Abstract
Recently, inflammatory processes have been shown to increase O2-sensitivity of the carotid body during chronic sustained hypoxia (Liu et al. 2009. Am. J. Physiol. Lung Cell Mol. Physiol. 296, L158–L166). We hypothesized that blocking inflammation with ibuprofen would reduce ventilatory acclimatization to hypoxia by blocking such increases in carotid body O2 sensitivity. We tested this in conscious rats treated with ibuprofen (4mg/kg IP daily) or saline during acclimatization to hypoxia (PIO2 = 70 Torr for 7 days). Ibuprofen blocked the increase in hypoxic ventilation observed in chronically hypoxic rats treated with saline; ibuprofen had no effects on ventilation in normoxic control rats. Ibuprofen blocked increases in inflammatory cytokines (IL-1β, IL-6) in the brainstem with chronic hypoxia. The data supports our hypothesis and further analysis indicates that ibuprofen also blocks inflammatory processes in the central nervous system contributing to ventilatory acclimatization to hypoxia. Possible mechanisms linking inflammatory and hypoxic signaling are reviewed.
Keywords: chronic hypoxia, hypoxic ventilatory response, inflammatory cytokines, nonsteroidal anti-inflammatory drugs
1. Introduction
Ventilatory acclimatization to hypoxia is a time-dependent increase in ventilation during chronic sustained hypoxia (Powell et al., 1998). Several laboratories have demonstrated that this involves increased O2-sensitivity of carotid body chemoreceptors resulting in increased afferent input to ventilatory chemoreflexes for a given arterial PO2 after chronic hypoxia (reviewed by Powell, 2007). Additionally, we have shown time-dependent changes in the central nervous system (CNS) processing of carotid body chemoreceptor input resulting in a greater respiratory motor output for a given afferent input (Dwinell and Powell, 1999). Plasticity in the carotid body-ventilatory chemoreflex with chronic hypoxia involves changes in neurotransmitters and ion channels in the carotid bodies and CNS (reviewed by Powell, 2007), and changes in gene expression controlled by Hypoxia Inducible Factor 1, HIF-1 (Kline et al., 2002, Powell and Fu, 2008).
Recently, inflammatory processes have been shown to be important for the increased O2-sensitivity of carotid bodies with chronic sustained hypoxia (Liu et al., 2009). Using an in vitro rat carotid body preparation, these investigators showed that chronic sustained hypoxia increases the frequency of action potentials recorded in the carotid sinus nerve when PO2 is lowered in a solution superfusing the carotid body. They also found increased mRNA expression for inflammatory cytokines in carotid bodies from chronically hypoxic rats. The increased cytokine expression, as well as the increased carotid body neural response to acute hypoxia, was blocked by ibuprofen and dexamethasone treatment during the chronic hypoxia.
To test the physiological significance of these inflammatory signals for plasticity in chronically hypoxic carotid bodies, we studied the effects of a nonsteroidal anti-inflammatory drug, ibuprofen, on ventilatory acclimatization to hypoxia in conscious rats. Also, we measured the effects of chronic hypoxia and ibuprofen on cytokine expression in the central nervous system (CNS). Ibuprofen decreased ventilatory acclimatization to hypoxia and cytokine gene expression in the CNS, providing evidence that inflammatory signaling in the CNS contributes to ventilatory acclimatization to hypoxia.
2. Methods
2.1 Experimental animals
Adult, male rats (Sprague-Dawley, Charles River) were housed in standard rat cages in a vivarium, with a 12:12-h light-dark cycle and fed a standard rat diet ad libitum. All experiments were approved by the University of California, San Diego, Animal Care and Use Committee. The experiments conformed to national standards for the care and use of experimental animals as well as the American Physiological Society's "Guiding Principles in the Care and Use of Animals." At the end of an experiment, rats were euthanized with an overdose of sodium pentobarbital (Sleep Away, 150mg/kg IV) and death was confirmed by open chest exsanguination via cardiac ventricular incision.
2.2 Experimental Groups
Animals were housed in individual cages in either normoxia (N) or chronic hypoxia (CH). Rats acclimatizing to CH were placed in a hypobaric chamber for 7 days at 0.5 atm (PIO2 ≈ 70 Torr); N rats were housed in the same room outside the chamber. CH rats were significantly lighter than N rats at the time of experiments (268 ± 8g vs. 349 ± 7g). The chamber was opened once daily for ~20 min for regular cage maintenance and drug injection or for removing animals for experimentation. Four experimental groups were studied: (1) NS = chronic normoxia with saline injections, (2) NI = normoxia with ibuprofen injections, (3) HS = CH injected with saline, and (4) HI = CH injected with ibuprofen.
2.3 Experimental Drugs
Ibuprofen solution (2 mg/ml) was prepared from commercially available syrup (20mg/mL, McNeil Children’s Motrin Berry-Flavored, Lot SHM046) diluted with sterile normal saline (0.9% NaCl). Ibuprofen injections (4mg/kg IP, ≈0.6 ml) and saline injections of a similar volume (0.6 ml) were made daily.
