Abstract
Despite numerous behavioral interventions designed for women, rates of HIV and STIs are increasing. Interventions are needed that reach a large number of at-risk individuals. This study was a randomized clinical trial of a HIV/STI behavioral intervention conducted in Baltimore, MD, USA. Heterosexual women (n=169) completed a baseline and 3 semiannual follow-up visits. Participants were randomized into a standard of care comparison condition or a Peer Mentor condition. At the 6-month follow-up, Peer Mentors were less likely to have multiple sex partners [AOR: 0.28 (95% CI: 0.13, 0.63)]. At the 18 month follow-up assessment, Peer Mentors increased their condom use during vaginal [AOR: 0.47 (95% CI: 0.25, 0.87)] and anal sex [AOR: 0.24 (95% CI: 0.09, 0.68)] as well as with main [AOR: 0.41 (95% CI: 0.21, 0.77)] and non-main partners [AOR: 0.33 (95% CI: 0.14, 0.79]. Peer education is a sustainable approach to change risky sexual behaviors.
INTRODUCTION
Despite numerous effective behavioral interventions designed specifically for women, rates of HIV and STIs continue to rise in this population (1). According to the Centers for Disease Control, in 2008, the rates of chlamydia and gonorrhea were higher among women compared to men in the United States (2). The World Health Organization has reported that among the 31 million people infected with HIV, 50% are women and heterosexual contact is the greatest risk factor (3). Many women who are at-risk may not seek traditional medical services to find out their serostatus or learn ways to lower their risk for HIV/STIs. Interventions are needed that can reach a large number of women to bring about sustainable behavior change.
The CDC has recognized over 30 interventions as effective at reducing risky behaviors among women (4). Most of the existing interventions have been at the individual level and tailored to address female specific factors hypothesized to be associated with sexual risk behavior such as race and culture, gender and power (5–8). Some have addressed relationship dynamics among dyads, such as sexual partnerships or incorporated community mobilization events (9–11).
Social networks are a key factor in HIV and STI transmission (12–14). Social network approaches, such as partner notification and cluster investigations, have been utilized to control STI epidemics (15). In a recent study, Neblett et al. (16) examined the social networks of African American women in relation to risky sex behaviors. They found that a larger total network, greater number of social network members to socialize with, and greater number of network members who provided financial support was associated with multiple sex partners in the past 90 days. In addition, having more social network members who used heroin or cocaine was associated with both having a risky sexual partner and multiple partners, even after controlling for individual drug use.
HIV prevention peer education interventions have been successfully implemented with populations including drug users, adolescents, and men who have sex with men (17–21). Peer education interventions that have been implemented with married women and female sex workers in developing countries have demonstrated success in increasing HIV/STI knowledge and safer sex behaviors (22–25).
Few interventions have focused on the social networks within which women, living in urban neighborhoods with high levels of drug activity, are embedded. Such neighborhoods may lead to high risk for acquiring HIV. In peer education interventions, a social-network approach, individuals are trained in HIV/STI risk reduction and conduct outreach to people in their social network. This approach is designed to bring about behavior change at the individual level, among the peer educator, as well as serve as a bridge to change social network level norms and affect a wider range of people.
The purpose of the present study is to evaluate the CHAT intervention, a social-network based intervention for heterosexual women who were trained to be Peer Mentors (i.e. peer educators). The CHAT intervention was a community-based intervention guided by several theoretical approaches: social influence, social identity, cognitive dissonance, social diffusion, and social learning (26–29). By talking about HIV/STI and risk reduction options, norms within the social networks were hypothesized to change towards promoting risk reduction, facilitating behavior change of the network members. In addition to changes among network members, it was hypothesized that Peer Mentors would change their own risky behaviors in order to practice what they preach.
This evaluation examined the effects of the CHAT intervention on risk behaviors of Peer Mentors. Specifically, we examined: 1) changes in number of sex partners and condom use among Peer Mentors; 2) changes in drug behaviors among Peer Mentors; and 3) changes in communication about HIV/STIs among Peer Mentors. Communication was examined as a mechanism to assess peer outreach. Although the primary outcomes were unprotected sex and number of partners, because the intervention was guided by harm reduction, which emphasizes a range of risk reduction options, we examined several risk behavior outcomes in the analyses.
METHODS
Sampling and Recruitment
The study was conducted in Baltimore, MD, USA. The sample consisted of women and their social network members. Women (referred to as index participants) were recruited through street outreach as well as at health clinics, and other local community agencies. Recruiters approached women and asked them if they were interested in learning about HIV and STIs and giving something back to their community. Interested persons were given a card with a toll-free number to call for a screening assessment, which lasted about 10 minutes. Because the emphasis was on recruiting women at high risk for sexual transmission of HIV, eligibility criteria included: 1) female, 2) age 18–55 years old, 3) did not inject drugs in the past 6 months, 4) self-reported sex with at least 1 male partner in the past 6 months, and 5) had at least one sexual risk factor including any of the following: a) more than 2 sex partners in the past 6 months, b) STI diagnosis in the past 6 months, and c) having a high risk sex partner in the past 90 days (i.e., injected heroin or cocaine, smoked crack, HIV seropositive, or man who has sex with men).
