Abstract
Objectives
Nearly all smokers who lapse experience a full blown relapse, but the mediating mechanisms that contribute to this relationship are not well understood. A better understanding of these mechanisms would help to advance more effective relapse prevention treatments for smokers. The purpose of this study is to experimentally evaluate the effects of a programmed smoking lapse on smoking relapse and the effects of post-lapse changes in craving on relapse.
Method
Adult smokers (n = 63) who quit smoking with a brief cognitive-behavioral intervention and self-help materials were randomly assigned to one of two experimental conditions after 48 hours of abstinence: No Lapse (a no smoking control/30 minute waiting period) or Lapse (smoking two cigarettes of their favored brand during a 30 minute period). All participants were then followed daily for 14 days. Craving and biochemically-verified self-reported abstinence were assessed on each follow-up day. Time (days) to relapse (seven consecutive days of smoking) was the main dependent measure.
Results
Results of Cox regression analysis revealed that participants in the lapse condition relapsed more quickly than participants in the no lapse condition (HR = 2.12, 95% CI [1.03, 4.35]). These effects were due in part to episodic increases in craving among participants in the lapse condition only (HR = 12.42, 95% CI [2.00, 77.1]).
Conclusions
Previously abstinent smokers who lapse are at risk for increased cigarette cravings and consequently, full-blown relapse. These results have implications for both cognitive-behavioral treatments for relapse prevention and for medications designed to help smokers manage cravings.
Keywords: Smoking cessation, relapse, craving
The overwhelming majority of smokers who attempt to quit using any method or delivery channel are unsuccessful (Shiffman, Brockwell, Pillitteri, & Gitchell, 2008). One of the central challenges for smokers who achieve initial abstinence is that they are at high risk for lapsing. A lapse is a circumscribed period of smoking after a smoker has achieved abstinence (see Hughes, Keely, Niaura, et al., 2003); after a lapse, individuals return to a period of abstinence. However, periods of abstinence following a lapse are typically short-lived: nearly every smoker who lapses eventually relapses (Brandon, Tiffany, Obremski, & Baker, 1990; Garvey et al., 1992; Chornock, Stitzer, Gross, & Leischow, 1992). Unfortunately, the mediating mechanisms that cause lapses to become relapses are poorly understood and interventions designed to stop the progression from lapse to relapse have not been especially effective. Advancing the field’s understanding of the mechanisms that turn lapses into relapses has been recommended as a way to improve smoking cessation treatments (Abrams, Emmons, Niaura, Goldsten, & Sherman, 1991; Niaura & Abrams, 2002; Piasecki, Fiore, McCarthy, & Baker, 2002; Shiffman, 2006). Once these mechanisms are identified treatments could be developed or existing treatments could be modified to target those mechanisms and improve cessation outcomes. This study experimentally investigates the role that craving plays in turning smoking lapses into relapses.
Craving plays a central role many theories of relapse (for reviews, see Brandon, Vidrine, & Litvin, 2007; Marlatt & Donovan, 2005; Skinner & Aubin, 2010; Tiffany,Warthen, & Goedeker, 2009). Two types of craving have been identified (see Shiffman, 2000). Background craving occurs on a more or less steady basis throughout the day, diminishing in intensity over time. Increasing levels of deprivation from smoking typically lead to stronger levels of background cravings (Payne, Smith, Sturges, & Holleran, 1996) and smokers with more intense levels of background craving have increased risk of relapse (Killen & Fortman, 1997; Shiffman et al., 1997). However, periodic episodes of intense surges in craving (episodic craving) can persist even as the intensity of background craving declines (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996b; Shiffman et al, 1997). Episodic craving can be triggered by contextual cues (Shiffman et al., 1996b) and can predict both lapses and relapses (e.g., Shiffman et al, 1996b). Owing to its importance in predicting smoking cessation outcomes and relapse, cognitive-behavioral treatments offer strategies to help smokers manage cravings (Brown, 2003). Pharmacological treatments for smoking cessation are thought to operate, in part, by blunting levels of background craving (Tiffany, Cox, & Elash, 2000). Some nicotine replacement medications (e.g., nicotine gum) can be used as “rescue” medications to help smokers manage episodic craving in response to provocative cues (Shiffman et al., 2003).
