Summary
Human bone marrow stromal cells (hMSCs) have the potential to differentiate into osteoblasts; there are age-related decreases in their proliferation and differentiation to osteoblasts. Parathyroid hormone (PTH), when applied intermittently in vivo, has osteoanabolic effects in a variety of systems. In this study, we compared PTH signaling and osteoanabolic effects in hMSCs from young and old subjects. There were age-related decreases in expression of PTH/PTHrP receptor type 1 (PTHR1) gene (p=0.049, n=19) and in PTH activation of CREB (p=0.029, n=7) and PTH stabilization of β-catenin (p=0.018, n=7). Three human PTH peptides, PTH1–34, PTH1-31C (Ostabolin-C, Leu27, Cyclo[Glu22-Lys26]-hPTH1–31), and PTH1–84 (10 nM) stimulated osteoblast differentiation with hMSCs. Treatment with PTH1–34 resulted in a significant 67% increase in alkaline phosphatase (ALP) activity in hMSCs obtained from younger subjects (<50-year-old, n=5), compared with an 18% increase in hMSCs from elders (>55-year-old, n=7). Both knockdown of CREB and treatment with a PKA inhibitor H-89 blocked PTH stimulation of osteoblast differentiation in hMSCs from young subjects. The PTH peptides significantly stimulated proliferation of hMSCs. Treatment with PTH1–34 resulted in an average of twice as many cells in cultures of hMSCs from young subjects (n=4), but had no effect with hMSCs from elders (n=7). Upregulation of PTHR1 by 24-hour pre-treatment with 100 nM dexamethasone rescued PTH stimulation of proliferation in hMSCS from elders. In conclusion, age-related intrinsic alterations in signaling responses to osteoanabolic agents like PTH may contribute to cellular and tissue aging of the human skeleton.
Keywords: PTH, Age, Signaling, Osteoblast, Proliferation, Differentiation, β-catenin, CREB, human MSCs
Introduction
Human bone marrow stromal cells (a.k.a. mesenchymal stem cells, hMSCs) have the potential to differentiate to lineages of mesenchymal tissues, including bone, cartilage, fat, tendon, and muscle (Prockop 1997; Pittenger et al., 1999; Krebsbach et al., 1999). Controlling the differentiation of hMSCs could be a useful approach to treat or prevent skeletal diseases (Nuttall & Gimble 2000) and other degenerative diseases (Abdallah & Kassem, 2009). Previously, we showed that there are age-related intrinsic changes in hMSCs and a decline in their differentiation to osteoblasts in vitro (Zhou et al., 2008).
Parathyroid hormone (PTH) is an 84-amino-acid polypeptide hormone that functions as a major mediator of bone remodeling and as an essential regulator of calcium homeostasis. Clinical studies indicate that the native human parathyroid hormone hPTH1–84, a peptide fragment hPTH1–34, and an analog hPTH1-31C (Ostabolin-C, Leu27, Cyclo[Glu22-Lys26]-hPTH(1–31)NH2) potently stimulate bone growth in both healthy and osteoporotic humans (Whitfield, 2006). Although chronic hyperparathyroidism is associated with increased bone resorption and reduced cortical bone mass, studies show clear and consistent anabolic skeletal effects by intermittent low-dose PTH (Watson et al., 1995; Neer et al., 2001). Several in vitro and in vivo models show that PTH significantly increases IGF-I mRNA and immunoreactive IGF-I, and that IGF-I is an anabolic factor that mediates PTH stimulation of bone formation (McCarthy et al., 1989; Linkhart & Mohan, 1989; Yakar et al., 2006; Wang et al., 2007; Elis et al., 2010). There is some information concerning Wnt-mediated mechanisms of PTH actions in mice (Tobimatsu et al., 2006; Guo et al., 2010; Weinstein et al., 2010; Jilka et al., 2010), rat osteoblast (Kulkarni et al., 2005; Wan et al., 2008), and human osteosarcoma cell line Saos-2 (Suzuki et al., 2008), but these effects have not been shown to apply in hMSCs. The major effects of PTH on differentiated osteoblasts are dependent upon binding to the transmembrane PTH/PTHrP receptor type 1 (PTHR1) and activation of transcription factors such as the activator protein-1 family, RUNX2, and cAMP response element binding protein (CREB); much of the regulation of these factors by PTH is protein kinase A (PKA)-dependent, independent of PKC activity (Swarthout et al., 2002).
These studies were designed to test the hypothesis that age influences the effects of PTH on hMSCs. In these in vitro studies with hMSCs from young and old subjects, we compared differentiation and proliferation effects of three PTH peptides, hPTH1–34, PTH1-31C, and hPTH1–84, we assessed the effects of age on PTH/PTHrP receptor type 1 (PTHR1) gene expression and on signaling mediators, and we tested the effect of CREB silencing and PTHR1 upregulation by dexamethasone on PTH responsiveness in hMSCs from young and old subjects, respectively.
Results
Constitutive expression of PTH/PTHrP receptor type1 in hMSCs declines with age of the subject
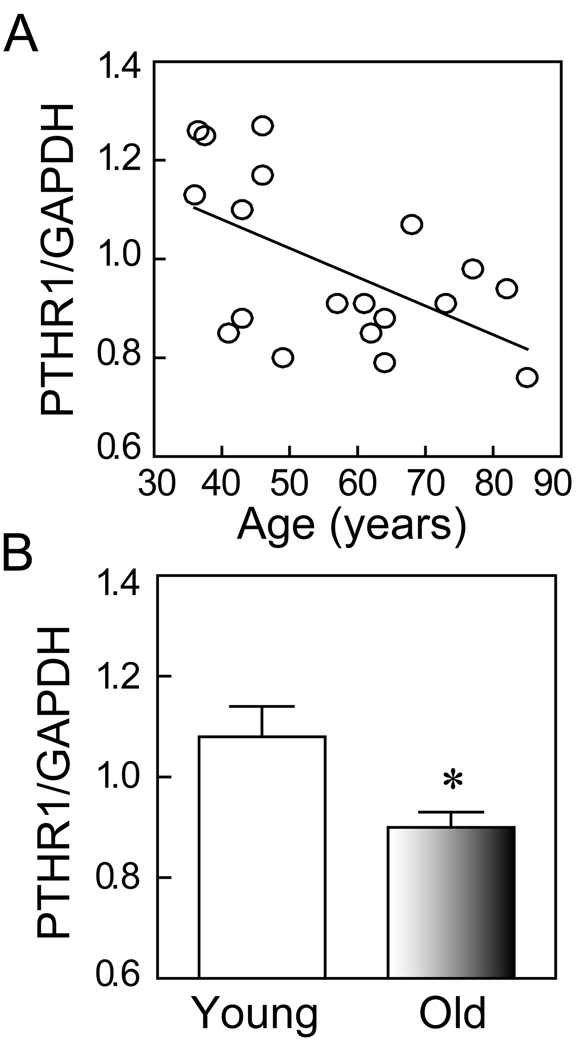
Analysis of the constitutive levels of expression of PTH/PTHrP receptor type 1 (PTHR1) gene in hMSCs showed a significant age-related decline (Spearman r=−0.46, p=0.049, n=19) (Fig. 1A). The mean level of expression of PTHR1 in hMSCs obtained from older subjects (≥ 55-years, n=10) was significantly lower (p=0.024, t-test; Fig. 1B) than in hMSCs from young subjects (<50-years, n=9).
FIG. 1.
Effects of age on expression of PTH/PTHrP receptor type 1 (PTHR1) in human MSCs. (A) The constitutive expression of PTHR1 gene in hMSCs was inversely correlated with age of subjects (Spearman r=−0.46, p=0.049, n=19). (B) The constitutive expression of PTHR1 gene was significantly higher in hMSCs obtained from young subjects (<50-year-old, n=9) than from older subjects (≥55-year-old, n=10) (p=0.024, t-test).