2.4 Surgical Procedures
After 5 days in designated experimental conditions, we catheterized animals to sample arterial blood gases. Anesthesia was induced with 5% isofluorane in 100% O2 and isofluorane was decreased to 2.5–3% for maintenance. A customized catheter (polyethylene PE-50 drawn out under heat to an end diameter approximately equivalent to PE-10 tubing) was inserted in the femoral artery through a skin incision. The catheter was advanced into the abdominal aorta, secured to the artery with suture (5.0 silk) tied around tubing glued to the outside of the catheter, filled with heparin (10,000 Units/mL) and heat-sealed at distal end. The distal end of the catheter was led subcutaneously along the back to emerge from a metal button (Instech) sutured to underlying muscle at the base of the neck. A spring sheath secured to the button protected the distal end of the catheter.
Finally, a telemetric thermometer (G-2 Emitter, Respironics) was implanted in the abdominal cavity through a midline incision. Muscle and skin were sutured together separately and antibiotic ointment (Fura-Zone, Squire) was placed over every incision site.
2.5 Physiological Measurements
After 1 week of acclimatization, a whole body plethysmograph (7L, plexiglass) was used to measure ventilatory responses to hypoxia and hypercapnia measurements as previously described (Reid and Powell, 2005). Briefly, flow (3 L/min) was maintained through the chamber during ventilatory measurements and pressure changes due to warming and humidification of inhaled gases were measured (MP45 with 2 cm H2O diaphragm, Validyne) and used to calculate tidal volume by the method of Drorbaugh and Fenn (1955). Arterial blood was sampled through polyethylene tubing (PE 50) leading out the top of the chamber and attached to the arterial catheter with 25 gauge hypodermic tubing. O2 and CO2 concentrations in the chamber were changed with a mass flow controller (Sable Systems MFC-4) and monitored with a mass spectrometer (Marquette 1100). Ventilatory data was digitized (Labview, National Instruments, v2.5.0) and analyzed using a custom Matlab-based program for VT, fR and their product, V̇I.
Ventilatory measurements were performed with baseline O2 levels equal to the environment in which the animals were housed the previous week (NS and NI =21%; HS and HI=10%). The animals acclimated to the box for 30min and were then given a 5–10min challenge (10% O2 for NS and NI; 21 or 30% O2 for HS and HI) to test responsiveness. Then the protocol began to measure ventilation in normoxia (21% O2), hypoxia (10% O2) and hypercapnia (7% CO2, 30% O2) with a return to baseline conditions (e.g. 21% O2 for NS and NI) for 15min between conditions. Different gas levels were maintained for 15min and ventilation was measured in a stable sample with at least 20 breaths between 10 and 15min after changing gas concentrations. Arterial blood was sampled (0.2mL) during ventilatory measurements for PO2, PCO2, pH and hematocrit analysis (GEM Premier 3000, Instrumentation Laboratory). Blood gas values were corrected to body temperature as measured with the telemeter.
In a separate cohort of rats (n = 3–5 per experimental group), we measured metabolic rates in normoxia and hypoxia (10% O2). The protocol was similar to that for the ventilatory measurements described above. O2 and CO2 concentrations were measured in the plethysmograph under steady state conditions and then gas flow into the chamber was blocked and the chamber was sealed for 3min. O2 and CO2 concentrations were measured at the end of this period, then flow through the chamber was restored and V̇O2 and V̇CO2 were calculated using mass balance.
2.6 Cytokin Gene Expression Measurements
In a third cohort of rats exposed to the same experimental conditions (n = 5 per group) we measured expression of mRNA for IL-1β, IL-6, and TNFα in biopsies of the nucleus tractus solitarii (NTS) using quantitative PCR (qPCR). Rats were euthanized and their brainstems were removed and immediately frozen in liquid nitrogen. Samples were allowed to thaw just enough to make a transverse section between the calamus scriptorius and 2mm rostral. This section was refrozen in liquid nitrogen and then allowed to thaw just enough to allow the dorsal region, containing the nucleus tractus solitarii (NTS), to be cut away from the remainder of the section. The NTS biopsies were placed in a 1.5mL Eppendorf tube and weighed. We extracted mRNA in 150µL buffer in the Eppendorf tubes (RNeasy Minikit, Qiagen, and rapid homogenization), adjusted the final volume to 600µL with buffer and performed an added step of on-column DNase digestion (Qiagen). Concentration of mRNA was determined with a micro-volume UV-vis spectrophotometer (Nanodrop 2000). A cDNA synthesizing kit (Invitrogen SuperScript III First-Strand Synthesis System for RT-PCR) was used to reverse transcribe the mRNA and prepare the samples for qPCR using Power SYBR Green (Applied Biosystems). qPCR reactions were performed in duplicate on the same day with reaction mix (SYBR green, primers, RNase free H2O, and cDNA) for each reaction made together to reduce pipetting errors. Primer sequences were as follows (FW, forward; RW, reverse):
| Rat IL-1β: | FW | CACCTCTCAAGCAGAGCACAG |
| RW | GGGTTCCATGGTGAAGTCAAC | |
| Rat IL-6: | FW | TCCTACCCCAACTTCCAATGCTC |
| RW | TTGGATGGTCTTGGTCCTTAGCC | |
| Rat TNFα | FW | AAATGGGCTCCCTCTCATCAGTTC |
| RW | TCTGCTTGGTGGTTTGCTACGAC |
Housekeeping genes used to normalize the above cytokines were Rat β-2 microglobulin (B2M) and ribosomal protein L13a (RPL13A), which are unchanged by hypoxia in the central nervous system (Tang et al., 2010). Primer sequences were as follows (FW, forward; RW, reverse):
| Rat B2M: | FW | CGTCGTGCTTGCCATTCAGA |
| RW | GACGGTTTTGGGCTCCTTCA | |
| Rat RPL13A: | FW | CCATTGTGGCCAAGCAGGTA |
| RW | GCTTTCGGAGAAAGGCCAGA |
2.9 Data Analysis
Ventilatory (VT, fR, V̇I), blood gas (PaO2, PaCO2, pHa) and metabolic (V̇O2, V̇CO2) data were analyzed with mixed-factorial ANOVA: 2 inspired O2 levels (21 and 10%) X 4 experimental groups (NS, NI, HS, and HI). When significant interactions (P < 0.05) were found, the LSD post-hoc test was used to determine which means were significantly different (P < 0.05).