Less well studied is what happens to craving after a smoking lapse and whether it prompts relapsing. Studies have suggested that craving can both decrease (Juliano, Donny, Houtsmuller, & Stitzer, 2006) and increase (Shiffman et al., 1996b; see also Shiffman & Paty, 2006) after a lapse. Post-lapse decreases in craving can be explained by negative reinforcement models (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). In this view, increased craving during an abstinent period (i.e., pre-lapse craving) represents an aversive state from which the smoker seeks relief by smoking (i.e., smoking reduces craving). A resumption of smoking (i.e., relapse) results from the drive to alleviate episodic increases in craving. Post lapse increases in craving can be explained by reinstatement models of drug use (Chiamulera, Borgo, Falchetto, Valerio, & Tessari, 1996; Shaham, Adamson, Grocki, & Corrigall, 1997; see Epstein, Preston, Stewart, & Shaham, 2006). In this view, the small priming dose of nicotine provided by a smoking lapse (perhaps combined with the context or cues associated with the lapse; see Niaura et al., 1988) creates an appetitive motivational state, indexed by increased craving, which then stimulates further smoking (Baker, Morse, & Sherman, 1986; Robinson & Berridge, 1993; Wise & Bozarth, 1987). Regardless of the direction of post-lapse changes in craving, no studies have examined whether these changes contribute to relapse.
The purpose of this study is to experimentally evaluate the effects of a programmed smoking lapse on smoking relapse and the effects of post-lapse changes in craving on relapse. We enrolled smokers who were motivated to quit smoking as participants. After 48 hours of biochemically confirmed abstinence (achieved with a minimal brief cognitive-behavioral intervention; see Shadel & Niaura, 2003), we experimentally manipulated lapse by randomly assigning participants to either a lapse or no-lapse condition, after which we tracked daily levels of craving and smoking behavior through a brief follow-up (14-days). Relapse was defined as the first instance of smoking at least one cigarette a day for seven consecutive days following the manipulation day (Hughes et al., 2003). We hypothesized that participants assigned to the lapse condition would relapse more quickly compared to participants in the no-lapse condition and that the effect of lapse on relapse would be explained (mediated) by post-lapse changes in craving.
Experimental studies of relapse such as the one reported in this paper permit stronger causal inferences compared to field studies and quasi experimental designs and allow for controlled evaluation of theoretical mechanisms that are thought to underlie an observed phenomenon (see Chow, 1995; see also McKee, 2009; Perkins, Stitzer, & Lerman, 2006). The current design was inspired by the only two smoking studies that have used experimental designs to model relapse in the laboratory (Chornick et al., 1992; Juliano et al., 2006). These studies manipulated lapses by having individuals smoke five cigarettes after an abstinent period (vs not smoking and/or smoking de-nicotinzed cigarettes) and measured time to first cigarette puff (after the manipulation) as the outcome. Findings from these studies suggested that lapse is causally related to subsequent smoking in recently quit individuals. However important these findings, these studies were limited by their use of individuals who were not motivated to quit, short follow-up periods (< one week), non-standard definitions of relapse, and lack of an attention to mechanisms that link lapse to relapse. The current study was designed to address these concerns with this previous work and to enhance the clinical utility of laboratory models of relapse.
Methods
Participants
This study was approved and monitored by the IRB at RAND Corporation. Individuals were eligible to participate in this study if they were: (1) between 18 and 65 years of age, (2) a current cigarette smoker, (3) smoking nearly every day for the last five years, (4) currently smoking ≥15 cigarettes a day, (5) have quit smoking for at least 48 hours at some point in their lives, and (6) were motivated to quit smoking, as indicated by a score in excess of 120 on their responses to two questions (each scaled from 0–100, where 0 is not at all and 100 is extremely; see Shiffman et al., 1996b): “How motivated are you right now to quit smoking?” and “How confident are you right now to quit smoking?” Individuals were excluded from participating if they: (1) were currently being treated with medications to help them quit smoking, (2) were receiving counseling to help them quit smoking, or (3) reported being or having been treated for any number of medical and/or psychological conditions within the last 12 months (e.g., arrhythmia, atherosclerosis, cancer [any type], angina pectoris, chronic bronchitis, coronary heart disease, congestive heart failure/congestive heart disease, diabetes, chronic obstructive pulmonary disease, myocardial infarction, hypertension, severe asthma, cerebrovascular accident, bipolar disorder, dementia, and schizophrenia). Women who were pregnant or who were planning to become pregnant in the next 30 days were also excluded.
Participants were recruited using media advertising (e.g., newspaper, billboards, fliers). A total of 548 individuals were screened for the study, of which 222 (41% of those screened) met inclusion criteria. The main reasons that participants were excluded were: smoking fewer than 15 cigarettes per day (31%), concurrent medical/psychiatric conditions (27%), and a failure to quit for 48 hours in the past (10%). Of those scheduled for a baseline visit, a total of 158 attended (a show rate of 71%). This paper examines the subset of n = 63 participants who were able to maintain 48 hours of abstinence during the study and thus eligible to be randomized to one of the two conditions (see results for additional information on the sample).