Baseline Data Collection
Eligible index participants were scheduled for a baseline visit which consisted of the consent process, survey administration and biological testing. During the visit, participants completed a personal social network inventory, which included questions about the people they interacted with and follow-up questions about the attributes of each person. Some examples included individuals who provided emotional support, provided financial support, had sex with, socialized with, etc. Based on this network inventory, eligible social network members were identified. Eligibility criteria for social network members were: 18 years and older, and one of the following: someone who injected drugs, sex partner of index, social network members whom the index participants felt comfortable talking to about HIV or STIs.
Index participants were given a list of eligible network members. They were then asked to approach the individuals on the list and invite them into the study. Network participants completed study interviews, but they did not participate in the intervention. While index participants were allowed to invite up to five eligible social network members into the study, each index participant was eligible to be randomized when at least one network member had completed a baseline visit. The average number of recruited network members was 1.6. The current study focuses on changes among index participants who were randomized into the 2 study arms.
Index participants received a remuneration of $10 for each network member who completed a baseline visit. The study visits lasted approximately 2 ½ hours and participants were paid $35. Part of the survey was conducted face-to-face by a trained interviewer while sections on HIV risk behaviors were administered through Audio-Computer Assisted Self Interviewing (ACASI). Also, participants provided two oral specimens; one of which was used to test for HIV antibodies using the Orasure® collection device (Orasure Technologies, Inc.), and the other was used to test for cocaine and heroin metabolites using Intercept® (Orasure Technologies, Inc). A urine sample was also collected to test for Chlamydia and Gonorrhea through ligase chain reaction (30). Baseline data were collected from September 2005 through July 2007. All protocols were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Randomization
On the day of randomization, after the index participants arrived at the center, they were randomized into one of the two study conditions. A computer program was used to assign conditions based on block randomization. Each block consisted of eight assignments. Thus, once eight women were randomized, 50% were in the intervention condition and 50% were in the comparison condition.
Intervention conditions
There were two study conditions- Peer Mentor intervention condition and standard of care comparison condition. Sessions were led by two female facilitators. The number of participants in each group ranged from 4 to 8 and each group session lasted about 2 hours. Participants received $20 for each group session that they attended.
Peer Mentor Condition: CHAT intervention
Prior to development of the intervention curriculum, we conducted focus groups and in-depth interviews to gain insight into women’s willingness to participate in a Peer Education training program and to obtain feedback on intervention activities. Through the formative research, we found that women were concerned about high rates of HIV and STIs in their community. They wanted to take an active role in preventing HIV/STIs and improving their community. In addition, women expressed interest in learning how to talk to younger family members and partners about HIV prevention.
We also discovered that women preferred the term “Peer Mentor” over “Peer Educator” because they were already familiar with and engaging in mentoring of family and friends. Many women considered themselves mentors of their children or friends so this concept seemed relevant. Peer Mentoring was viewed as an opportunity to have conversations with social network members (partners, family, and friends) about important issues in their lives. As a result, the CHAT intervention curriculum defined a Peer Mentor as Someone who has meaningful conversations with partners, friends, and other people in their social network. The intervention emphasized that the main difference between this program and their usual conversations was that as Peer Mentors in the CHAT program, they used a specific communication skills set to engage in meaningful conversations about HIV and STI risk reduction. The four communication skills, which also represent the intervention’s acronym, (CHAT) were: 1) Choose the right time and place; 2) Hear what the person is saying; 3) Ask Questions; and 4) Talk with respect.
The CHAT intervention consisted of five small group sessions and one individual session based on a harm reduction philosophy. Peer Mentors were encouraged to talk to their family, friends, and sex partners about a range of sex risk reduction options.
Table 1 provides a description of the main activities covered in the sessions. Each group session included facilitated discussions, presentation of new information, and Peer Mentoring practice activities like role-plays. Some of the prominent activities included:
Table 1.
Overview of Topics and Activities of the CHAT intervention
| Session | Topic | Activities |
|---|---|---|
| 1 | Introduction to Peer Mentoring and communication |
|
| 2 | HIV transmission and risk reduction |
|
| 3 | STI transmission and risk reduction |
|
| 4 | Sexual risk reduction options |
|
| 5 | Individual session |
|
| 6 | Graduation and sustainability of peer outreach |
|
HIV/STI prevalence activity: An activity using 2 types of candy in a bag that demonstrated the way in which living in an urban city with high rates of HIV/STIs increases the likelihood of coming in contact with an infected sex partner.
Risk reduction ladder that presented sex behaviors ranging from abstinence to unprotected anal sex and their levels of risk for HIV/STIs. The higher a behavior was placed on the ladder, the higher the transmission risk.
Problem-solving activities and role-plays: Peer Mentors were presented with a scenario such as having a friend who does not use condoms with her sex partners. As a group, participants discussed the safer sex options that the friend has to lower their risk for HIV/STIs. After the brainstorm, participants role-played conversations a Peer Mentor could have with her friends to discuss safer sex options.
At the end of each session, participants were given a homework assignment, which was an opportunity to practice their communication skills and disseminate risk reduction information. Participants were encouraged to conduct the homework assignment with the social network members from their social network inventory. While drug use was not an inclusion criterion, the sample was drawn from communities with high drug activity levels and many participants used non-injection drugs. Thus, information on the link between drugs and sexual behaviors was integrated into the sessions. For example, many role-play scenarios focused on a drug-related situation such as exchanging sex. Facilitators also discussed how drug use is linked to risky sexual risk behaviors such as unprotected sex.
An individual session was conducted in which the Peer Mentor met one-on-one with a facilitator to assess participants’ Peer Mentoring experiences, address barriers to peer outreach, identify personal risk for HIV/STIs, and develop a personalized risk reduction plan.