PTH activation of CREB signaling is diminished in hMSCs from elders
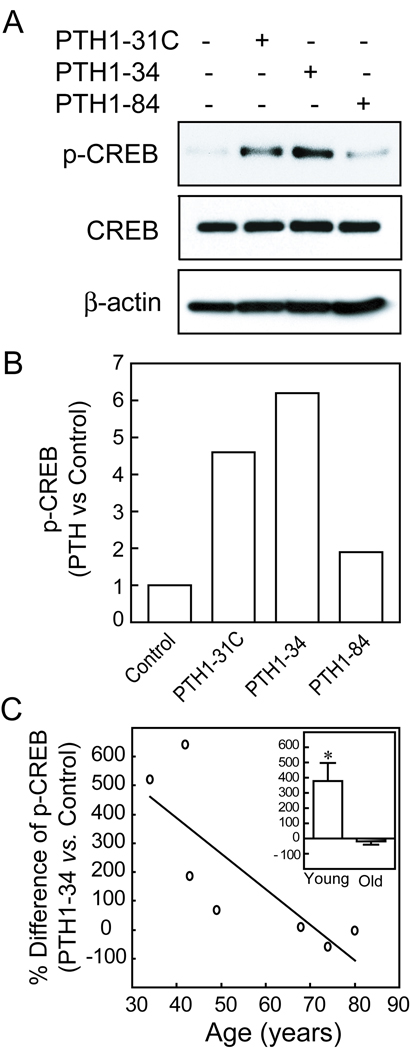
All three PTH peptides (hPTH1–34, hPTH 1-31C, and hPTH1–84) activated CREB signaling in hMSCs at concentrations of 10 nM, with increased phosphorylation of CREB at 6 hours, as shown by Western immunoblot (Fig. 2A, B).
FIG. 2.
Effects of age on CREB activation by PTH peptides in hMSCs. (A) Western immunoblot shows that after 6 hours treatment, hPTH1-31C, hPTH1–34, and hPTH1–84 (10 nM) increased phospho-CREB levels, but not CREB, in hMSCs (representative immunoblot with hMSCs from a 34-year-old female subject). (B) Quantitative data from Western immunoblot are presented as fold-difference of p-CREB with PTH treatment compared with vehicle control after normalization to each β-actin internal control. (C) The effect of PTH1–34 on p-CREB was inversely correlated with subject age (Pearson r=−0.80, p=0.0294, n=7). INSET: The mean effect of PTH1–34 on p-CREB was significantly higher in the young group (<50 years, n=4) than in the group of subjects older (>55 years, n=3) (*p<0.05, Mann-Whitney test).
There was an age-related decline in the magnitude of activation of phospho-CREB by PTH1–34 in hMSCs (Pearson r=−0.80, p=0.029, n=7) (Fig. 2C). The mean activation of phospho-CREB was significantly greater in hMSCs from four younger subjects (379% ± 238, compared with untreated control) than in hMSCs from three older subjects (−19% ± 37) (Fig. 2C, Inset; p<0.05, Mann-Whitney test).
PTH stabilization of β-catenin is diminished in hMSCs from elders
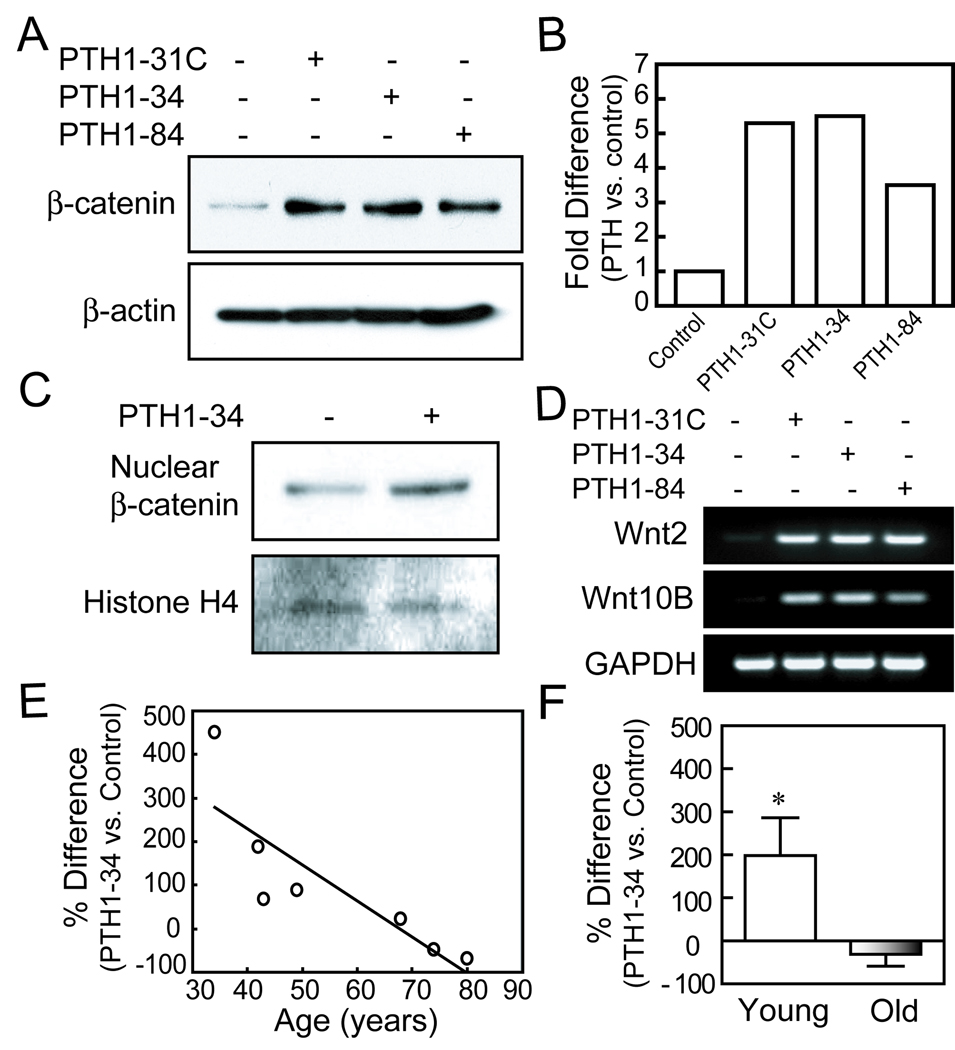
All three PTH peptides increased the levels of β-catenin, a key member in the canonical Wnt signaling pathway, as shown by Western immunoblotting. PTH1–34, PTH1-31C, and PTH1–84 (10 nM) increased total β-catenin levels at 6 hours to a similar degree (Fig. 3A, B). PTH1–34 was shown to increase nuclear β-catenin in hMSCs, as demonstrated by Western immunoblot (a representative result was shown in Fig. 3C). In addition, all PTH peptides upregulated expression of canonical Wnt2 and Wnt10B genes at 3 days (Fig. 3D).
FIG. 3.
Effects of age on stabilization of β-catenin by PTH analogs in hMSCs. (A) Western immunoblot shows that after 6 hours treatment, PTH1-31C, 1–34, and 1–84 (10 nM) increased β-catenin levels in hMSCs (representative immunoblot with hMSCs from a 34-year-old female subject). (B) Quantitative data from Western immunoblots are presented as fold-difference of β-catenin with PTH treatment compared with vehicle control after normalization to each β-actin internal control. (C) PTH1–34 (10 nM) increased nuclear β-catenin in hMSCs as shown by Western immunoblot with equal loading of 10 µg in each sample. Histone H4 served as an internal control. (D) Electrophoretogram of RT-PCR products shows upregulation of Wnt2 and Wnt10B genes in hMSCs (34F), after 3 days treatment with PTH analogs (10 nM) in osteogenic medium. GAPDH was used as an internal control. (E) The effect of PTH1–34 on β-catenin in hMSCs was inversely correlated with subject age (Pearson r=−0.84, p=0.018, n=7). (F) The mean effect of PTH1–34 on β-catenin was significantly higher in the group younger than 50 years (n=4) than in the group of subjects older than 55 years (n=3) (*p<0.05, Mann-Whitney test).
There was an age-related decline in the effect of PTH1–34 on total β-catenin accumulation (Fig. 3E, Pearson r=−0.84, p=0.018, n=7). The mean accumulation of β-catenin by PTH1–34 was significantly greater in hMSCs from four younger subjects (198% ± 176, compared with untreated control) than in hMSCs from 3 older subjects (−32% ± 48) (Fig 3F; p<0.05, Mann-Whitney test).