Procedures
Overview
Total length of participation in the study was 20 consecutive days. The study involved a baseline session, pre-manipulation phase, a manipulation day, and a post-manipulation follow-up period. On week days, participants attended daily individual sessions during which they completed assessments; during each of the three weekends that fell within the timeframe of the study, participants completed a single assessment. Participants could be paid up to $310 for attending the sessions and completing the study procedures. The payment schedule was as follows: $40 for the baseline session; $20 for each pre-manipulation session ($60 total); $10 for each post-manipulation/follow-up session ($100); and $10 for each weekend assessment ($30). Participants could earn a $30 bonus if they remained abstinent during the pre-quit/pre-manipulation phase and a $50 bonus for attending all of the post-manipulation/follow-up sessions. Parking and transportation expenses were also reimbursed.
Baseline session
Participants attended a baseline session on a Friday, during which all aspects of the study were described to them, including that they could be assigned to a study condition in which they would be asked to smoke two cigarettes after quitting (the lapse condition).1 After providing written informed consent, participants completed measures of their smoking and quitting history and smoking-related psychosocial variables. At this baseline session, all participants were asked to provide the researchers with two of their own cigarettes on the chance that they would be assigned to the lapse condition. At the end of the baseline session, all participants set a quit date for the following Monday and received a brief (20-minute) quit smoking intervention and a self-help booklet for help with quitting (see Shadel & Niaura, 2003).
Quitting/pre-manipulation phase
Participants completed one take-home follow-up assessment over the weekend. Monday was their target quit day. They attended in-person sessions on Monday and Tuesday during which they reported on their smoking status (abstinence was confirmed with expired air carbon monoxide [CO]) and completed other assessments.
Manipulation day
Wednesday was designated as the manipulation day. Participants who were able to maintain 48-hour abstinence (Monday through Wednesday, confirmed with expired air CO) were randomized to one of the two experimental conditions: no lapse (a no smoking, 30-minute waiting period) or lapse (smoking two cigarettes of their favored brand during a 30-minute period). They completed assessments both before and after the manipulation (i.e., the pre- and post-assessments were 30 minutes apart). Neither participants nor staff that had contact with participants was aware of condition assignment until the manipulation day.
Post-manipulation/follow-up phase
Participants attended daily visits to the laboratory on the 10 weekdays that followed the manipulation day (Thursday through Friday; Monday through Friday; Monday through Wednesday) and completed two weekend assessments during this time. Smoking status was tracked daily during this 14-day follow-up interval; expired air CO was used to verify self-reported abstinence at all study points.
Study exit/clinical treatment option
Participants were thoroughly debriefed during the last follow-up. They had the option to attend eight group-based cognitive behavioral smoking cessation classes following their participation to assist them with their efforts at quitting smoking. The sessions consisted of monitoring participants’ progress and a 30-minute lecture on a theme typical of state-of-the-science smoking cessation programs (e.g., self-monitoring, stimulus control, cognitive coping; for details of this treatment approach, see Brown, 2003).
Measures
Demographics
Gender, age, race/ethnicity, and education were assessed at baseline.
Smoking and quitting history
Number of lifetime and past year 24 hour quit attempts, number of years smoked, and current daily smoking rate were assessed at baseline.
Nicotine dependence
The Nicotine Dependence Syndrome Scale (NDSS; see Shiffman et al. 2004) was used to assess nicotine dependence at baseline. We used the total scale factor score to assess nicotine dependence in this study (e.g., see Gwaltney, Shiffman, Balabanis, & Paty, 2005). Higher scores reflect greater levels of nicotine dependence.
Craving
A five-item scale was used to assess craving at each study visit (see Shiffman et al., 1996b): “I have a desire for a cigarette right now”; “If it were possible, I would smoke right now”; “All I want right now is a cigarette”; “I have an urge for a cigarette”; and “I crave a cigarette right now”. Responses were made on a 1 to 10 scale for each item (1 = not true of me; 10 = extremely true of me) and these responses were averaged to produce a total craving score for that day. Alpha coefficients exceeded .951 across all measurement points in this study.
Smoking status
Smoking status during each visit was determined by asking participants whether they had smoked since the last visit (i.e., within the last 24 hours). Self-reports of abstinence were confirmed using expired air CO values < 12 ppm. No participant who reported abstinence needed to be reclassified as a smoker based on the results of their CO assessment.