Comparison condition
In addition to high quality Voluntary Counseling and Testing (VCT) based on Project RESPECT (31), the comparison condition consisted of one group session delivered by a female facilitator. The session, which lasted about 90 minutes, focused on HIV and STIs transmission and risk reduction information. This session consisted of didactic presentations and a culminating game that synthesized information presented. Participants received $20 for attending the session.
Follow-up Data Collection
Participants completed 6, 12, and 18-month follow-up visits. Due to the low STI burden at baseline, only a survey was administered at the follow-up visits. Baseline data were collected from September 2005 through July 2007. The final 18-month follow-up assessments ended in February 2010.
Measures
Sexual risk behaviors
Sexual behaviors in the past 90 days were collected via ACASI software. All participants reported the total number and type (main or non-main) of sex partners in the prior 90 days. A dichotomous variable of having multiple partners was created based on having two or more sex partners in the prior 90 days.
Since risk behaviors are based on type of partner and type of sex act, we examined both condom use during vaginal and anal sex (regardless of type of partner) and condom use with specific partners. Dichotomized variables of unprotected sex were created based on the frequency of condom use for anal or vaginal sex. Unprotected sex was defined as less than 100% condom use versus 100% condom use. Participants who had sex partners in the prior 90 days were also asked if they knew or suspected that any of their sex partners: 1) injected drugs, 2) smoked crack in the last 90 days, 3) were HIV seropositive, 4) had a STI, or 5) a male partner who had sex with other men. A dichotomized variable of having risky sex partners was created if participants reported at least one sex partner who had any of these risks.
Drug use behaviors
A dichotomized variable was created to indicate participants’ use of cocaine or heroin (regardless of route) in the past 6 months. We also examined crack use in the past 6 months as a separate variable due to the high level of crack use in the population and based on prior research demonstrating a strong link between HIV and STIs with crack use (32–34).
HIV/STIs communication
At the baseline, participants were asked “How often do you talk to your family members/sex partners/friends about HIV or STIs?” (separate items for each relationship). These items were measured on a scale from “never” to “more than once a day.” Dichotomized variables were created if participants reported they had talked about HIV or STIs or never talked. In the follow-ups, participants were asked “Have you talked with family members/sex partners/friends about HIV or STIs in the past 6 months?” (separate items for each relationship). Finally, an overall dichotomized communication variable was created if participants had talked to anyone about HIV or STIs in the past 6 months.
Analysis
Data were analyzed on an intent-to-treat basis. Demographic differences at baseline between index participants in the intervention and comparison conditions were assessed using chi-square or t-tests. Longitudinal analyses were conducted by fitting repeated measures logistic regression models using Generalized Estimating Equations (GEE) for the behavioral outcomes (35). An exchangeable correlation structure was assumed, and standard error was calculated using the Huber/White/sandwich estimator to decrease the correlation misspecification (36, 37). For all models, basic effects included intervention assignment, follow-up visits and the baseline value of the behavior if a difference was found between the intervention and comparison condition participants at the baseline visit. Time by intervention interactions were added to the basic model which evaluated the intervention effect at a specific time point. Since we found the prevalence of having exchanged sex was different between index participants in the intervention and the comparison group, this variable was added as time-varying covariates in the models of HIV-related sexual risk behaviors. Overall intervention effects, intervention effects at each follow-up visit and time by intervention interactions were assessed from the models by computing Wald tests. All analyses were conducted in STATA version 10.0 (Stata Corporation: College Station, TX, 2007).
RESULTS
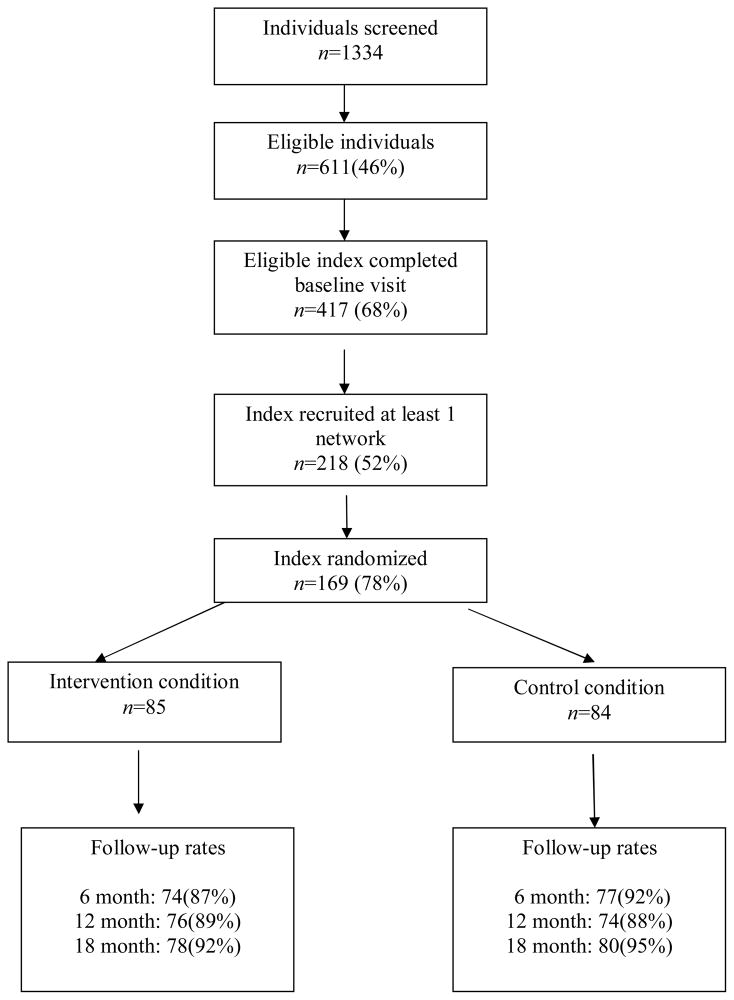
Figure 1 displays the flow of index participants through the study. There were no differences between index participants who successfully referred network members to the study but were not randomized and those participants who were randomized. The average number of sessions attended by Peer Mentors was 4.8 sessions, with 73% of participants attending 5 or 6 sessions.