PTH stimulation of osteoblast differentiation is diminished in hMSCs from elders
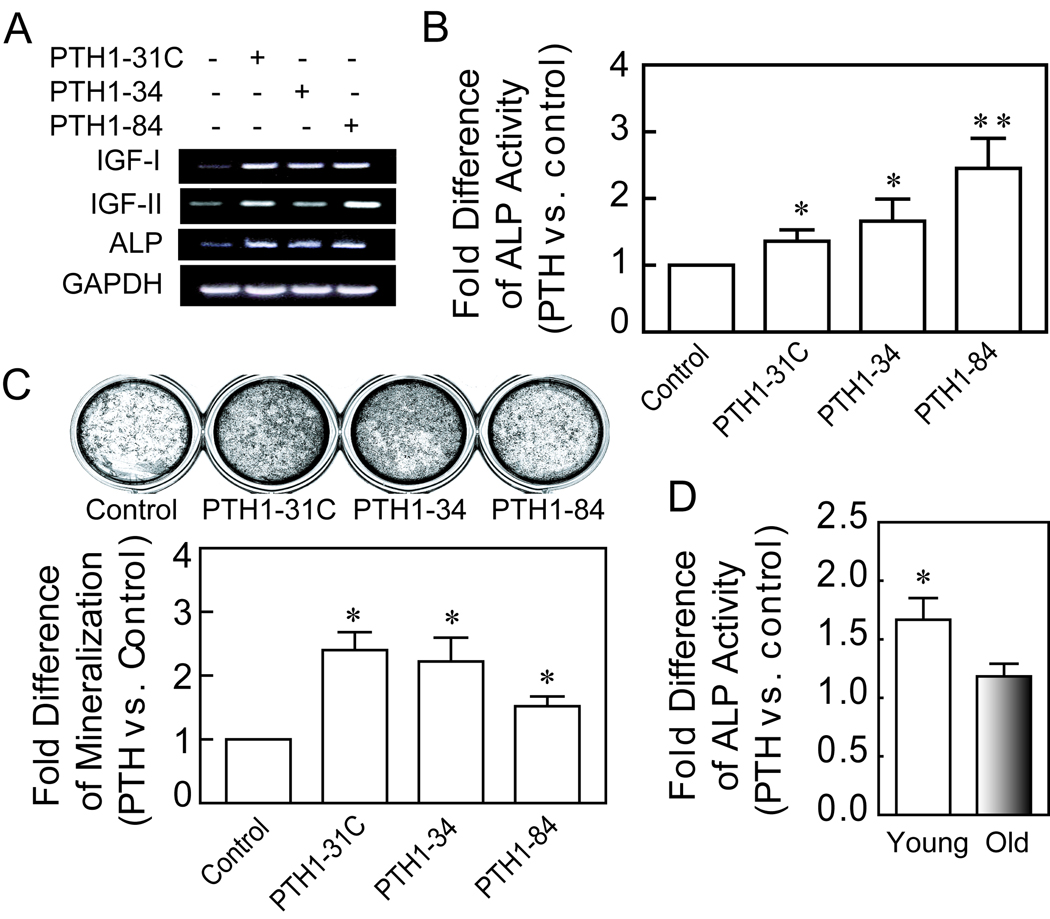
The effects of PTH peptides (10 nM) on osteoblast differentiation with hMSCs were analyzed by expression of ALP and IGF genes at day 3, by biochemical measurement of activity of the osteoblast signature enzyme, alkaline phosphatase (ALP) at day 7, and by measurement of mineralization at day 21. For these studies, confluent cultures of hMSCs were incubated with osteogenic medium, with and without 10 nM of PTH1–34, PTH1-31C, or PTH1–84, added with fresh medium twice weekly. Analysis of gene expression in hMSCs after 3 days treatment in osteogenic medium revealed that all PTH peptides increased IGF-I, IGF-II, and ALP gene expression (Fig. 4A). For the longer duration studies, serum concentration was used at 1% to minimize possible differences in proliferation. At 7 days, all PTH peptides significantly stimulated ALP activity in hMSCs (Fig. 4B). At 21 days, all PTH peptides significantly enhanced mineralization of the cultures (Fig. 4C). The fold-effects of PTH 1-31C, 1–34, and 1–84 on mineralization were, respectively, 2.4, 2.2, and 1.5 (*p<0.05, compared with untreated control, ANOVA).
FIG. 4.
Effects of age on stimulation of osteoblastogenesis by PTH peptides in hMSCs. (A) Electrophoretogram of RT-PCR products shows upregulation of IGF-I, IGF-II and ALP genes in hMSCs (34F), after 3 days treatment with PTH peptides (10 nM) in osteogenic medium. GAPDH was used as an internal control. (B) The PTH peptides (10 nM) produced significant increases in ALP activity at 1 week (*p<0.05, **p<0.01, ANOVA). (C) The PTH peptides (10 nM) produced significant increases in mineralization at 3 weeks (*p<0.05, ANOVA). (D) PTH1–34 (10 nM) stimulated ALP activity, compared with control, set at 1.0, in cells from young subjects (<50-year-old, n=5) 3.7-fold more than in cells from older subjects (>55-year-old, n=7) (p=0.042, Mann-Whitney test).
Treatment with 10 nM PTH1–34 resulted in a significant 67% increase in ALP activity in hMSCs obtained from younger subjects (n=5), compared with an 18% increase in the cells obtained from elders (n=7). The 3.7-fold difference in stimulation in hMSCs from young and older subjects was statistically significant (Fig. 4D; p=0.042, Mann-Whitney test).
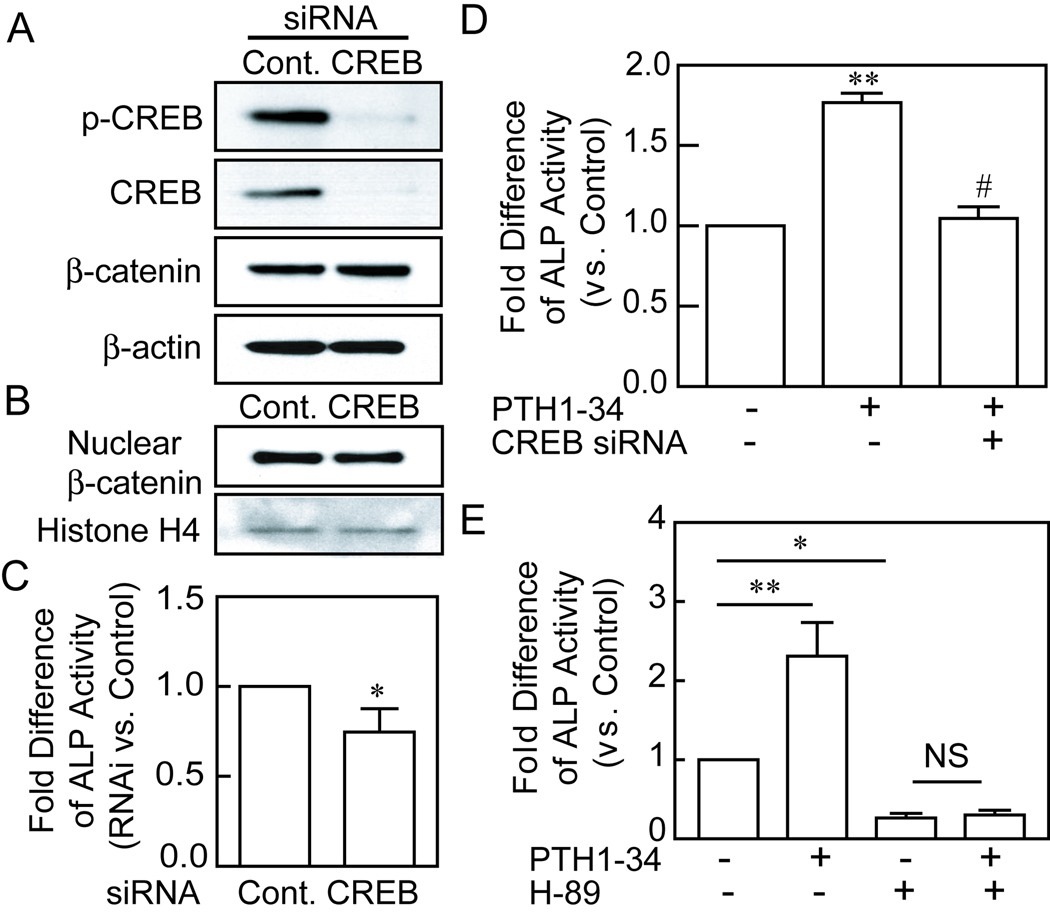
CREB is necessary for PTH stimulation of osteoblastogenesis in hMSCs
To model for the role of diminished CREB signaling in the age-related decline in PTH stimulation of osteoblast differentiation, we transfected CREB siRNA and control siRNA into hMSCs obtained from a 47-year-old female subject. The gene-specific transfection with CREB siRNA obliterated the levels of phospho-CREB and CREB, without significant effects on the baseline level of β-catenin protein (Fig. 5A) or nuclear β-catenin (Fig. 5B). Knockdown of CREB with 100 pmole of siRNA resulted in significantly lower baseline osteoblast differentiation after 7 days in osteoblastogenic medium, as measured by ALP activity (Fig. 5C, p<0.05). There was a similar decrease in ALP activity after CREB knockdown in hMSCs obtained from a 69-year-old female subject (data not shown). The stimulation of osteoblast differentiation by PTH1–34 was completely blocked in the cells with CREB knockdown (Fig. 5D). This shows that an intact CREB pathway is necessary for PTH stimulation of osteoblast differentiation with hMSCs.