Results
Descriptive Characteristics of Randomized Participants
Table 1 presents baseline characteristics between participants in the two conditions (there were no significant differences between these randomized participants and the 95 individuals who were unable to maintain the 48 hour abstinence period on any of the variables in Table 1). Across conditions, participants were mostly similar, though participants in the no-lapse condition had significantly higher levels of nicotine dependence compared to participants in the lapse condition. Several of these variables were included in later analyses as covariates to improve model fit and to control for possible selection imbalance.
Table 1.
Baseline characteristics between participants in the two conditions.
| No Lapse (n = 32) |
Lapse (n = 31) |
p | |
|---|---|---|---|
| M Age (SD) | 43.0 (12.8) | 43.5 (13.5) | 0.90 |
| Gender (% female) | 59% | 52% | 0.54 |
| Race | 0.52 | ||
| % Caucasian | 53% | 55% | |
| % African-American | 38% | 26% | |
| % Other | 9% | 16% | |
| % > high school education | 75% | 77% | 0.82 |
| M NDSS total scale (factor) score (SD) | −.156 (.750) | −.608 (.920) | 0.04 |
| M cigarettes smoked/day (SD) | 24.3 (12.1) | 19.6 (11.2) | 0.12 |
| M number of years smoked (SD) | 22.1 (11.8) | 25.3 (15.8) | 0.52 |
| M number of 24 hour quit attempts: past year (SD) | 2.2 (2.9) | 2.4 (2.3) | 0.34 |
| M number of 24 hour quit attempts: lifetime (SD) | 7.4 (6.3) | 21.1 (32.5) | 0.07 |
| M craving scores (SD) a | 5.9 (2.3) | 4.7 (2.3) | 0.05 |
Baseline craving scores are defined as the average of craving scores from baseline through the pre- manipulation day craving score.
Manipulation Check
Everyone in the lapse group smoked two cigarettes (confirmed by collecting the smoked cigarette butts) and no one in the no lapse group smoked (confirmed by researcher observation) during the manipulation phase. Assessment of changes in expired air CO pre- to post-manipulation confirmed these observations: a 2 (condition: no lapse, lapse) X 2 (time: pre-manipulation, post-manipulation) analysis of variance revealed a significant condition X time interaction (interaction F (1, 60) = 33.945, p < .0001). Participants in the no lapse condition had no changes in their CO during the manipulation interval (Ms = 2.8 vs 2.8, p = .908) whereas participants in the lapse condition had significant increases in CO during the same interval (Ms = 4.1 vs 9.1, p < .0001). Participants in the lapse condition had significantly higher post-manipulation CO levels compared to participants in the no lapse condition (p < .0001).
Craving Change as a Function of the Manipulation
The amount of change in participants’ craving (from baseline to immediately after the manipulation) depended on the experimental manipulation that participants received. A 2 X 2 (condition: no lapse, lapse) X 2 (time: baseline, post-manipulation) analysis of variance revealed a significant condition X time interaction (interaction F (1, 60) = 14.01, p < .0001). Unlike participants in the no lapse condition, who experienced no changes in their craving during the manipulation interval (Ms = 5.9 vs 5.4, p = .168), participants in the lapse condition experienced a significant decrease in craving pre- to post-manipulation (Ms = 4.7 vs 2.2, p < .0001).
Mediation Analyses
We hypothesized that participants assigned to the lapse condition would relapse more quickly compared to participants in the no-lapse condition and that the effect of lapse on relapse would be mediated by post-lapse changes in craving. Our meditation analyses are based on the MacArthur approach (Kraemer Kiernan, Essex, & Kupfer, 2008), a modified version of the Baron and Kenny approach (1986) that is appropriate for analyzing mediation in clinically-relevant experimental data. The MacArthur approach was proposed specifically as a way to test mediation in the context of randomized clinical trials in which alternate treatment conditions may affect mediators (and thus outcomes) differently depending on condition. Although the current study is not a clinical trial, the logic of the approach for testing mediation is the same. That is, we expected that inducing a lapse among participants in the lapse condition would cause changes in craving that would in turn hasten relapse. Among participants in the no lapse condition, we expected craving to follow its natural course given that we did not intervene to affect it. The key assumptions underlying the MacArthur approach are that the independent variable temporally precedes the mediating variable and that the independent variable causally impacts the mediator. Under these assumptions, a statistically significant interaction between the independent variable and the mediator is interpreted as evidence of mediation (for additional details on this approac, see Kraemer et al., 2008).