Figure 1.
Flow of Index study participants in the CHAT study, Baltimore, Maryland, 2005–2010
Sample characteristics of index participants
Table 2 presents the baseline demographic and risk behavior characteristics of the randomized index participants. The sample was majority African American (98%), heterosexual (86%), unemployed (92%), with a mean age 42.7 years (SD=6.5). Over half of the sample had multiple sex partners (55%). Unprotected sex was common; 23.9% reported unprotected anal sex and 76% reported unprotected vaginal sex. Regarding condom use with a specific partner type, 68% reported unprotected sex with a main partner and 42% reported unprotected sex with a non-main partner. Less than one third reported exchanging sex (30.3%) and almost two-thirds reported having a risky sex partner (65%). The majority of the sample reported at least one risky sexual behavior (95%) in the past 90 days. Approximately 76% reported using heroin or cocaine in the past 6 months and 66.3% smoked crack. Among participants who provided an oral specimen to test for HIV antibodies, 10.3% were seropositive. In addition, there were low rates of gonorrhea (0.8%) and Chlamydia (0.8 %).
Table 2.
Baseline Characteristics of Randomized Index Participants, (n=169)
| Characteristics (n, %) | Total (n=169) | Comparison (n=84) | Intervention (n=85) | Test statistics+ |
|---|---|---|---|---|
| Mean age, years (SD) | 42.7 (7) | 43.3 (7) | 42.1 (7) | 1.43 |
| Race | ||||
| African American | 165(98) | 81(96) | 84(99) | |
| White | 3(2) | 2(2) | 1(1) | |
| Other | 1(1) | 1(1) | 0(0) | 1.38 |
| Education: Less than high school diploma | 82(49) | 41(49) | 41(49) | 0.01 |
| Sexual orientation | ||||
| Heterosexual | 146(86) | 72(86) | 74(87) | |
| Gay/homosexual | 3(2) | 2(2) | 1(1) | |
| Bisexual | 20(12) | 10(12) | 10(12) | 0.35 |
| Relationship status | ||||
| Married | 11(7) | 6(7) | 5(6) | |
| In a committed relationship | 53(31) | 23(27) | 30(35) | |
| Separated | 14(8) | 9(11) | 5(6) | |
| Divorced | 10(6) | 6(7) | 4(5) | |
| Widowed | 3(1) | 3(4) | 0(0) | |
| Single | 78(46) | 37(44) | 41(48) | 5.76 |
| Currently having main partners | 141(83) | 72(86) | 69(81) | 0.63 |
| Currently unemployed | 156(92) | 80(95) | 76(89) | 2.02 |
| Income: >=$500 | 88(52) | 45(54) | 36(42) | 1.93 |
| Homeless (past 6 months) | 58(34) | 30(36) | 28(33) | 0.14 |
| Recent incarceration (past 6 months) | 23(14) | 12(14) | 11(13) | 0.07 |
| Self report HIV positive1 | 21 (12) | 12 (14) | 9 (11) | 0.53 |
| Orasure test for HIV positive | 15 (10) | 8 (11) | 7 (10) | 1.07 |
| Urine test for gonorrhea positive | 1(1) | 1(2) | 0(0) | 1.04 |
| Urine test for Chlamydia positive | 1(1) | 0(0) | 1(2) | 0.98 |
| Sex risk behaviors (past 90 days) | ||||
| >=2 sex partners | 89(55) | 42(51) | 47(58) | 0.76 |
| Unprotected anal sex | 39(24) | 20(24) | 19(24) | 0.02 |
| Unprotected vaginal sex | 123(76) | 63(77) | 60(74) | 0.17 |
| Unprotected sex with main partner | 111(68) | 58(71) | 53(65.) | 0.53 |
| Unprotected sex with non-main partner | 68(42) | 37(45) | 31(38) | 0.79 |
| Had exchange partner | 49(30) | 18(22) | 31(39) | 5.42* |
| Any high risk sexual behavior | 154(95) | 78(95) | 76(94) | 0.13 |
| Drug use behaviors (past 6 months) | ||||
| Crack use | 112(66) | 59(70) | 53(62) | 0.90 |
| Use of heroin or cocaine (any route of administration) | 128 (76) | 64 (76) | 64 (75) | 0.02 |
| HIV communication (past 6 months) | ||||
| Talked to family members about HIV or STIs | 128 (76) | 63 (75) | 65 (77) | 0.05 |
| Talked to sex partners about HIV or STIs | 135 (80) | 69 (82) | 66 (78) | 0.53 |
| Talked to friends about HIV or STIs | 112 (66) | 51 (61) | 61 (72) | 2.30 |
| Talked about HIV or STIs | 158(94) | 78(93) | 80(94) | 0.11 |
Please note: Some participants who were aware of their HIV seropositivity chose not to take the HIV Orasure test during the baseline visit.