Cytokine qPCR data was analyzed with 1-way ANOVA and when significant interactions were detected (P < 0.05), a post-hoc Tukey’s test was used to determine which means were significantly different (P < 0.05) from each other (Keppel and Wickens, 2004).
All results are reported as mean ± standard error of the mean (SEM). Statistical analysis was performed using SPSS software (PASW Statistics, v17.0) and 2-tailed tests were used unless stated otherwise.
3. Results
3.1 Ventilatory Response to Hypoxia
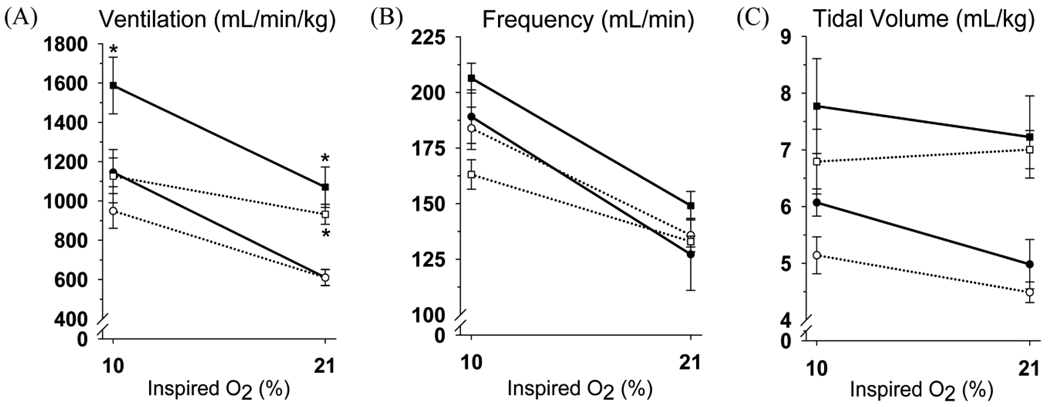
In normoxic rats, ibuprofen had no effect on ventilation (V̇I) or its components, frequency and tidal volume (fR and VT, respectively), breathing normoxic or hypoxic gas (Fig. 1). Chronic hypoxia increased V̇I in saline treated rats breathing normoxic and hypoxic gas, as expected for ventilatory acclimatization to sustained hypoxia. Ibuprofen had no effect on the persistent hyperventilation in chronically hypoxic rats breathing normoxic gas but ibuprofen blocked the increase in V̇I breathing hypoxic gas (Fig. 1). The increase in fR with chronic hypoxia was smaller with ibuprofen treatment although there were no significant interactions between experimental groups and O2 level being breathed for either fR or VT (Fig. 1).
Figure 1.
Ventilatory responses to hypoxia in normoxic (circles) and chronically hypoxic rats (squares) treated with saline (filled symbols) or ibuprofen (open symbols). Ibuprofen significantly reduces V̇I in chronically hypoxic rats breathing 10% but not 21% O2 (*p≤0.02 vs. NS at a given O2%, n = 6 except NS n = 5).
3.2 Arterial Blood Gases and Metabolism
Table 1 shows that PaO2 decreased with inspired O2 but it was not significantly affected by chronic hypoxia or ibuprofen. However, PaCO2 was significantly lower with chronic hypoxia versus normoxia breathing 21% or 10% O2 with no differences between ibuprofen or saline treatment. Arterial pH was lower also in all conditions with chronic hypoxia versus normoxia, as expected for the metabolic compensation for the chronic respiratory alkalosis with chronic hypoxia. Hematocrit increased significantly with chronic hypoxia (44.1±1.1 versus 35.6±0.8% for controls, p<0.05).
Table 1.
Arterial blood gases and pH of normoxic and chronically hypoxic rats treated with saline (NS and HS, respectively) or ibuprofen (NI and HI, respectively). For given inspired O2, PaCO2 and pHa decreased with chronic hypoxia but there was no effect of ibuprofen vs. saline and PaO2 was not different.
| Group | O2 (%) |
PaO2 (Torr) |
PaCO2 (Torr) |
pHa |
|---|---|---|---|---|
| NS (n=5) | 21 | 86.8±1.9 | 38.0±0.6* | 7.44±0.01 |
| NI (n=6) | 86.5±3.9 | 34.2±1.0† | 7.45±0.01 | |
| HS (n=2) | 86.5±4.5 | 18.0±1.0 †* | 7.39±0.02* | |
| HI (n=3) | 83.3±1.5 | 19.3±0.9†* | 7.41±0.00 | |
| NS (n=5) | 10 | 40.6±2.2 | 22.6±0.2 | 7.55±0.01 |
| NI (n=6) | 39.0±1.3 | 22.5±0.6 | 7.56±0.01 | |
| HS (n=2) | 39.0±3.0 | 14.5±0.5†* | 7.48±0.02†* | |
| HI (n=3) | 38.3±1.7 | 16.7±1.8†* | 7.50±0.01†* | |
p < 0.01 vs. NS,
p < 0.03 vs. NI.