Effect of lapse on time to relapse
The first question we addressed was whether participants in the lapse condition relapsed more quickly than participants in the no lapse condition. We used Cox proportional hazards analysis (Cox regression) to model time to relapse, with number of days post-manipulation as the time scale. The first opportunity participants had to relapse (i.e., to begin a 7-day period of smoking following the 48-hour abstinence period) was the day of the manipulation, following their visit to the laboratory. Thus, in our Cox regression model, we defined Day 0 as the period from the baseline session to the end of the post-manipulation assessment. Day 1 was defined as any time after the post-manipulation assessment on the day of the manipulation (Wednesday) up until the point of assessment the following day (Thursday). Day 2 covered the period from the first follow-up assessment (Thursday) to the second (Friday), and so on through Day 14. Time to relapse was defined as the number of measurement occasions (days) between day 0 and relapse. Relapse was defined using a standard definition of seven consecutive days of smoking at least one cigarette each day (Hughes et al., 2003); time to relapse was defined as the number of days to the first of seven consecutive days of smoking. 2 Although we observed participants for 14 days post-manipulation, censoring occurred at 9 days post-manipulation. In other words, participants who had not begun smoking by the ninth day post-manipulation were no longer at risk of being observed to relapse in our study (due to the 14-day length of the observation period) and were therefore censored on that day. Censoring is a common feature of time-to-event data, which, if ignored, can lead to substantial bias in the analysis. Survival analysis properly takes censoring into account (Selvin, 2004).
Our initial Cox regression model included relapse study condition (no lapse = 0, lapse = 1) and the following baseline (time-fixed) covariates as predictors of time-to-relapse: participant age (in years), nicotine dependence as assessed by the NDSS (Shiffman et al., 2004), and the four smoking and quitting history variables from Table 1 (number of years smoked, number cigarettes smoked per day, number of past year 24 hour quits, number of lifetime 24 hour quits).
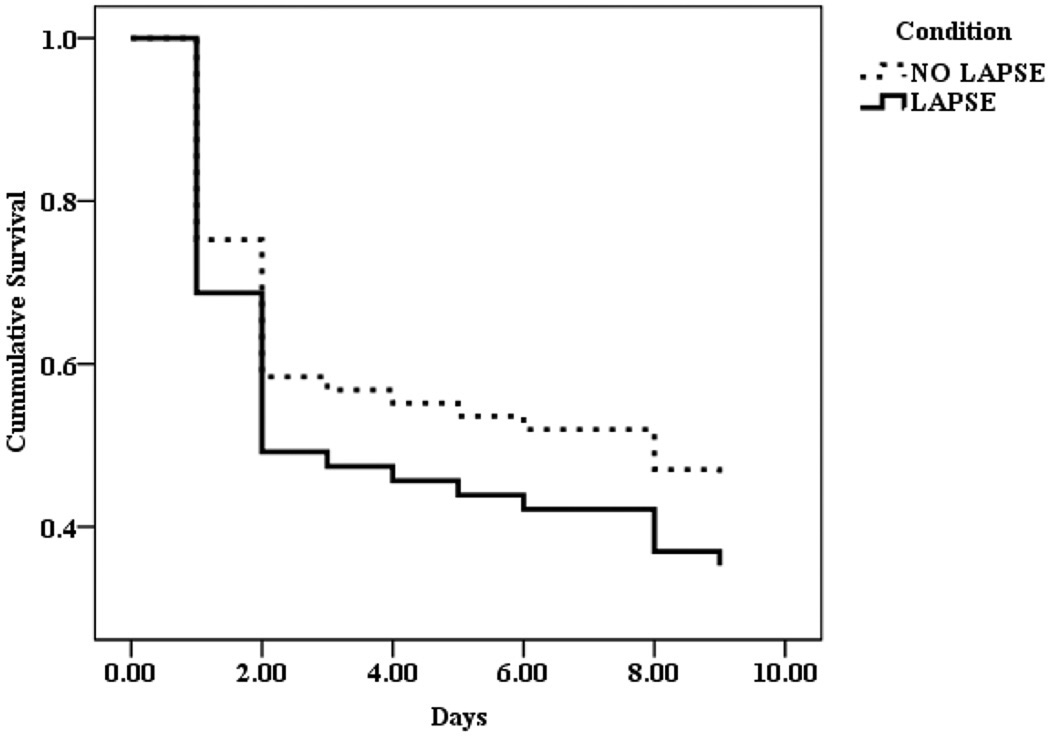
Table 2 displays the results for this analysis. After adjustment for baseline covariates, the risk of relapse was estimated to be more than two times as high among participants in the lapse condition as it was among participants in the control condition (HR = 2.12, 95% CI [1.03, 4.35]). Whereas 53.1% of participants in the no lapse condition relapsed, 65.5% of participants in the lapse condition did so. Figure 1 displays the unadjusted survival function for participants in the lapse and no lapse conditions. These results clearly show that an increased risk of relapse (decrease in time to relapse) among those in the lapse condition compared to those in the no lapse condition.