F test for continuous variables and Chi-square test for categorical variables.
p <.05
The only significant difference at baseline between the intervention and comparison condition index participants was that there was a significantly higher prevalence of having exchange sex in the intervention group, as compared to the comparison group (39% vs. 22%, p=0.02).
Intervention effect on index participants’ HIV risk behaviors
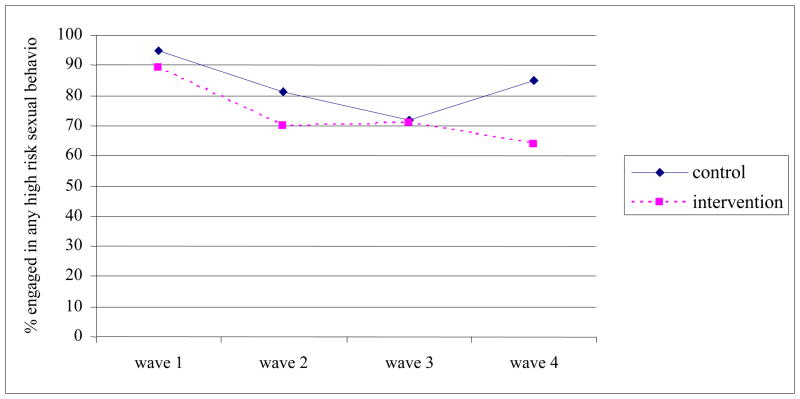
Figure 2 presents the percentage of index participants in intervention and comparison conditions who engaged in any sex risk behavior over the 18 month follow-up period. Table 3 shows results of time specific and overall (wave 1–4) GEE logistic regression modeling of the intervention effect on specific HIV-related risk behaviors. The overall GEE model without the time-interaction term showed a significant intervention effect for having unprotected sex with non-main sex partner, and the intervention effect significantly varied across time. At Wave 2 (6 month), the intervention condition index participants had reduced odds of having multiple sex partners as compared to index participants in the comparison condition (Adjusted Odds Ratio [AOR]: 0.28; 95% Confidence Interval [CI]: 0.13, 0.63).
Figure 2.
Comparisons of Index Participants in Intervention versus Control condition on changes in sex risk behavior over 18 months The CHAT Study, Baltimore Maryland.
Table 3.
Overall and Time-specific Intervention Effect on HIV Risk Behaviors of Index Participants as Compared to the Comparison Index Participants, CHAT study (n=169)
| Time Specific 1 | Overall 2 (Wave1–4) | |||
|---|---|---|---|---|
| (Wave 2) | (Wave 3) | (Wave 4) | ||
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Sex risk behaviors (past 30 days) | ||||
| >=2 sex partners | 0.28 (0.13,0.63)** | 1.28(0.61,2.71) | 0.98(0.42,1.92) | 0.78(0.47,1.32) |
| Unprotected anal sex | 1.61(0.68,3.78) | 0.40(0.16,1.01)+ | 0.24(0.09,0.68)** | 0.70(0.40,1.25) |
| Unprotected vaginal sex | 0.82(0.43,1.57) | 0.64(0.34,1.22) | 0.47(0.25,0.87)* | 0.64(0.40, 1.02)+ |
| Unprotected sex with main partner | 1.15(0.60,2.20) | 0.74(0.39,1.42) | 0.41(0.21,0.77)** | 0.70(0.45,1.10) |
| Unprotected sex with non-main partner | 0.91(0.44,1.87) | 0.36(0.16,0.84)* | 0.33(0.14,0.79)* | 0.59 (0.36,0.95)* |
| Any high risky sexual behavior | 0.50(0.23,1.07)+ | 0.92 (0.45,1.87) | 0.30(0.14,0.64)** | 0.52(0.31, 0.85)* |
| Drug use behaviors (past 6 months) | ||||
| Crack use | 1.30(0.71,2.38) | 1.08(0.59,1.97) | 0.77(0.41,1.42) | 0.95(0.60,1.51) |
| Used any cocaine or heroin | 1.12(0.60,2.10) | 1.20(0.64,2.25) | 0.71(0.38,1.33) | 0.98(0.61,1.57) |
| HIV communication (past 6 months) | ||||
| Talk to family about HIV or STIs | 2.68(1.22,5.89)* | 2.82(1.41,5.64)** | 2.41(1.25,4.65)** | 2.14(1.34,3.42)** |
| Talk to partner about HIV or STIs | 2.10(0.73,6.06) | 0.97(0.47,2.01) | 1.12(0.56,2.24) | 1.03(0.63,1.67) |
| Talk to friends about HIV or STIs | 2.04(0.90,4.63)+ | 1.23(0.60,2.51) | 1.90(0.94,3.83)+ | 1.65(1.04,2.61)* |
| Talked about HIV or STIs | 7.45(0.80,69.02)+ | 1.57(0.53,4.66) | 2.58(0.93,7.14)+ | 2.06(0.98,4.31)+ |
Longitudinal models included effects for intervention assignment, wave, and wave by intervention assignment interaction
Longitudinal models were fit as above excluding wave by intervention assignment interaction
p<.10,
p<.05,
p<.01
At Wave 3 (12 months), the intervention index participants had statistically significant lower odds of having unprotected sex with a non-main sex partner (AOR: 0.36, 95%CI: 0.16, 0.84), as compared to index participants in the comparison condition.