To determine if decreased metabolic rate could explain the similar PaCO2 with decreased V̇I in HI versus HS groups, we measured V̇O2 and V̇CO2 in a new cohort of rats using the same experimental treatments. Table 2 shows that acute hypoxia (10% O2) decreased metabolic rate in all groups but there were no differences between groups. Acute hypoxia tended to increase R and it exceeded 1.0, indicating a non-steady state as CO2 is washed out of the body during acute hypoxic hyperventilation. V̇I and PaCO2 were not significantly different in this small cohort of chronically hypoxic rats with ibuprofen (n=3) versus saline (n=3) treatment either: V̇I = 1076±116 vs. 1160±282 mL/(min•kg) and PaCO2 = 17.7±0.7 vs. 17.0±2.0 Torr, respectively. However, we did not observe a difference in V̇O2 or V̇CO2 with ibuprofen treatment before or after chronic hypoxia.
Table 2.
O2 consumption, CO2 production and respiratory exchange ratio (V̇O2, V̇CO2, and R) of normoxic and chronically hypoxic rats treated with ibuprofen (NI and HI, respectively) or saline (NS and HS, respectively). Acute hypoxia (10% O2) significantly decreased V̇O2 and V̇CO2 (*p<0.02) but there were not differences between experimental. R tended to increase with acute hypoxia (p = 0.06).
| Group | O2 (%) |
V̇O2 (ml/(min•100g)) |
V̇CO2 (ml/(min •100g)) |
R |
|---|---|---|---|---|
| NS (n=4) | 21 | 2.59±0.08 | 2.29±0.05 | 0.89±0.04 |
| NI (n=5) | 2.69±0.16 | 2.41±0.20 | 0.89±0.03 | |
| HS (n=3) | 2.94±0.05 | 2.49±0.08 | 0.85±0.01 | |
| HI (n=3) | 2.42±0.35 | 2.43±0.27 | 1.04±0.20 | |
| NS (n=4) | 10 | 1.98±0.11* | 2.09±0.10* | 1.05±0.03 |
| NI (n=5) | 1.92±0.12* | 2.08±0.10* | 1.09±0.03 | |
| HS (n=3) | 2.00±0.14* | 2.23±0.11* | 1.12±0.03 | |
| HI (n=3) | 2.19±0.14* | 2.30±0.12* | 1.05±0.02 | |
3.3 Response to CO2
The effect of ibuprofen to blunt the ventilatory response in chronically hypoxic rats was unique to hypoxic stimulation. There were no significant effects of ibuprofen on the ventilatory response to hypercapnia (7% CO2 in 30% O2) in normoxic rats (V̇I for NS (n=5) 1675±153 vs. NI (n=6) 1312±78 mL/(min•kg)) or chronically hypoxic rats (V̇I for HS (n=6) 2162±103 vs. HI (n=5) 1858±171 mL/(min•kg)). Also, there were no significant differences in arterial blood gases or pHa with ibuprofen vs. saline in normoxic rats (PaO2 = 145±7.9 vs. 137.5±6.0 Torr, PaCO2 = 47.4±2.6 vs. 45.0±1.3 Torr, pHa = 7.32±0.01 vs. 7.33±0.01, n = 5 vs. 6, respectively) or chronically hypoxic rats (PaO2 = 159.0±1.0 vs. 140.5±12.5 Torr, PaCO2 = 39.5±1.5 vs. 33.5±1.5 Torr, pHa = 7.12±0.02 vs. 7.23±0.01, n = 2 vs. 2, respectively).