Table 2.
Results of Cox Regression Testing the Effect of Study Condition (lapse, no lapse) on Relapse
| Predictor | β | SE | Hazard ratio | 95% CI | p |
|---|---|---|---|---|---|
| Condition a | 0.75 | 0.37 | 2.12 | [1.03, 4.35] | 0.04 |
| Age (years) | 0.00 | 0.02 | 1.00 | [0.95, 1.04] | 0.94 |
| NDSS total scale (factor) score | 0.39 | 0.22 | 1.48 | [0.95, 2.30] | 0.08 |
| Number of years smoked | 0.00 | 0.02 | 1.00 | [0.97, 1.03] | 0.97 |
| Number cigarettes smoked per day | 0.02 | 0.11 | 1.02 | [0.82, 1.26] | 0.87 |
| Number of 24 hour quits: past year | −0.02 | 0.01 | 0.98 | [0.96, 1.01] | 0.19 |
| Number of 24 hour quits: lifetime | 0.75 | 0.37 | 2.12 | [1.03, 4.35] | 0.04 |
Note. N = 62, 34 events (relapse) and 28 censored cases; Likelihood ratio chi-square (7 df) = 10.15, p = 0.18.
0 = no lapse, 1 = lapse
Figure 1.
Survival function (unadjusted) for the two study conditions.
Effect of lapse on change in craving
The next question we sought to answer was whether participants in the lapse condition were more likely to experience changes in craving compared with those in the no lapse condition. Change in craving was calculated as the difference between a participant’s craving score on each post-manipulation measurement occasion and the participant’s initial craving score, which we defined as the average of a participant’s craving scores from the four measurement occasions occurring prior to the manipulation. Thus, in the model, change in craving is a time-varying outcome and each participant contributes as many observations to the model as he or she had post-manipulation days (up to 9) without relapsing (i.e., up to the first day of the relapse). Preliminary analysis suggested that the relationship between relapse condition and craving change was not linear. To account for the non-linearity in the association, we recoded craving change into an ordinal scale. The ordinal scale was a good fit to the data and there were enough observations at each level of the ordinal scale on which to base meaningful inferences. We categorized change in craving at each measurement occasion post-baseline as (a) decreasing if craving was at least 1 point less than baseline craving, (b) stable if craving was within 1 point (in either direction) of baseline craving, and (c) increasing if craving was at least 1 point greater (on the 8-point craving scale) than baseline craving. This coding of the craving outcome allowed us to use ordinal logistic regression to test the association between study condition and change in craving. In the ordinal logistic model, we used the following values to represent these categories: −1 for decrease in craving, 0 for stable craving, and 1 for increase in craving. The stable craving group was used as the reference group. Predictors in the model included all covariates from the initial Cox regression model plus participants’ baseline craving scores. Standard errors for model coefficients were adjusted to account for clustering of observations within participants using the Huber-White sandwich estimator, a variance estimation approach implemented in the Surveylogistic procedure in SAS.
Table 3 displays the results of this model. Compared with participants in the no lapse condition, participants in the lapse condition had 1.85 times the odds of having no change in craving compared to a decrease in craving and they also had 1.85 times the odds of having an increase in craving compared to stable craving (OR = 1.85, 95% CI [1.00, 3.47]). Because these analyses control for baseline craving levels through the pre-lapse period, the results establish an episodic surge in craving after a lapse (i.e., episodic craving) as a candidate mediator of the lapse-relapse relationship that we observed in the initial Cox regression model.
Table 3.