FIG. 5.
The roles of CREB and PKA in PTH stimulation of osteoblastogenesis. (A) Western immunoblot shows absence of p-CREB and CREB proteins after transfection of CREB siRNA (100 pmole per million cells) into hMSCs (from a 47-year-old woman), compared with transfection with control siRNA. (B) Knockdown of CREB did not significantly affect nuclear β-catenin in hMSCs as shown by Western immunoblot with equal loading of 30 µg of each nuclear protein sample. Histone H4 served as an internal control. (C) There was a significant reduction in baseline generation of alkaline phosphatase (ALP) activity in hMSCs after transfected with CREB siRNA (*p<0.05, relative to control siRNA, t-test). (D) Stimulation of ALP activity by 10 nM of PTH1–34 was absent in hMSCs with CREB knockdown (**p<0.001, PTH vs. control; #p<0.001, PTH/CREB siRNA vs. PTH; ANOVA). (E) The PKA inhibitor H-89 (5 µM) antagonized the stimulation of ALP activity by PTH1–34 in hMSCs from a 42-year-old woman (**p<0.01, PTH vs. control; *p<0.5, H-89 vs control; ANOVA).
The role of PKA in osteoblast differentiation was evaluated with a selective inhibitor of protein kinase A (H-89, 10 µM). Blocking PKA pathway with H-89 diminished the stimulation of ALP activity by PTH1–34 (p<0.01, PTH vs. control; no significant difference between H-89 plus PTH vs. H-89; n=4, ANOVA) (Fig. 5E). Treatment with H-89 alone also reduced control levels of ALP activity (p<0.05, H-89 vs. control). These results suggest that PKA is necessary for PTH stimulation on osteoblastogenesis in hMSCs.
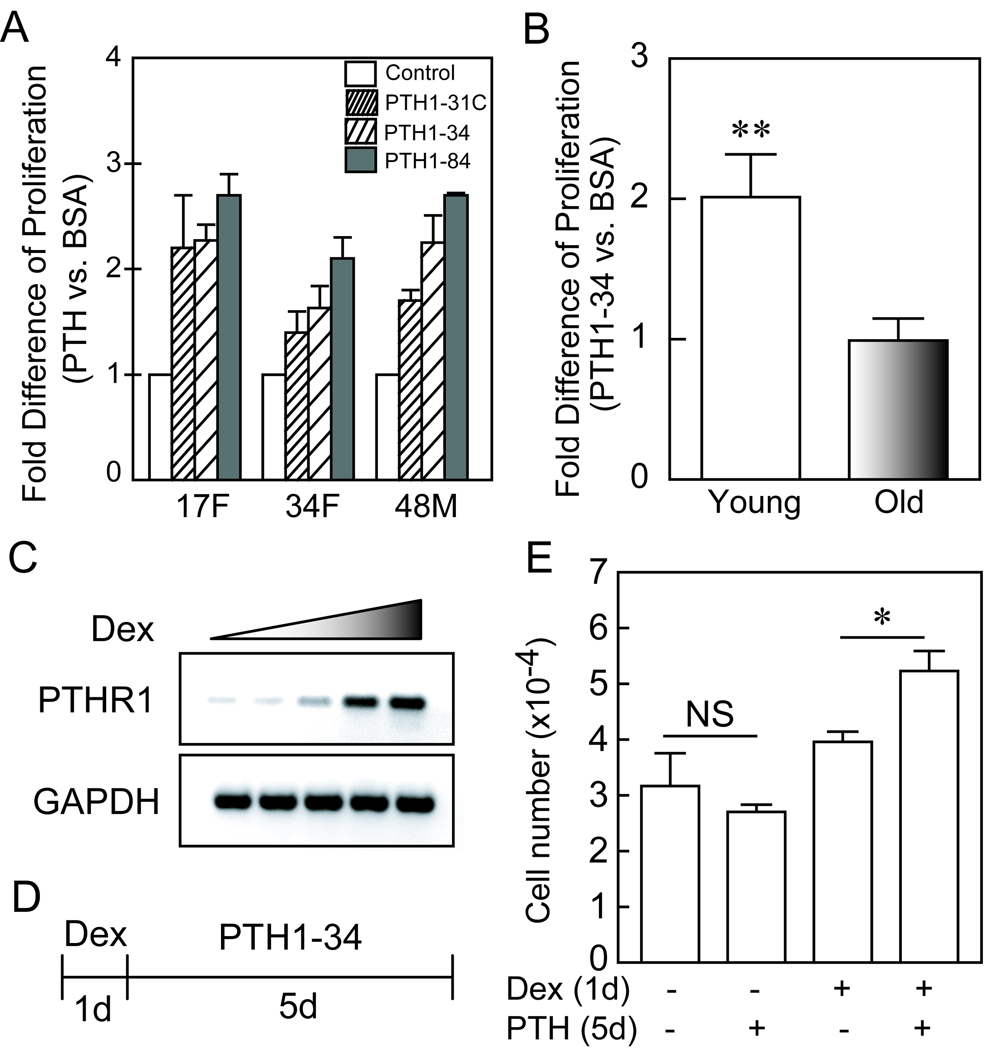
Restoring PTH/PTHrP receptor type I in hMSCs of elderly subjects rescues PTH stimulation of proliferation
For assessment of effects of PTH on the numbers of cells, hMSCs were cultured in MEM-α with 10% FBS-HI and antibiotics. Effects of PTH were evaluated with short-term (5-day) cultures of freshly isolated hMSCs from young and older subjects. We tested the effect of 10 nM of each of three PTH peptides (PTH1–34, 1-31C, and 1–84) on proliferation of hMSCs. All three PTH peptides significantly increased the numbers of hMSCs, compared with control medium (Fig. 6A). For a series of 3 samples, the mean stimulation of proliferation was greatest with PTH1–84 (250% ± 20), less with PTH1–34 (205% ± 21), and least with PTH1-31C (177% ± 23). The differences between the peptides were not statistically significant, with the numbers of samples tested. Treatment with PTH1–34 resulted in an average of twice as many cells in cultures of hMSCs from young subjects (n=4), but had no effect on hMSCs from elders (n=7) (Fig. 6B).
FIG. 6.