3.4 Inflammatory Cytokines in the NTS
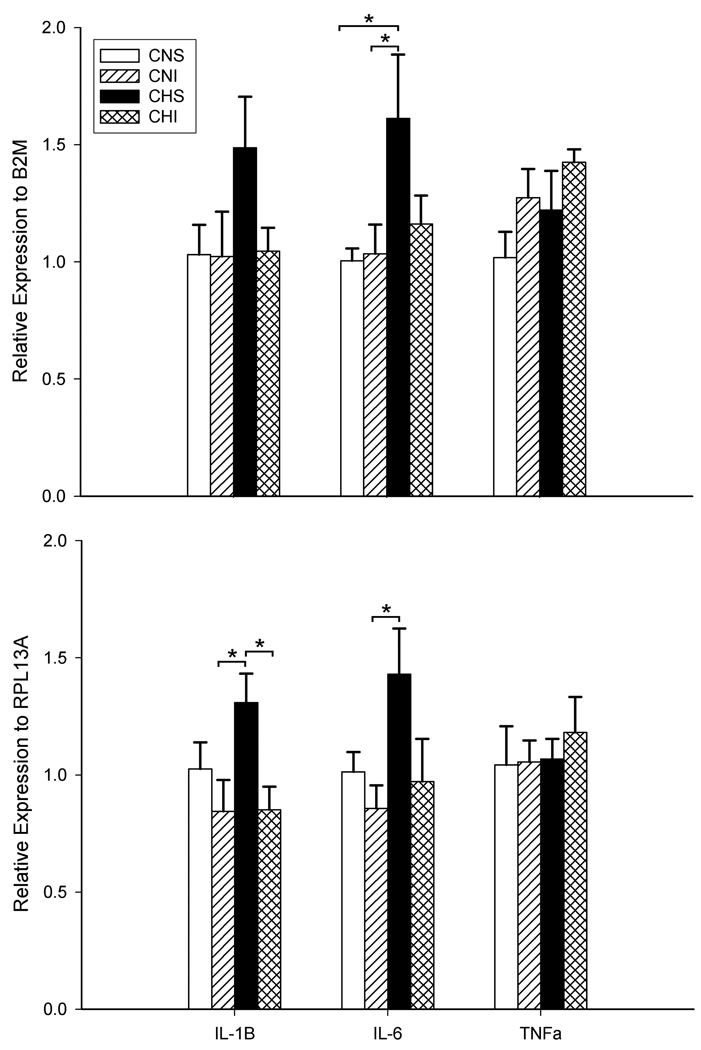
Figure 2 shows cytokine expression (mRNA) levels in the NTS for the four experimental groups. The data is normalized to two housekeeping genes previously shown to be unaffected by hypoxia (Tang et al., 2010). IL-6 and IL-1β behaved similarly to V̇I with increases in chronic hypoxia that were blocked by ibuprofen, while ibuprofen had no effect in normoxic rats. TNFα was not affected by this duration of chronic hypoxia or ibuprofen.
Figure 2.
mRNA levels for IL-1β, IL-6 and TNFα in biopsies of the nucleus tractus solitarii in control (N) rats and after 7 days of chronic hypoxia (H). Cytokine expression is normalized to B2M in the top panel and RPL13A in the lower panel. TNFα was not affected significantly by any treatment. Chronic hypoxia significantly increased IL-1β and IL-6 (HS vs. NS) and this was blocked by ibuprofen (HI vs. HS). * p<0.05, 1-tailed, n = 5 except n= 4 for IL-1β in HS.
4. Discussion
The results showed an effect of ibuprofen on ventilation during hypoxia in chronically hypoxic rats but not normoxic rats (Fig. 1). However, there were no significant effects of ibuprofen on ventilation in rats breathing normoxic or hypercapnic gas before or after acclimatization to chronic hypoxia. Hence, the data indicate that the previously demonstrated effect of ibuprofen to block the time-dependent increase in carotid body discharge with chronic hypoxia (Liu et al., 2009) is physiologically significant in terms of blocking the time-dependent increase in hypoxic ventilation during chronic hypoxia.
4.1 Critique of Methods
Although ibuprofen blocked the increase in V̇I in acute hypoxia after chronic hypoxia, PaCO2 was not significantly increased by ibuprofen treatment (Table 1) as predicted if only ventilation changed. One possible explanation is that ibuprofen decreased CO2 production but we did not measure any significant differences when we measured V̇CO2 in a separate group of rats (Table 2). There were only three rats in each chronically hypoxic group for these measurements, so we do not have strong statistical power to indicate that V̇CO2 is not changing with ibuprofen. Also, we did not observe a significant effect of ibuprofen on V̇I in this small cohort. Hence, further experiments will be necessary to resolve the role of metabolic responses in the effects of ibuprofen in ventilatory acclimatization to hypoxia.
Another possible explanation for decreased ventilation without increased PaCO2 is a change in physiological dead space with ibuprofen treatment. Olson (1994) reported increased physiological dead space, or decreased alveolar ventilation, in rats during acute hypoxia. Physiological dead space returned to normal values after 4 to 7 days of chronic hypoxia so we would not expect to see differences in our chronically hypoxic rats. However, if such an increase in physiological dead space involved inflammatory responses, then the time course and resolution of such changes could differ between experiments depending upon the immune status of the animals being studied. If ibuprofen blocked an increase in physiological dead space, then PaCO2 would be lower for any overall V̇I, which is in the direction of our results. Hence, this is another problem warranting further investigation.
Although not significant, PaCO2 tended to be higher in the chronically hypoxic rats treated with ibuprofen versus saline breathing 10% O2 (16.7 ±1.8 vs. 14.5±0.5 Torr, respectively) or 21% O2 (19.3±0.9 vs 18.0±1.0 Torr, respectively). Similar trends were not observed in normoxic rats, and in fact, PaCO2 was significantly lower with ibuprofen in normoxic rats breathing room air for unexplained reasons (Table 1). Summarizing, the most conservative explanation of the results is that ibuprofen blunts the time-dependent increase in hypoxic ventilation. However, these experiments do not have the statistical power to distinguish potential effects of the initial immune status of the animals or ibuprofen on changes in metabolic rate or gas exchange efficiency with chronic hypoxia.
The changes in cytokine gene expression we measured may not necessarily reflect changes in protein levels but they agree well with previous observations for carotid bodies (Lam et al., 2008; Liu et al., 2009). Similar to Liu and co-workers, we referenced cytokine gene expression to house-keeping genes shown to be stable in chronic hypoxia (Tang et al., 2010). We also selected house-keeping genes (B2M and RPL13A) with expression levels similar to that of the cyokines being studied, to reduce errors from normalizing to extremely large values.