Results of Ordinal Logistic Regression Testing the Effect of Study Condition (lapse, no lapse) on Change in Craving
| Predictor | β | SE | Odds ratio |
95% CI | p |
|---|---|---|---|---|---|
| Study condition a | 0.61 | 0.32 | 1.85 | [1.00, 3.47] | 0.05 |
| Baseline craving | 0.14 | 0.09 | 1.15 | [0.96, 1.38] | 0.14 |
| Age (years) | 0.05 | 0.02 | 1.05 | [1.02, 1.09] | <0.01 |
| NDSS total scale (factor) score | −0.05 | 0.23 | 0.95 | [0.61, 1.50] | 0.83 |
| Number of years smoked | −0.01 | 0.01 | 0.99 | [0.96, 1.02] | 0.36 |
| Number cigarettes smoked per day | −0.02 | 0.01 | 0.98 | [0.97, 1.00] | 0.06 |
| Number of 24 hour quits: past year | 0.21 | 0.07 | 1.23 | [1.07, 1.42] | <0.01 |
| Number of 24 hour quits: lifetime | −0.01 | 0.006 | 0.99 | [0.98, 1.00] | 0.04 |
Note. N = 601 observations; Likelihood ratio chi-sqare (8 df) = 83.95, p < .001.
0 = no lapse, 1 = lapse
Effect of lapse and changes in craving on time to relapse
The final question we sought to answer was whether increased craving experienced by those in the lapse condition explained (mediated) their increased risk of relapse. To test this hypothesis, we estimated a second Cox regression model in which we evaluated the joint effect of study condition and change in craving (from baseline to the day before relapse) on relapse (i.e., an interaction model). This model included all of the predictors from the initial Cox regression model plus change in craving, which was treated as a time-varying covariate and had three levels (decreasing, stable, and increasing) as defined above. We modeled the joint effect of condition and change in craving by categorizing participants into conjoint levels of the two characteristics with dummy variables (i.e., representing different levels of an interaction). Specifically, we used study condition (no lapse or lapse) and change in craving (increasing, decreasing, or stable) to assign each observation to one of six condition by change-in-craving groups (as in the ordinal logistic regression model, each participant contributed as many observations as he or she had post-manipulation days without relapsing; n’s in parentheses represent number of observations): (1) no lapse/ decreasing craving (n = 176); (2) no lapse/stable craving (n = 136); (3) no lapse/increasing craving (n = 63); (4) lapse/decreasing craving (n = 111); (5) lapse/stable craving (n = 122); and (6) lapse/increasing craving (n = 64). Condition by change-in-craving group membership was represented in the regression model with 5 dummy variables using the “no lapse/stable craving” group as the natural reference category. To estimate our Cox regression model, we used the PHREG procedure in SAS, which is designed to accounts for time-varying covariates and the repeated observations within participants (Hosmer & Lemeshow 1999).
Table 4 displays the results for this model. Among participants in the lapse condition, the hazard of relapse was estimated to be twelve times higher (HR = 12.42, 95% CI = [2.00, 77.1], p = .007) after experiencing an increase in craving than it was among participants in the control condition who experienced stable craving. Thus, an episodic surge in craving significantly increased the likelihood of relapse but only among participants in the lapse condition.
Table 4.
Results of Cox Regression Testing the Joint Effect of Study Condition (lapse, no lapse) and Change in Craving on Relapse
| Predictor | β | SE | Hazard ratio |
95% CI | p |
|---|---|---|---|---|---|
| Baseline craving | 0.17 | 0.12 | 1.18 | [0.94, 1.49] | 0.15 |
| Condition × change in cravinga | |||||
| No lapse/decreasing craving | 0.79 | 0.79 | 2.20 | [0.46, 10.4] | 0.32 |
| No lapse/increasing craving | 0.78 | 0.94 | 2.17 | [0.35, 13.66] | 0.41 |
| Lapse/decreasing craving | 1.44 | 0.81 | 4.22 | [0.86, 20.74] | 0.08 |
| Lapse/stable craving | 1.43 | 0.88 | 4.18 | [0.74, 23.46] | 0.10 |
| Lapse/increasing craving | 2.52 | 0.93 | 12.42 | [2.00, 77.1] | 0.01 |
| Age (years) | 0.00 | 0.03 | 1.00 | [0.95, 1.05] | 0.93 |
| NDSS total scale (factor) score | 0.03 | 0.31 | 1.03 | [0.56, 1.87] | 0.93 |
| Number of years smoked | 0.00 | 0.02 | 1.00 | [0.97, 1.04] | 0.97 |
| Number cigarettes smoked per day | 0.01 | 0.02 | 1.01 | [0.98, 1.04] | 0.66 |
| Number of 24 hour quits: past year | 0.04 | 0.12 | 1.04 | [0.82, 1.33] | 0.73 |
| Number of 24 hour quits: lifetime | −0.03 | 0.02 | 0.97 | [0.94, 1.01] | 0.10 |
Note. N = 601 observations; Likelihood ratio chi-square (12 df) = 16.38, p = 0.17.