At Wave 4 (18 months), the intervention condition index participants had statistically significant lower odds of having unprotected anal sex, unprotected vaginal sex, unprotected sex with a main sex partner, and unprotected sex with a non-main sex partner. Reductions of any sexual risk behavior were observed in Wave 4 and in the overall GEE logistic regression (AOR: 0.52, 95%CI: 0.31, 0.85). No intervention effect on reduction of drug use behaviors was observed.
Table 3 also presents results of time specific and overall GEE logistic regression modeling of the intervention effect on HIV/STI communication among randomized index participants. Being in the intervention condition was significantly associated with talking to family about HIV/STIs at all follow-up time points (wave2–4) and in the overall GEE logistic regression, as compared to the comparison condition. There was an overall intervention effect of having conversations with friends about HIV/STIs among index participants in the intervention condition (AOR: 1.65, 95%CI: 1.04, 2.61).
DISCUSSION
The CHAT intervention integrated peer education, risk reduction and communication skills. Peer Mentors, compared to women in the comparison condition, reduced a greater number of their risky sexual behaviors over time. Peer Mentors were less likely to have two or more sexual partners at the 6-month follow-up. One possible explanation is that Peer Mentors engaged in numerous activities to communicate information about the rates of HIV and STIs in their communities, such as the candy activity, which influenced their personal behavior to be consistent with their peer outreach conversations. Cognitive dissonance theory suggests that individuals who publicly promote risk reduction would be more likely to engage in risk reduction as compared to individuals who do not publicly advocate. Public commitment to HIV/STI prevention may have led to reductions in number of sex partners and increases in condom use among Peer Mentors.
Significant changes in condom use were observed among Peer Mentors. Peer Mentors were less likely to have unprotected vaginal or anal sex, regardless of type of partner. CHAT intervention messages focused on a variety of condoms such as latex condoms, polyurethane, female condoms, and flavored condoms, and condoms were distributed to participants. Awareness of the diversity of condoms available, as well as easy access to condoms, may have persuaded participants to try a condom that they had not used previously and talk to partners and other network members about condom use.
In addition to increasing condom use during specific sexual acts, Peer Mentors were less likely to have unprotected sex with both main and non-main partners. Over 80% of participants in both groups reported having a main sex partner. The intervention group was almost 60% less likely to have unprotected sex with a main partner at the18-month follow up. Consistent condom use with a main partner is challenging since use of condoms may suggest infidelity or lack of trust. In a qualitative study of heterosexual couples at high risk for HIV, Corbett and colleagues (38) found that many couples feel that not using condoms are signs of trust and intimacy as well as “placing their love for their partner and other emotional needs over concerns about their health”. The present study findings suggest that training women to have conversations with numerous individuals in their social networks may enable them to practice these communication skills with sex partners in comfortable, natural settings and practice safe sex.
In addition to communication skills to talk with both main and non-main partners, participants were taught to solve problems regarding common complaints about condom use such as reduced pleasure or loss of erection. While the skills training was used for peer outreach, some Peer Mentors also used this information to persuade their own partners to use condoms. Participants were encouraged to use their participation in a Peer Mentor program as a reason to use condoms, such as stating “Remember that program I told you I was in, they gave us this bag of condoms and asked us to try them out. Can we use one so I can do my homework?” Training women in these communication skills options may have empowered women to persuade their partner to use condoms.
Many of the index participants were non-injection drug users. While there was a trend of reduction of cocaine and heroin use, as well as crack use, significance was not reached. Drug use was integrated into the intervention material. However, since information on sexual risk reduction was more salient, Peer Mentors have focused on their own sex behaviors rather than considering how drug use impacted their own HIV/STIs risk behavior. Future interventions should focus on both types of risks because any successful change in one type of behavior may not make a huge difference if the individual is still practicing other risky behaviors. Focus on both types of risk in social network-based interventions is also an effective way to diffuse risk reduction information to sex partners and other network members who use drugs.
Overall, women in both conditions reported high rates of communication with family, friends, and partners about HIV/STIs. Peer Mentors, as compared to those in the comparison group, were more likely to talk to their family members and this trend remained stable over time. In the intervention sessions, many participants noted the importance of sharing this information with their children. In addition, index participants in the intervention group were more likely to talk with their friends about HIV and STIs. Talking to individuals who were not partners may have been viewed as less problematic, especially for women Previous research has shown that higher levels of communication are associated with perceived norms about safer behaviors (39). Continued conversations with family members or friends may have reinforced the role of Peer Mentor and led to development of safer sex norms and ultimately safer behaviors. More research is needed on the quality and frequency of these conversations about HIV.
There are limitations of the study that should be noted. First, sampling selection bias may be present since participants volunteered to be Peer Mentors. Through participation in the intervention, participants may have underreported their risk behaviors due to social desirability bias. As a result, the sample may have appeared to be less risky than intended, which means behavior change may be a challenge. There may have been some contamination as a result of some index participants in the intervention group promoting risk reduction with index participants in the comparison group. This dynamic may have reduced the differences between the two conditions.
Many HIV/STI prevention interventions have demonstrated behavior changes at 6 month or less follow-up assessments (40,41). Our study found that a Peer Education intervention is effective at reducing individual risk behaviors, including reductions in unprotected anal sex, unprotected vaginal sex, and unprotected sex with main or non-main partners over the 18-month follow-up period.