Restoring PTH/PTHrP receptor type I rescues the stimulation of PTH on proliferation in hMSCs of elderly subjects. (A) All three PTH peptides significantly increased the numbers of hMSCs, compared with control medium (p<0.05, Mann-Whitney Test). (B) Treatment with PTH1–34 (10 nM) resulted in an average of twice as many cells in cultures of hMSCs from young subjects (n=4), but had no effect on hMSCs from elders (n=7) (p=0.0061, Mann-Whitney test). (C) After 24 hours treatment, dexamethasone (Dex; 0, 1, 10, 100, and 1000 nM) dose-dependently up-regulated PTH/PTHrP receptor type I (PTHR1) gene expression in hMSCs (from a 57-year-old male subject); GAPDH was used as an internal control in RT-PCR. (D) The hMSCs were pre-treated with 100 nM Dex or ethanol control for 24 hours, and then treated with 10 nM PTH1–34 or BSA control in fresh MEM-α with 10% FBS-HI for 5 days. (E) Dexamethasone rescued the effect of PTH1–34 (10 nM) on numbers of hMSC (from a 70-year-old female subject) (*p<0.05, PTH vs BSA control in Dex pre-treated group; no significant difference between PTH and BSA control in ethanol pre-treated group; ANOVA).
There was a significant age-related decline in constitutive expression of PTHR1 gene in hMSCs (Fig. 1). Because it is known that dexamethasone (Dex) can up-regulate PTHR1 gene expression in cells such as UMR-106 osteoblast-like cells (Haramoto et al., 2007), we tested the effects of 24 hours of treatment with different doses of Dex (0, 1, 10, 100, and 1000 nM) on PTHR1 gene expression in hMSCs obtained from a 57-year-old male subject (Fig. 6C). Similar results were obtained with hMSCs from another subject (Data not shown). The hMSCs from a 70-year-old female subject were pre-treated with 100 nM dexamethasone or ethanol-control for 24 hours, and then transferred to fresh MEM-α with 10% FBS-HI for 5 days with either 10 nM PTH1–34 or BSA control (Fig. 6D). There was no effect of PTH on cell number in control hMSCs (pre-treated with ethanol), but PTH significantly increased cell number with the hMSCs that were pre-treated with Dex (Fig. 6E; p<0.05, PTH vs BSA control in Dex pre-treated group; no significant difference between PTH and BSA control in ethanol pre-treated group; ANOVA). Similar results were obtained with hMSCs from a 57-year-old female subject (Data not shown).
Discussion
Recombinant human parathyroid hormone PTH1–34 was the first anabolic agent available in USA for treatment of osteoporosis, and recombinant human PTH1–84 is available in Europe (Deal, 2009; Lane & Silverman, 2010). Intermittent administration of PTH has anabolic skeletal effects and reduces fracture risk in postmenopausal women with osteoporosis, but the cellular and structural mechanisms by which these effects are mediated have not been fully established (Compston, 2007). The native human parathyroid hormone PTH1–84, a fragment hPTH1–34, and an analog hPTH1-31C potently stimulate bone growth, reinforce bone microstructure weakened by estrogen deprivation, reduce further fracturing, and may also promote the repair of existing fractures and implant anchorage in both healthy and osteoporotic humans (Whitfield, 2006). Rat enterocytes have been used as a model to characterize the effects of aging on PTH regulation of intestinal signal transduction and growth (Gentili et al., 2003; Russo de Boland 2004). Our novel studies with human cells document and begin to unravel the mechanisms of age-related declines in the effects of PTH peptides on intracellular signaling and osteoblast differentiation in hMSCs.
PTH is a peptide ligand that initiates its effects by interacting with the heterotrimeric G protein-coupled PTH/PTHrP receptor type 1 (PTHR1); this receptor is characterized by seven transmembrane domains, a long amino-terminal extracellular domain, and a long carboxyl-terminal intracellular tail (Abou-Samra et al., 1992). It was reported that PTHR1 protein expression in rat intestinal cells decreased with aging (Russo de Boland, 2004). Although there was a range of expression in younger subjects, we found an significant age-related decline in constitutive gene expression of PTHR1 in hMSCs. This may explain the diminished functional responses in hMSCs obtained from elders to PTH activation of CREB and stabilization of β-catenin signaling and its stimulation of osteoblastogenesis.
We assessed hMSC response to PTH by measurement of phosphorylated CREB, a component in the PTH signal pathway. Activation of p-CREB was dramatically diminished in hMSCs from elders. Other studies with osteoblast-like cells from rats (Egrise et al., 1992; Qin et al., 2004) and humans (Wong et al., 1994) showed an age-related decrease in cAMP accumulation in response to PTH. It was suggested that with aging there is a decreased sensitivity of rat osteoblastic cells to PTH (Donahue et al., 1997).
Our data show that PTH peptides activated canonical Wnt signaling as demonstrated by increased stabilization of total β-catenin, a key member in the canonical Wnt signaling pathway and its nuclear accumulation , and PTH1–34 increased the stabilization of β-catenin in hMSCs from young subjects, but not in hMSCs from elders. In addition, these PTH peptides upregulated expression of canonical Wnt2 and Wnt10B genes in hMSCs from a 34-year-old subject; this suggest a possible autocrine mechanism for PTH activation of the Wnt/β-catenin signaling pathway. The Wnt signaling pathway is activated by PTH in rat osteoblastic osteosarcoma cells (Kulkani et al., 2005), rat osteoblast (Kulkarni et al., 2005; Wan et al., 2008), human osteoblasts (Suzuki et al., 2008), and in rats (Li et al., 2007; Wan et al., 2008) and mice (Tobimatsu et al., 2006; Guo et al., 2010; Weinstein et al., 2010; Jilka et al., 2010). Clinical investigations show that mutations in LRP-5, a Wnt co-receptor, are associated with bone mineral density and fractures (Gong et al., 2001; Little et al., 2002; Boyden et al., 2002). Studies of knockout and transgenic mouse models for Wnt pathway components, such as Wnt-10b, LRP-5/6, secreted frizzled-related protein-1, dickkopf-2, Axin-2, and β-catenin, demonstrated that canonical Wnt signaling modulates many aspects of osteoblast physiology including proliferation, differentiation, bone matrix formation, mineralization, and apoptosis, as well as coupling to osteoclastogenesis and bone resorption (Bodine and Komm, 2006). It was reported that canonical Wnts, (Wnt1, Wnt2, and Wnt3a) increased ALP activity in murine C3H10T1/2 and ST2 cells, and that non-canonical Wnts, i.e. Wnt5a and Wnt4Shimizu, H., Julius, M.A., Giarre, M., Zheng, Z., Brown, A.M. and Kitajewski, J., 1997. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 8, pp. 1349–1358. View Record in Scopus | Cited By in Scopus (215), did not increase ALP activity (Gong et al., 2001). Wnt10b, a canonical Wnt, increased postnatal bone formation by enhancing osteoblast differentiation in mice (Bennett et al., 2005 & 2007). Canonical Wnt signaling appears to either suppress or promote osteoblastogenesis of MSCs that may depend on differences in the cellular background, the species employed, the experimental conditions and stimuli applied, the level of Wnt activity, or the stage of target MSCs (Ling et al., 2009). Our recent data show that activation of canonical Wnt signaling with LiCl, a surrogate for Wnt treatment, and/or TGF-β1 inhibit osteoblastogenesis in hMSCs (Zhou, 2011). Detailed analysis of constitutive expression of Wnt genes in hMSCs revealed age-related decreases in canonical Wnt 2, 7B, 13, and 14, and other gender-specific differences (Shen et al., 2009). It was reported that PTH-PKA signaling facilitates canonical Wnt effects by inactivation of glycogen synthase kinase-3β in human osteoblastic Saos-2 cells (Suzuki et al., 2008) and that PTH needs PKA to stabilize β-catenin in murine osteoblasts and osteoclasts (Wan et al., 2008; Romero et al., 2010). Our data show that PTH stabilized and increased nuclear accumulation of β-catenin and, because knockdown of CREB did not significantly change the level of β-catenin, it appears that PTH-PKA-GSK-3β signaling involves stabilization of β-catenin independent of CREB in hMSCs.