4.2 Physiological Mechanisms
Liu and co-workers (2011) propose that chronic hypoxia evokes some of the same mechanisms in the carotid body that are responsible for hyperalgesia, i.e. increased pain sensation to a noxious stimulus. This is based on several observations, including their own (Liu et al., 2009). Hyperalgesia depends on cytokines that are released from activated immune cells and glia, such as IL-1β, IL-6 and TNF-α, which increase excitability in sensory nerve endings (Watkins and Maier, 2002). These cytokines increase in the carotid bodies with chronic hypoxia (Liu et al., 2009) and in sensory neurons they can up-regulate genes coding for specialized transduction molecules (e.g. acid sensitive ion channels, ASICs) and voltage gated ion channels (e.g. NaV1.7) that are involved in carotid body chemoreception (reviewed by Liu et al., 2011). Also, the time course of changes in inflammatory cytokines and increased O2-sensitivity in the carotid body with chronic hypoxia are similar and both are blocked with ibuprofen (Liu et al, 2009). Finally, the dose of ibuprofen used in the isolated carotid body studies (Liu et al., 2009) was the same as we used, and has been shown to suppress phenotypic changes in rat primary sensory neurons induced by inflammation (Voilley et al., 2001). Considered together, the results support the idea that the effects of ibuprofen to block inflammatory-induced increases in carotid body O2-sensitivity, which share common mechanisms with peripheral nervous system plasticity during chronic inflammatory pain, contribute to the changes in ventilatory acclimatization to hypoxia that we observed with ibuprofen.
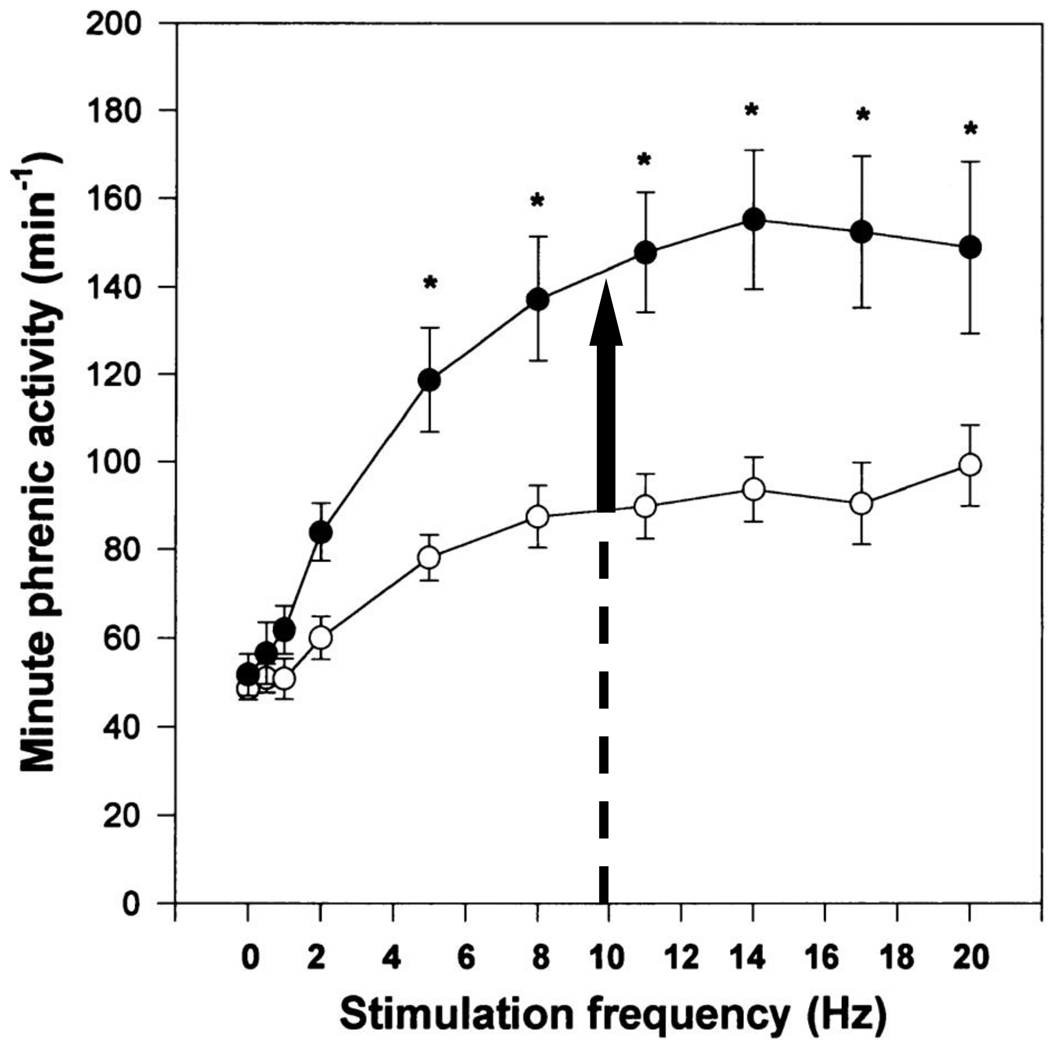
Inflammatory cytokines also enhance synaptic transmission between primary afferents and neurons in the CNS in neuropathic pain, which involves both presynaptic and postsynaptic changes (Tsuda et al., 2005). We hypothesize that inflammatory signaling contributes to the increased ventilatory sensitivity to carotid body chemoreceptor afferent input with chronic hypoxia we observed previously (Dwinell and Powell, 1999; Wilkinson et al., 2010). Fig. 3 shows how we measured an “increased CNS gain of the hypoxic ventilatory response (HVR)” in anesthetized rats (Dwinell and Powell, 1999). Minute phrenic activity (the neural analog of ventilation), was greater in chronically hypoxic rats compared to normoxic controls for any given stimulation frequency of the carotid sinus nerve (the neural analog of hypoxic stimulation). In our current experiments, chronically hypoxic rats treated with ibuprofen would be predicted to have the same frequency of carotid body discharge as normoxic controls at any PaO2 (cf. Liu et al., 2009). Hence, the increased CNS gain of the HVR should have increased hypoxic ventilation in our chronically hypoxic rats treated with ibuprofen but we observed no difference from normoxic controls (Fig. 1). This implies that ibuprofen blocked the increased CNS gain of the HVR, in addition to carotid body sensitization. Given the effect of ibuprofen to block increases in IL-1β and IL-6 with chronic hypoxia in the brainstem (Fig. 2), we propose that inflammatory signals are necessary for CNS plasticity contributing to ventilatory acclimatization to hypoxia.