By taking on the role of Peer Mentor, women lowered their own risk for HIV and STIs. While all of the index participants were women, over half of the network participants were men. Thus, Peer Mentor interventions for women are also an avenue to reach men. In addition, through dissemination of risk reduction resources and information in a social network, norms about risk reduction may prevail.
Community-based organizations should consider implementation of peer education interventions whose reach goes beyond the people who attend their programs. Peer education interventions capitalize on naturally occurring social influence process and can sustain behaviors for an extended period of time as they influence dynamics of the social environment, such as norms, in addition to the individual.
Acknowledgments
This work was funded by the National Institute on Mental Health (Grant# R01 MH66810).
References
- 1.Aral SO, Fenton KA, Holmes KK. Sexually transmitted diseases in the USA: temporal trends. Sex Transm Infect. 2007 Jul;83(4):257–266. doi: 10.1136/sti.2007.026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Accessed August 4, 2010];Sexually Transmitted Diseases in the United States, 2008: National Surveillance Data for Chlamydia, Gonorrhea, and Syphilis. 2009 Available at: http://www.cdc.gov/std/stats08/trends.htm.
- 3.Joint United Nations Programme on HIV/AIDS and World Health Organization. AIDS Epidemic Update. Nov, 2009. [Google Scholar]
- 4. [Accessed 8/3/2010, 2010];2009 Compendium of Evidence-Based HIV Prevention Interventions. 2009 Available at: http://www.cdc.gov/hiv/topics/research/prs/evidence-based-interventions.htm.
- 5.Shain RN, Piper JM, Holden AE, Champion JD, Perdue ST, Korte JE, et al. Prevention of gonorrhea and Chlamydia through behavioral intervention: results of a two-year controlled randomized trial in minority women. Sex Transm Dis. 2004 Jul;31(7):401–408. doi: 10.1097/01.olq.0000135301.97350.84. [DOI] [PubMed] [Google Scholar]
- 6.Jemmott LS, Jemmott JB, 3rd, O’Leary A. Effects on sexual risk behavior and STD rate of brief HIV/STD prevention interventions for African American women in primary care settings. Am J Public Health. 2007 Jun;97(6):1034–1040. doi: 10.2105/AJPH.2003.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiClemente RJ, Wingood GM. A randomized controlled trial of an HIV sexual risk-reduction intervention for young African-American women. JAMA. 1995 Oct 25;274(16):1271–1276. [PubMed] [Google Scholar]
- 8.Wingood GM, DiClemente RJ, Mikhail I, Lang DL, McCree DH, Davies SL, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: The WiLLOW Program. J Acquir Immune Defic Syndr. 2004 Oct 1;37( Suppl 2):S58–67. doi: 10.1097/01.qai.0000140603.57478.a9. [DOI] [PubMed] [Google Scholar]
- 9.El-Bassel N, Witte SS, Gilbert L, Wu E, Chang M, Hill J, et al. The efficacy of a relationship-based HIV/STD prevention program for heterosexual couples. Am J Public Health. 2003 Jun;93(6):963–969. doi: 10.2105/ajph.93.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikkema KJ, Kelly JA, Winett RA, Solomon LJ, Cargill VA, Roffman RA, et al. Outcomes of a randomized community-level HIV prevention intervention for women living in 18 low-income housing developments. Am J Public Health. 2000 Jan;90(1):57–63. doi: 10.2105/ajph.90.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauby JL, Smith PJ, Stark M, Person B, Adams J. A community-level HIV prevention intervention for inner-city women: results of the women and infants demonstration projects. Am J Public Health. 2000 Feb;90(2):216–222. doi: 10.2105/ajph.90.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellen JM, Gaydos C, Chung SE, Willard N, Lloyd LV, Rietmeijer CA. Sex partner selection, social networks, and repeat sexually transmitted infections in young men: a preliminary report. Sex Transm Dis. 2006 Jan;33(1):18–21. doi: 10.1097/01.olq.0000187213.07551.a6. [DOI] [PubMed] [Google Scholar]
- 13.Klovdahl AS, Potterat JJ, Woodhouse DE, Muth JB, Muth SQ, Darrow WW. Social networks and infectious disease: the Colorado Springs Study. Soc Sci Med. 1994 Jan;38(1):79–88. doi: 10.1016/0277-9536(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 14.Wylie JL, Jolly A. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis. 2001 Jan;28(1):14–24. doi: 10.1097/00007435-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Engelgau MM, Woernle CH, Rolfs RT, Greenspan JR, O’Cain M, Gorsky RD. Control of epidemic early syphilis: the results of an intervention campaign using social networks. Sex Transm Dis. 1995 Jul–Aug;22(4):203–209. [PubMed] [Google Scholar]
- 16.Neblett RC, Davey-Rothwell MA, Chander G, Latkin CA. Social Network Characteristics and HIV Sexual Risk Behavior among Urban African American Women. J Urban Health. doi: 10.1007/s11524-010-9513-x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003 Jul;22(4):332–339. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- 18.Garfein RS, Golub ET, Greenberg AE, Hagan H, Hanson DL, Hudson SM, et al. A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C virus infection in young injection drug users. AIDS. 2007 Sep 12;21(14):1923–1932. doi: 10.1097/QAD.0b013e32823f9066. [DOI] [PubMed] [Google Scholar]