Our data show that the three PTH analogs stimulated osteoblast differentiation of hMSCs as demonstrated by up-regulation of ALP gene expression, ALP activity, and matrix mineralization. By stimulating osteoblast function in vivo, anabolic PTH increases bone formation on cancellous, endocortical, and periosteal bone surfaces (Kousteni & Bilezikian, 2008). IGF-I is required for PTH’s anabolic effects in mice, although stimulation of some osteoblast markers was found to be independent of IGF (Bikle et al., 2002). Other studies with mouse marrow cultures showed that intermittent but not continuous PTH1–34 increased IGF-I mRNA and osteoblast differentiation (Locklin et al., 2003). The IGF-IR null mutation in mature murine osteoblasts results in less bone and decreased bone formation, in part because of the requirement for the IGF-IR in mature osteoblasts to enable PTH to stimulate osteoprogenitor cell proliferation and differentiation (Wang et al., 2007). Different pathways mediate different effects of PTH. In bone cultures, PTH stimulation of collagen synthesis required IGF-I, but its mitogenic effects did not (Canalis, et al. 1989). We recently showed that IGF-I mediates some of the effects of PTH1–34 on vitamin D synthesis and osteoblast differentiation in hMSCs (Geng et al., 2010). This study showed that PTHs increase IGF-I and IGF-II gene expression in hMSCs; this is consistent with the role of IGF as a potential mediator of the osteoanabolic effects of PTH in human osteoblast progenitors.
We (Mueller & Glowacki, 2001; Zhou et al., 2008) and others (D'Ippolito et al., 1999; Nishida et al., 1999) reported that there is an age-related decline in osteoblast differentiation with hMSCs. This study indicates that PTH stimulation of osteoblast differentiation with hMSCs declined with age, consistent with the age-related decline in PTH receptor expression and PTH signaling. It is important to emphasize that these studies were conducted with 10 nM PTH, a concentration that stimulated cells from younger subjects significantly more than cells from older subjects. This concentration was used to reveal the mechanism of the age-related effect. This concentration is in comparison with our other studies that used 100 nM PTH1–34 to acutely stimulate vitamin D synthesis in hMSCs from an 83-year-old subject (Geng et al., 2010). The higher concentration may overcome lower receptor levels in cells from elders to some degree, but there are no human studies available about the effects of age on dose responsiveness to PTH. Jilka et al. recently showed in vivo and in vitro conditions in which PTH rescued some of the skeletal effects of age in mice (Jilka et al., 2010). They reported that daily injections of PTH for 28 days produced greater increases in vertebral bone mineral density and the rate of bone formation in the more deficient bones of 26-month mice, compared with 6-month-old mice. Further, 50 nM PTH treatment in vitro protected osteoblasts from acute oxidative-stress-related effects.
We used genetic and pharmacological approaches to determine the roles of CREB in osteoblast differentiation. Both knockdown of CREB and inhibition of PKA decreased basal differentiation of osteoblasts. In addition, knockdown of CREB and inhibition of PKA with H-89 (Lee & Lorenzo, 2002) obliterated the stimulation of ALP by PTH; this indicates that PKA/CREB signaling is necessary for PTH to stimulate osteoblastogenesis. These findings suggest that the mechanism by which PTH stimulates osteoblastogenesis in hMSCs may involve the PTH-PTHR1-PKA-CREB axis.
It was necessary to assess the effects of PTH on cell numbers. If PTH affects the proliferation or viability of cells to different degrees, interpretation of other effects may be misinterpreted. In addition, proliferating cells may not express differentiation genes. These studies indicate that all three PTH peptides increased the numbers of hMSCs, but do not reveal the signaling mechanism(s) for those effects. Experimental evidence indicates a role for PKC in PTH stimulation of cell numbers. The effects of PTH1–31 on membrane-associated PKC activity was shown to depend on the cell type (Jouishomme et al., 1994; Whitfield et al., 1999). It was shown for rat osteoblast-like cells (Swarthout et al., 2001 & 2002), opossum kidney cells (Cole, 1999), and human periodontal ligament cells (Lossdorfer et al., 2006) that stimulation of proliferation by PTH is PKC-dependent.
Previously, we reported that there is an age-related decline in proliferation in hMSCs (Zhou et al., 2008). This study showed that PTH stimulation of proliferation in hMSCs declined with age, consistent with the age-related decline in PTH receptor expression. After up-regulation of PTH/PTHrP receptor type I with dexamethasone, hMSCs from elderly subjects were rescued their response to PTH1–34 stimulation on proliferation. These findings suggest the possibility of restoring sensitivity to osteoanabolic PTH in hMSCs from elders by correcting age-related dysregulation of PTH signaling.
In sum, the effects of PTH peptides on activation of CREB, β-catenin, osteoblastogenesis, and proliferation were diminished in hMSCs from elders. The age-related decline in PTH/PTHrP receptor gene expression in hMSCs may explain these observations. PTH stimulated osteoblastogenesis in hMSCs from young subjects by activating CREB and IGF and required PKA signaling pathway. With genetic and pharmacological means, we found that the effects of age were reproduced by blocking PKA or CREB signaling. Restoring PTH/PTHrP type I receptor rescued the stimulation of PTH on hMSCs of elderly subjects. These studies begin to define the mechanisms by which age influences the osteoanabolic effects of PTH. Intrinsic alterations in signaling responses to hormones and drugs may explain cellular and tissue aging of the human skeleton. These findings suggest the possibility of restoring sensitivity to osteoanabolic PTH in hMSCs from elders by correcting the age-related dysregulation of PTH signaling.
Experimental Procedures
Reagents
Human PTH1–34 was purchased from Bachem Americas, Inc. (Torrance, CA) and human PTH1–84 was purchased from American Research Products (Belmont, MA). Human PTH1-31C (Ostabolin-C, Leu27, Cyclo[Glu22-Lys26]-hPTH1-31NH2) was a gift from Zelos Therapeutics (West Conshohocken, PA). They were dissolved in 0.1% BSA in phosphate-buffered saline (PBS). The specific protein kinase A inhibitor, H-89 (Lee & Lorenzo, 2002), was obtained from Sigma (MO, USA). It was dissolved as stock solutions in dimethylsulfoxide (DMSO).
Preparation of Human Marrow Stromal Cells (hMSCs)
Bone marrow samples were obtained with IRB approval as femoral tissue discarded during orthopedic surgery for non-inflammatory conditions, with inclusion and exclusion criteria as described previously (Zhou et al., 2010). Low-density marrow mononuclear cells were isolated by density centrifugation on Ficoll/Histopaque 1077 (Sigma, MO) (Zhou et al., 2008). This procedure enriches for undifferentiated cells and includes a population of non-adherent hematopoietic cells and a fraction capable of adherence and differentiation into musculoskeletal cells. Non-adherent cells were removed at 24 hours after seeding and the adherent human MSCs were expanded in monolayer culture with phenol red-free α-MEM medium, 10% Fetal Bovine Serum-Heat Inactivated (FBS-HI), 100 U/mL penicillin, and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA). We define passage 0 as when the initial hMSCs proliferate to ∼80% confluence. At each passage, contents of each dish were divided into 5 dishes. In each experiment, standardized conditions were used: passage 2, identical seeding density, and identical reagents.
To determine whether there are intrinsic effects of in vivo age on hMSCs, we used hMSCs at passage 2. This approach avoids changes in cell behaviors that are associated with prolonged culture, such as in vitro senescence or culture stress (Zhou et al., 2008). Like other normal mammalian cells, when cultured for many passages, MSCs display what is termed “in vitro senescence”, i.e. decreased proliferation, replicative quiescence, enlargement, increase in SA-β-gal activity, and erosion of telomeres (Fehrer & Lepperdinger, 2005; Sethe et al., 2006). Further, there is a link between the accumulation of DNA damage and loss of multipotency of human MSCs with time of culture (Alves et al., 2009). Cellular senescence can contribute to the physiological processes of normal organismal aging (Jeyapalan & Sedivy, 2008). The results obtained herein, however, should reflect the effects of in vivo age because cells from young and old individuals were treated the same way and evaluated at early passage.
RNA Isolation and RT-PCR
Total RNA was isolated from human MSCs with Trizol reagent (Invitrogen). For RT-PCR, 2 µg of total RNA was reverse-transcribed into cDNA with SuperScript II (Invitrogen), following the manufacturer’s instructions. Concentration of cDNA and amplification conditions were optimize to reflect the exponential phase of amplification. In general, one-twentieth of the cDNA was used in each 50 µL PCR reaction (30–40 cycles of 94° C for 1 minute, 55–60° C for 1 minute, and 72° C for 2 minutes) as described (Zhou et al., 2008). The gene-specific primers for human ALP (Winn et al., 1999), IGF-I (Gordeladze et al., 2002), IGF-II (Sayer et al., 2005), and PTH/PTHrP type I receptor (PTHR1) (Winn et al., 1999) were used for amplification. PCR products were quantified by densitometry of captured gel images with KODAK Gel Logic 200 Imaging System and measured by KODAK Molecular Imaging Software, following the manufacturer’s instructions (KODAK, Molecular Imaging Systems, New Haven, CT, USA). Quantitative data were expressed by normalizing the densitometric units to GAPDH (internal control).
