettsii was observed intranuclearly and in digestive organelles of the host cell as well as in the cytoplasm. Cells infected with ornithosis virus contained several forms representing the stages in its life cycle. The “initial bodies,” made up of ribosomes and DNA strands, were morphologically similar to the rickettsiae. In cultures infected with M. hominis, most of the cells became large and multinucleate. Although the Mycoplasma organisms were readily cultivated from these cultures, only a few could be found in the electron microscope preparations. These organisms were extracellular and lacked a cell wall, being bound only by a unit membrane. Again, the internal components were ribosomes and DNA strands. Under the uniform preparative conditions employed here, the three groups of organisms were morphologically distinguishable from one another.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. A., REED S. E. NATURE AND ORIGIN OF INITIAL BODIES IN LYMPHOGRANULOMA VENEREUM. Nature. 1964 Jan 25;201:371–373. doi: 10.1038/201371a0. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Barile M. F. Ultrastructure of Mycoplasma hominis. J Bacteriol. 1965 Jul;90(1):180–192. doi: 10.1128/jb.90.1.180-192.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARILE M. F., YAGUCHI R., EVELAND W. C. A simplified medium for the cultivation of pleuropneumonia-like organisms and the L-forms of bacteria. Am J Clin Pathol. 1958 Aug;30(2):171–176. doi: 10.1093/ajcp/30.2_ts.171. [DOI] [PubMed] [Google Scholar]
- CARO L. G., JACKSON E. B., SMADEL J. E., WISSIG S. L. Electron microscopic observations on intracellular rickettsiae. Am J Pathol. 1956 Nov-Dec;32(6):1117–1133. [PMC free article] [PubMed] [Google Scholar]
- GIBBONS I. R., GRIMSTONE A. V. On flagellar structure in certain flagellates. J Biophys Biochem Cytol. 1960 Jul;7:697–716. doi: 10.1083/jcb.7.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- ITO S., VINSON J. W. FINE STRUCTURE OF RICKETTSIA QUINTANA CULTIVATED IN VITRO AND IN THE LOUSE. J Bacteriol. 1965 Feb;89:481–495. doi: 10.1128/jb.89.2.481-495.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISAO T., KOBAYASHI Y., SHIRAKAWA M. La mononucléose infectieuse (fièvre ganglionnaire). Sang. 1957;28(9):785–805. [PubMed] [Google Scholar]
- MITSUI Y., FUJIMOTO M., KAJIMA M. DEVELOPMENT AND MORPHOLOGY OF TRACHOMA AGENT IN THE YOLK SAC CELL AS REVEALED BY ELECTRON MICROSCOPY. Virology. 1964 May;23:30–45. doi: 10.1016/s0042-6822(64)80005-2. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R., ALLISON A. C. Cell-wall constituents of rickettsiae and psittacosis-lymphogranuloma organisms. J Gen Microbiol. 1963 Mar;30:469–480. doi: 10.1099/00221287-30-3-469. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., BOZEMAN F. M., SMADEL J. E. Study on the growth of Rickettsiae. II. Morphologic observations of living Rickettsiae in tissue culture cells. Virology. 1957 Feb;3(1):160–172. doi: 10.1016/0042-6822(57)90030-2. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., TOUSIMIS A. J., COHN Z. A., ROSEN H., CAMPBELL J., HAHN F. E. Morphological, chemical, and serological studies of the cell walls of Rickettsia mooseri. J Bacteriol. 1957 Dec;74(6):822–829. doi: 10.1128/jb.74.6.822-829.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]