- 19.Cai Y, Hong H, Shi R, Ye X, Xu G, Li S, et al. Long-term follow-up study on peer-led school-based HIV/AIDS prevention among youths in Shanghai. Int J STD AIDS. 2008 Dec;19(12):848–850. doi: 10.1258/ijsa.2008.008129. [DOI] [PubMed] [Google Scholar]
- 20.Mahat G, Scoloveno MA, De Leon T, Frenkel J. Preliminary evidence of an adolescent HIV/AIDS peer education program. J Pediatr Nurs. 2008 Oct;23(5):358–363. doi: 10.1016/j.pedn.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Williamson LM, Hart GJ, Flowers P, Frankis JS, Der GJ. The Gay Men’s Task Force: the impact of peer education on the sexual health behaviour of homosexual men in Glasgow. Sex Transm Infect. 2001 Dec;77(6):427–432. doi: 10.1136/sti.77.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu I, Jana S, Rotheram-Borus MJ, Swendeman D, Lee SJ, Newman P, et al. HIV prevention among sex workers in India. J Acquir Immune Defic Syndr. 2004 Jul 1;36(3):845–852. doi: 10.1097/00126334-200407010-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong H, Ji GP, Ye DQ. Long-term follow-up of a peer-led HIV/AIDS prevention program for married women in rural China. Int J Gynaecol Obstet. 2009 Jul;106(1):69–70. doi: 10.1016/j.ijgo.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.O’Hara Murdock P, Garbharran H, Edwards MJ, Smith MA, Lutchmiah J, Mkhize M. Peer led HIV/AIDS prevention for women in South African informal settlements. Health Care Women Int. 2003 Jul;24(6):502–512. doi: 10.1080/07399330303975. [DOI] [PubMed] [Google Scholar]
- 25.Williams BG, Taljaard D, Campbell CM, Gouws E, Ndhlovu L, Van Dam J, et al. Changing patterns of knowledge, reported behaviour and sexually transmitted infections in a South African gold mining community. AIDS. 2003 Sep 26;17(14):2099–2107. doi: 10.1097/00002030-200309260-00011. [DOI] [PubMed] [Google Scholar]
- 26.Fisher JD, Misovich SJ. Social Influence and AIDS-Preventive Behavior. In: Edwards J, Tindale RS, Heath L, Posavac EJ, editors. Social Influence Processes and Prevention. New York: Plenum Press; 1990. pp. 39–70. [Google Scholar]
- 27.Tajfel H. Human Groups and Social Categories: Studies in Social Psychology. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- 28.Festinger L. A Theory of Social Comparison Processes. Human Relations. May 01;7(2):117–140. [Google Scholar]
- 29.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- 30.Quinn TC, Welsh L, Lentz A, Crotchfelt K, Zenilman J, Newhall J, et al. Diagnosis by AMPLICOR PCR of Chlamydia trachomatis infection in urine samples from women and men attending sexually transmitted disease clinics. J Clin Microbiol. 1996 Jun;34(6):1401–1406. doi: 10.1128/jcm.34.6.1401-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamb ML, Fishbein M, Douglas JM, Jr, Rhodes F, Rogers J, Bolan G, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998 Oct 7;280(13):1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 32.Edlin BR, Irwin KL, Faruque S, McCoy CB, Word C, Serrano Y, et al. Intersecting epidemics--crack cocaine use and HIV infection among inner-city young adults. Multicenter Crack Cocaine and HIV Infection Study Team. N Engl J Med. 1994 Nov 24;331(21):1422–1427. doi: 10.1056/NEJM199411243312106. [DOI] [PubMed] [Google Scholar]
- 33.Wilson T, DeHovitz JA. STDs, HIV, and crack cocaine: a review. AIDS Patient Care STDS. 1997 Apr;11(2):62–66. doi: 10.1089/apc.1997.11.62. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman JA, Klein H, Eber M, Crosby H. Frequency and intensity of crack use as predictors of women’s involvement in HIV-related sexual risk behaviors. Drug Alcohol Depend. 2000 Mar 1;58(3):227–236. doi: 10.1016/s0376-8716(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–130. [PubMed] [Google Scholar]
- 36.The behavior of maximum likelihood estimates under nonstandard conditions. Berkeley, CA: University of California Press; 1967. [Google Scholar]
- 37.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–838. [Google Scholar]
- 38.Corbett AM, Dickson-Gomez J, Hilario H, Weeks MR. A little thing called love: condom use in high-risk primary heterosexual relationships. Perspect Sex Reprod Health. 2009 Dec;41(4):218–224. doi: 10.1363/4121809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davey-Rothwell MA, Latkin CA. HIV-related communication and perceived norms: an analysis of the connection among injection drug users. AIDS Educ Prev. 2007 Aug;19(4):298–309. doi: 10.1521/aeap.2007.19.4.298. [DOI] [PubMed] [Google Scholar]
- 40.Wechsberg WM, Lam WK, Zule WA, Bobashev G. Efficacy of a woman-focused intervention to reduce HIV risk and increase self-sufficiency among African American crack abusers. Am J Public Health. 2004 Jul;94(7):1165–1173. doi: 10.2105/ajph.94.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford K, Wirawan DN, Suastina SS, Reed BD, Muliawan P. Evaluation of a peer education programme for female sex workers in Bali, Indonesia. Int J STD AIDS. 2000 Nov;11(11):731–733. doi: 10.1258/0956462001915156. [DOI] [PubMed] [Google Scholar]