Figure 3.
Effects of chronic hypoxia on the phrenic response to carotid sinus nerve stimulation in anesthetized rats (after Dwinell and Powell, 1999). Minute phrenic ventilation (the product of phrenic burst frequency and rectified, integrated phrenic amplitude) increases significantly more with increasing frequency of electrical stimulation of the carotid sinus nerve in chronically hypoxic rats (filled symbols) than in normoxic controls (open symbols). Vertical arrow at carotid sinus nerve stimulation frequency = 10 Hz shows how ventilation is predicted to increase in chronically hypoxic rats when the increase in carotid body O2-sensitivity with chronic hypoxia is blocked by ibuprofen. By contrast, we observed no increase in hypoxic ventilation with ibuprofen in chronic hypoxia (cf. Fig. 1) implying ibuprofen blocks plasticity in the CNS with chronic hypoxia too.
Although chronic hypoxia increases inflammatory mediators in both the peripheral and central nervous system, the sources may differ in the carotid bodies and CNS respiratory centers. In carotid bodies, there is evidence for cytokines from invading immune cells, type I glomus cells and glial-like type II cells (Lam et al., 2008; Liu et al., 2009). We did not isolate the cellular source of cytokines with chronic hypoxia in our NTS biopsies but both neurons and glia are known to produce cytokines in the CNS (Watkins and Maier, 2002; Tsuda et al., 2005). In the carotid body, the role of resident versus migrating macrophages with chronic hypoxia (cf. Liu et al., 2009) remains to be determined. The source of migrating immune cells in the carotid body is unknown but the highly vascularized nature of carotid bodies and their fenestrated endothelium (Fidone and Gonzalez, 1986) may render them more susceptible to migrating immune cells than other sites. Differences in the observed time-course of cytokine changes with chronic hypoxia between carotid bodies and the NTS also suggest different sources, stimuli or responses. After 7 says of chronic hypoxia, IL-1β returned to control levels in carotid bodies (Liu et al., 2009) but it was elevated in the NTS (Fig. 2). Finally, differential inflammatory responses to chronic hypoxia are suggested also by the different effect of ibuprofen on hypoxic versus normoxic ventilation after chronic hypoxia (Fig. 1). Ventilatory acclimatization to hypoxia involves both an increased hypoxic ventilatory response, and persistent hyperventilation when normoxia is restored (Bisgard and Neubauer, 1995). The increased normoxic ventilatory drive involves altered regulation of arterial PCO2 (Weil, 1986) and potentially different sites of respiratory plasticity (Nichols et al., 2009). Further studies are needed to determine spatially and temporally specific inflammatory responses to chronic hypoxia in cardiorespiratory control pathways.
The most fundamental question raised by these results is, how does chronic hypoxia signal an inflammatory response? One candidate is HIF-1α, which increases with chronic hypoxia in both carotid bodies and CNS respiratory centers (reviewed by Powell and Fu, 2008). Recently, HIF-1α has been shown to regulate innate immunity and it is proposed that this relationship evolved to allow phagocytic cells to operate efficiently in the hypoxic microenvironments of infected tissues (Zinkernagel et al., 2007). Conditional deletion of HIF-1α in myeloid cells blocks the normal inflammatory response (Cramer et al., 2003) and HIF-1α increases TNF-α by a nitric oxide-dependent mechanism (Peyssonnaux et al., 2005). Hypoxia also indirectly activates NF-κB, which promotes transcription of TNF-α and other cytokines. In its inactive state in the cytosol, NF-κB is bound to the inhibitory protein IκB. When IκB is phosphorylated by IκB kinase (IKK-β), it separates from NF-κB and is degraded. Then NF-κB translocates to the nucleus and binds to DNA response elements to promote transcription. In normoxia, IKK-β is inhibited by prolyl hydroxylases that depend on O2 but in hypoxia, IKK-β is disinhibited, thereby activating NF-κB (Rius et al., 2008).