Western Immunoblot
Human MSCs were cultured in 100-mm dishes. After confluence, the medium was changed to α-MEM with 1% FBS-HI for 2 days. The cells were treated with 10 nM of human PTH analogs (hPTH1–34, hPTH1-31C, or hPTH1–84) for 6 hours before harvest for whole-cell or nuclear proteins. Pilot time-course experiments showed a peak in signaling at 6 hours. The whole-cell lysates were prepared with high salt lysis buffer containing 150 mM NaCl, 3 mM NaHCO3, 0.1% Triton x-100 and a mixture of protease inhibitors (Roche Diagnostics, CA). The whole-cell lysates were homogenized with a Kontes’ Pellet Pestle and separated from insoluble cell materials by centrifugation at 16,000 g in a bench-top Eppendorf centrifuge for 15 minutes at 4° C. Nuclear fractions were obtained according to a protocol modified from Hocevar et al., 2003 (Zhou et al., 2004 & 2005). In brief, cells were homogenized in a 2-ml Dounce homogenizer with hypotonic lysis buffer (10 mM Tris-HCl, pH 7.4, 1x Roche cocktail, 5 mM NaF, 2 mM Na3VO4). The lysates were transfered into a 1.5-ml Eppendorf tube and centrifuged for 10 min at 3300 rpm (900×g) in Eppendoff 5415C at 4°C. The supernatants were discarded and the nuclear pellets were re-suspend with high salt lysis buffer and homogenized with a Kontes’ Pellet Pestle as described in whole-cell lysates. Protein concentrations were determined with the BCA system (Pierce, Rockford, IL). Proteins were resolved on 4–12% SDS-PAGE (NuPAGE® Bis-Tris gel, Invitrogen) and transferred onto PVDF membranes (Amersham Biosciences, UK). The membranes were blocked with 5% nonfat milk in PBS buffer containing 0.1% Tween-20 (PBST-M) for 2–3 hours at room temperature, and then incubated with primary antibodies at 4° C for overnight. The primary antibodies anti-β-catenin (E5) and anti-histone H4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); anti-phospho-CREB-1 (Ser133) was purchased from Millipore (Billerica, MA); anti-CREB was purchased from Cell Signaling (Danvers, MA); and anti-β-actin was purchased from Sigma (St. Louis, MO). After removal of unbound primary antibodies by three 10-minute washes with PBST, the membranes were incubated with Horseradish Peroxidase (HRP)-conjugated secondary antibodies for 1 hour in PBST-M at room temperature and washed 3×10 minutes with PBST. The anti-mouse IgG-HRP was purchased from Amersham, and anti-rabbit IgG-HRP was purchased from Santa Cruz Biotechnology. The antibody-associated protein bands were revealed with the ECL-plus Western blotting system (Amersham Biosciences, UK).
Osteoblast Differentiation Assays
For each sample assayed for osteogenic gene expression by RT-PCR, cells were cultured in 100-mm dishes in α-MEM with 10% FBS-HI until confluence; this required different times depending upon age and rates of proliferation. Upon confluence, medium was changed to osteogenic medium (phenol red-free α-MEM with 10% charcoal-stripped FBS-HI, 100 U/mL penicillin, 100 µg/mL streptomycin plus 5 mM β-glycerophosphate, 50 µg/mL ascorbate-2-phosphate) with or without 10 nM of human PTH analogs (hPTH1-31C, hPTH1–34, or hPTH1–84) for 3 days.
For each sample assayed for Alkaline Phosphatase (ALP) activity and mineralization, 2×104 cells/well were first seeded in triplicate in 12-well-plates or quadruplicate in 24-well-plates in α-MEM with 10% FBS-HI. The next day, the medium was replaced with osteogenic medium (α-MEM with 1% FBS-HI, 100 U/mL penicillin, 100 µg/mL streptomycin plus 10 nM dexamethasone, 5 mM β-glycerophosphate, 50 µg/mL ascorbate-2-phosphate) with or without 10 nM of PTHs. Reduction of serum to 1% for differentiation was designed to minimize possible differences in proliferation during the experiment that could confound interpretation of effects on osteoblastogenesis. Alkaline Phosphatase activity was assayed after 7 days of treatment with PTHs; data were calculated as hydrolysis of p-nitrophenyl phosphate (µmole) per minute at 37 °C per gram of total cellular protein and expressed as fold-difference for each treatment/control (Zhou et al., 2008). Mineralization was assayed after 21 days of treatment (Gregory et al., 2004) with data calculated as nmole/well of Alizarin Red S and expressed as fold-difference for each treatment/control.
Enumeration of Cells
Human MSCs were seeded at 1×104 cells in each of three 35-mm dishes in α-MEM with 10% FBS-HI. After overnight incubation, cells were treated with vehicle control (0.1% BSA in PBS) or 10 nM of human PTH peptides (hPTH1–34, hPTH1-31C, or hPTH1–84). After 5 days, cells were suspended with 0.5 ml of 0.05% trypsin-EDTA (Invitrogen) and cell number was determined by hemacytometer.
Transient Transfection of CREB siRNA
Transient transfection of CREB siRNA (Stealth RNAi duplex siRNA, Invitrogen) into hMSCs was performed by electroporation with the Human MSC Nucleofector Kit (Lonza, MD) according to the manufacturer’s instruction and as described (Aslan et al., 2006). In brief, hMSCs were harvested by trypsinization and resuspended at one million cells in 100 µL of human MSC nucleofector solution with either 10 or 100 pmole of CREB siRNA or control siRNA (SiRNA-A, Santa Cruz Biothech., Inc.). Electroporation was performed in Nucleofector™ II with program U-23 provided by Lonza/Amaxa Biosystems (Lonza, MD). Immediately after electroporation, the cells were transferred to 6-well plates for Western immunoblot assays or to 12-well plates. They were incubated with complete growth medium until confluence, and then the medium was changed to osteogenic medium for 7 days for ALP enzyme assays.
Statistical Analyses
All experiments were performed at least in triplicate. Group data were presented as mean values ± SEM. Quantitative data were analyzed by GraphPad InStat® (GraphPad Software, Inc.). When the data were validated for Gaussian distributions, parametric tools were used, either group comparisons by t-test with Welch correction or multiple comparisons by one-way ANOVA followed by Tukey post hoc analysis, or Pearson correlation coefficient. Otherwise, non-parametric tools were used, either the Mann-Whitney test for group comparisons or Spearman correlation test. All analyses used unpaired and two-tail methods; a value of p<0.05 was considered significant.
Acknowledgments
This study was supported by grants from the National Institutes of Health R01 AG 025015 and R01 AG 028114, a research grant from Zelos Therapeutics, Inc., PA, USA (to J. G.), and the American Federation for Aging Research A09052 (to S. Z.). The discarded marrow was obtained and studied with approval and annual review from the Partners Human Research Committee.
Footnotes
This study was presented in part at the American Society for Bone and Mineral Research 2009 Annual Meeting, Denver, Colorado, USA. The authors greatly appreciate help from K. D. Johnson and S. Anderson for aspects of these experiments, and Dr. Regina O’Sullivan for critically reviewing the manuscript.
Author Contributions
SZ and JG were responsible for the conception, design, data analysis, interpretation of the data, and preparing the manuscript and figures. SZ, EMB, SWK, IA, LS, JH, and IB performed different experiments. PM contributed to experimental design comparing effects of the PTH peptides. All authors read and approved the final manuscript.