Conversely, NF-κB promotes expression of HIF-1α, linking the evolutionarily ancient stress responses of hypoxia and innate immunity (Rius et al., 2008). Ibuprofen blocks nuclear translocation of NF-κB, which would block NF-κB transcription of IL-6, IL-1β and TNF-α (Stuhlmeier et al., 1999). In other words, ibuprofen has anti-inflammatory effects independent of its classical effect to inhibit cyclooxygenase 1 and 2, which control the production of inflammatory prostanoids that can sensitize nociceceptors. However, a role for changes in prostaglandin modulation of the carotid bodies with ibuprofen treatment has not been ruled out (reviewed by Liu et al., 2011).
4.3 Significance and Future Directions
The dose of ibuprofen used in this study is equivalent to the therapeutic does used for pain in humans so it is interesting to consider how NSAIDs could affect people experiencing sustained hypoxia. Acute Mountain Sickness (AMS) is very common at in otherwise healthy people who travel rapidly to altitudes above 2000m (West et al., 2007). Headache is a universal feature of AMS, which is commonly treated with nonsteroidal anti-inflammatory drugs (NSAID) such as ibuprofen. AMS resolves as acclimatization to high altitude improves arterial oxygenation but our results predict that NSAID could block acclimatization. Hence, ibuprofen as a common treatment for AMS has the potential to prolong the problem by blocking or delaying the mechanisms of ventilatory acclimatization to hypoxia.
It is not known if patients with chronic hypoxemia, for example with chronic obstructive pulmonary disease, exhibit ventilatory acclimatization to hypoxia and have neural plasticity in ventilatory chemoreceptor chemoreflexes. Furthermore, it is not known if ibuprofen can reverse ventilatory acclimatization to hypoxia or if it just blocks its induction. Hence, further study is necessary to determine if NSAIDs may also be contraindicated for patients with chronic hypoxemia.
Acknowledgments
This study was supported by RO1HL081823, 1P01HL098053 and White Mountain Research Station. The authors would like to thank Drs. Moh Malek and Sue Hopkins, for their assistance with statistical analysis and Drs. Kechun Tang and Ellen Breen for their assistance with the ctyokine expression measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York, Basel, Hong Kong: Marcel Dekker, Inc.; 1995. pp. 617–618. [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–86. [PubMed] [Google Scholar]
- Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J. Appl. Physiol. 1999;87:817–823. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]
- Fidone SJ, Gonzalez C. Initiation and control of chemoreceptor activity in the carotid body. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology: The Respiratory System - Control of Breathing. Baltimore, MD: Waverly Press, Inc; 1986. pp. 247–312. [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. Upper Saddle River, N.J.: Pearson Prentice Hall; 2004. [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc. Natl. Acad. Sci. U. S. A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SY, Tipoe GL, Liong EC, Fung ML. Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem. Cell Biol. 2008;130:549–559. doi: 10.1007/s00418-008-0437-4. [DOI] [PubMed] [Google Scholar]
- Liu X, He L, Stensaas L, Dinger B, Fidone S. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:L158–L166. doi: 10.1152/ajplung.90383.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, He L, Dinger B, Gonzalez C, Stensaas L, Fidone S. A chronic pain: inflammation-dependent chemoreceptor adaptation in rat carotid body. Respir. Physiol. Neurobiol. 2011 doi: 10.1016/j.resp.2011.03.006. This volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats. Respir. Physiol. Neurobiol. 2009;168:272–280. doi: 10.1016/j.resp.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB., Jr Physiological dead space increases during initial hours of chronic hypoxemia with or without hypocapnia. J. Appl. Physiol. 1994;77:1526–1531. doi: 10.1152/jappl.1994.77.3.1526. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Datta V, Cramer T, Doedens Al, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL. The influence of chronic hypoxia upon chemoreception. Respir. Physiol. Neurobiol. 2007;157:154–161. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Fu Z. HIF-1 and ventilatory acclimatization to chronic hypoxia. Respir. Physiol. Neurobiol. 2008;164:282–287. doi: 10.1016/j.resp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir. Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Reid SG, Powell FL. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J. Appl. Physiol. 2005;99:2108–2114. doi: 10.1152/japplphysiol.01205.2004. [DOI] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmeier KM, Li H, Kao JJ. Ibuprofen: new explanation for an old phenomenon. Biochem. Pharmacol. 1999;57:313–320. doi: 10.1016/s0006-2952(98)00301-3. [DOI] [PubMed] [Google Scholar]
- Tang K, Xia FC, Wagner PD, Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir. Physiol. Neurobiol. 2010;170:16–22. doi: 10.1016/j.resp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, Schoene RB, Milledge JS. High Altitude Medicine and Physiology. 4th ed. London: Hodder; 2007. [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Weil J. Ventilatory control at high altitude. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology: The Respiratory System - Control of Breathing. Baltimore, MD: Waverly Press, Inc; 1986. pp. 703–728. [Google Scholar]
- Wilkinson KA, Huey K, Dinger B, He L, Fidone S, Powell FL. Chronic hypoxia increases the gain of the hypoxic ventilatory response by a mechanism in the central nervous system. J. Appl. Physiol. 2010;109:424–430. doi: 10.1152/japplphysiol.01311.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]