References
- Abdallah BM, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J. Cell. Physiol. 2009;218:9–12. doi: 10.1002/jcp.21572. [DOI] [PubMed] [Google Scholar]
- Alves H, Munoz-Najar U, de Wit J, Renard AJ, Hoeijmakers JH, Sedivy JM, van Blitterswijk C, de Boer J. A link between the accumulation of DNA damage and loss of multipotency of human mesenchymal stromal cells. J. Cell. Mol. Med. 2009 doi: 10.1111/j.1582-4934.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, Li JZ, Helm GA, Gazit D, Gazit Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J. Bone Miner. Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J. Bone Miner. Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev. Endocr. Metab. Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J. Clin. Invest. 1989;83:60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JA. Parathyroid hormone activates mitogen-activated protein kinase in opossum kidney cells. Endocrinology. 1999;140:5771–5779. doi: 10.1210/endo.140.12.7173. [DOI] [PubMed] [Google Scholar]
- Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodeling and structure. Bone. 2007;40:1447–1452. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Deal C. Potential new drug targets for osteoporosis. Nat. Clin. Pract. Rheumatol. 2009;5:20–27. doi: 10.1038/ncprheum0977. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- Donahue HJ, Zhou Z, Li Z, McCauley LK. Age-related decreases in stimulatory G protein-coupled adenylate cyclase activity in osteoblastic cells. Am. J. Physiol. 1997;273:E776–E781. doi: 10.1152/ajpendo.1997.273.4.E776. [DOI] [PubMed] [Google Scholar]
- Egrise D, Martin D, Vienne A, Neve P, Schoutens A. The number of fibroblastic colonies formed from bone marrow is decreased and the in vitro proliferation rate of trabecular bone cells increased in aged rats. Bone. 1992;13:355–361. doi: 10.1016/8756-3282(92)90451-2. [DOI] [PubMed] [Google Scholar]
- Elis S, Courtland HW, Wu Y, Sun H, Rosen CJ, Yakar S. Elevated serum IGF-1 levels synergize PTH action on the skeleton only when the tissue IGF-1 axis is intact. J. Bone Miner. Res. 2010 doi: 10.1002/jbmr.100. PubMed PMID: 20499370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp. Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Geng S, Zhou S, Glowacki J. Age-related declines in expression of CYP27B1 and in osteoblast differentiation in human MSCs. J. Bone Mineral Res. 2010;25S:107. [Google Scholar]
- Gentili C, Picotto G, Morelli S, Boland R, Russo de Boland A. Effect of ageing in the early biochemical signals elicited by PTH in intestinal cells. Biochim. Biophys. Acta. 2003;1593:169–178. doi: 10.1016/s0167-4889(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J. Cellular Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Analytical Biochemistry. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11:161–171. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto N, Kawane T, Horiuchi N. Upregulation of PTH receptor mRNA expression by dexamethasone in UMR-106 osteoblast-like cells. Oral Dis. 2007;13:23–31. doi: 10.1111/j.1601-0825.2006.01234.x. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Regulation of Wnt signaling pathway by disabled-2 (Dab2) EMBO J. 2003;22:3084–3094. doi: 10.1093/emboj/cdg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech. Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, as compared to the young, murine skeleton by parathyroid hormone. Aging Cell. 2010;9:851–867. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouishomme H, Whitfield JF, Gagnon L, Maclean S, Isaacs R, Chakravarthy B, Durkin J, Neugebauer W, Willick G, Rixon RH. Further definition of the protein kinase C activation domain of the parathyroid hormone. J. Bone Miner. Res. 1994;9:943–949. doi: 10.1002/jbmr.5650090620. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr. Osteoporos. Rep. 2008;6:72–76. doi: 10.1007/s11914-008-0013-9. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit. Rev. Oral. Biol. Med. 1999;10:165–181. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell. Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- Lane NE, Silverman SL. Anabolic Therapies. Curr. Osteoporosis Rep. 2010;8:23–27. doi: 10.1007/s11914-010-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lorenzo JA. Regulation of receptor activator of nuclear factor-κ B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase A pathway in murine bone marrow cultures. Bone. 2002;31:252–259. doi: 10.1016/s8756-3282(02)00804-9. [DOI] [PubMed] [Google Scholar]
- Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J. Biol. Chem. 2007;282:33086–33097. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S. PTH stimulated the release of IGF-I and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology. 1989;125:1484–1491. doi: 10.1210/endo-125-3-1484. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J. Cell. Biochem. 2003;89:180–190. doi: 10.1002/jcb.10490. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E. PTH enhances the transcript and polypeptide levels of IGF-I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–1253. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J. Bone Miner. Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone. 2000;27:177–184. doi: 10.1016/s8756-3282(00)00317-3. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Qin L, Raggat LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trents Endo. Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC, Friedman PA. Parathyroid hormone receptor directly interacts with dishevelled to regulate β-Catenin signaling and osteoclastogenesis. J. Biol. Chem. 2010;285:14756–14763. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo de Boland A. Age-related changes in the response of intestinal cells to PTH. Mech. Ageing Develop. 2004;125:877–888. doi: 10.1016/j.mad.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Sayer RA, Lancaster JM, Pittman J, Gray J, Whitaker R, Marks JR, Berchuck A. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol. Oncol. 2005;96:355–361. doi: 10.1016/j.ygyno.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Shen L, Zhou S, Glowacki J. Effects of age and gender on WNT gene expression in human bone marrow stromal cells. J. Cell. Biochem. 2009;106:337–343. doi: 10.1002/jcb.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ozono K, Kubota T, Kondou H, Tachikawa K, Michigami T. PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3β in osteoblastic Saos-2 cells. J. Cell. Biochem. 2008;104:304–317. doi: 10.1002/jcb.21626. [DOI] [PubMed] [Google Scholar]
- Swarthout JT, D'Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- Swarthout JT, Doggett TA, Lemker JL, Partridge NC. Stimulation of extracellular signal-regulated kinases and proliferation in rat osteoblastic cells by parathyroid hormone is protein kinase C-dependent. J. Biol. Chem. 2001;276:7586–7592. doi: 10.1074/jbc.M007400200. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Mori T, Imabayashi H, Kiyono T, Gojo S, Miyoshi S, Hida N, Ita M, Segawa K, Ogawa S, Sakamoto M, Nakamura S, Umezawa A. Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation? J. Gene Med. 2004;6:833–845. doi: 10.1002/jgm.583. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K. Parathyroid hormone increases β-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147:2583–2590. doi: 10.1210/en.2005-1627. [DOI] [PubMed] [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J. Bone Miner. Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JF, Isaacs R, MacLean S, Morley P, Barbier JR, Willick GE. Stimulation of membrane-associated protein kinase-C activity in spleen lymphocytes by hPTH-(1–31)NH2, its lactam derivative, [Leu27]-cyclo(Glu22-Lys26)-hPTH-(1–31)NH2, and hPTH-(1–30)NH2. Cell Signal. 1999;11:159–164. doi: 10.1016/s0898-6568(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. Osteoporosis-treating parathyroid hormone peptides: What are they? What do they do? How might they do it? Curr. Opin. Investig. Drugs. 2006;7:349–359. [PubMed] [Google Scholar]
- Winn SR, Randolph G, Uludag H, Wong SC, Hair GA, Hollinger JO. Establishing an immortalized human osteoprecursor cell line: OPC1. J. Bone Miner. Res. 1999;14:1721–1733. doi: 10.1359/jbmr.1999.14.10.1721. [DOI] [PubMed] [Google Scholar]
- Wong MM, Rao LG, Ly H, Hamilton L, Ish-Shalom S, Sturtridge W, Tong J, McBroom R, Josse RG, Murray TM. In vitro study of osteoblastic cells from patients with idiopathic osteoporosis and comparison with cells from non-osteoporotic controls. Osteoporos. Int. 1994;4:21–31. doi: 10.1007/BF02352257. [DOI] [PubMed] [Google Scholar]
- Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, Cohen P, Hwang D, Boisclair Y, Leroith D, Rosen CJ. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J. Endocrinol. 2006;189:289–299. doi: 10.1677/joe.1.06657. [DOI] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J. Bone Mineral Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- Zhou S, Lechpammer S, Greenberger J, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires TGFβ/Smad3 signaling. J. Biol. Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011 doi: 10.1002/jcb.23079. [Epub ahead of print]. PubMed PMID: 21344492. [DOI] [PMC free article] [PubMed] [Google Scholar]