No lapse, stable craving is the reference category
Discussion
The results of the current study, in combination with the results of other experimental studies (Chornock et al., 1992; Juliano et al., 2006), firmly establish that lapse has a causal relationship to relapse in smokers. The current study goes beyond these previous studies in that it provides new information about mediating mechanisms that link smoking lapses to relapses. Compared with participants in the no lapse condition, participants who were assigned to lapse experienced an initial acute decrease in craving followed by a significant surge in craving. The surges in craving experienced by those in the lapse condition, which were observed after controlling for their baseline craving levels, explained their faster rate of relapse relative to participants in the control condition.
The field of tobacco control has long struggled to understand why smoking lapses nearly always lead to relapses. As a result, cognitive-behavioral and pharmacological interventions have had little success in helping smokers to avoid relapse. Research that clearly identifies the mechanisms that govern the relationship between lapse and relapse would have a substantial impact on smoking cessation and relapse prevention treatments, pointing the way toward the development of new treatments and modification of existing ones to target those mechanisms for change (Shiffman, 2006). Our results therefore have implications for relapse prevention treatments in addition to having implications for theories that explain why lapses almost inevitably lead to relapses.
Cognitive-behavioral treatments (Brown, 2003) and medications for smoking cessation (Shiffman et al., 2003; Tiffany et al., 2000) target craving to help smokers maximize their successes with quitting, but neither is currently employed specifically to prevent lapses from turning into relapses. Currently, cognitive-behavioral treatments emphasize management of the abstinence violation effect that is thought to result from lapses (Brown, 2003), even though data supporting the existence of the abstinence violation effect are not particularly strong (Shiffman, Kassel, Gwaltney, & McChargue, 2005). The results of this study suggest that cognitive-behavioral treatment efforts could, instead, focus on helping smokers prepare for and manage episodic increases in craving that follow lapses. For example, cognitive-behavioral craving management strategies such as relaxation or guided imagery, or cognitive restructuring to manage cue provoked cravings after smokers quit (see Brown, 2003) could be included in advice to smokers on how to manage increases in craving that follow a lapse. Medications like the nicotine patch are ineffective at blunting episodic surges of craving that result from exposure to smoking cues (Tiffany et al., 2000), but faster acting nicotine replacement therapies such as the nicotine gum (Shiffman et al., 2003) are effective at reducing episodic cravings. As such, nicotine gum could potentially be used immediately after a lapse to manage the episodic surge in craving that follows a lapse, that is, to “rescue” the smoker from the negative consequences of a lapse. Of course, these suggestions would need to be evaluated in controlled clinical trials to ensure safety and efficacy.
The study results are neither fully consistent with negative reinforcement models (Baker et al., 2004) nor are they fully consistent with nicotine reinstatement models (Epstein et al., 2006). The initial craving relief experienced by participants in the lapse condition is predicted by negative reinforcement models (see Juliano et al., 2006). However, the subsequent increases in craving participants experienced between the programmed lapse and relapse, and the finding that this increase was related to subsequent relapse, are more consistent with reinstatement models than with negative reinforcement models (see Shiffman et al., 1996b). Though not consistent with either theory individually, the findings are broadly consistent with the nicotine regulation model of smoking (reviewed in Benowitz et al., 2008; see also Chandra, Scharf & Shiffman, in press; Koob & Le Moal, 1997) which incorporates elements of both negative reinforcement models (Baker et al., 2004) and nicotine reinstatement models (Epstein et al., 2006). According to the nicotine regulation model, smoking will alleviate craving related to nicotine deprivation (i.e., lapse), but only for a short time. Once nicotine has been metabolized and blood levels begin to drop, craving will re-emerge motivating the person to smoke again (i.e., relapse). These rises and falls in levels of nicotine and corresponding changes in craving are hypothesized to maintain long-term patterns of smoking. Additional research is needed to further refine the conceptual explanations that best account for the role that craving plays in explaining the link between smoking lapse and relapse.
There are limitations to this study. First, the generalizability of the study is limited given that the sample was composed of reactively recruited, healthy, and heavier smokers. The results may not apply to lighter smokers and/or to those with more significant health problems. The extent to which these results generalize to smokers quitting on their own in the field and/or with medications or more intensive cognitive-behavioral treatments is also not known. Second, there are several processes likely involved in lapsing and relapsing (see Marlatt & Donovan, 2005) and this paper only examined craving. Third, we inferred the motivational effect of increased craving but did not assess the affective qualities of craving. Thus, we cannot draw conclusions about the relative positive or negative valence of participants’ experience of craving following the lapse (see Baker et al., 1986; Ferguson et al., 2006). Fourth, the lapse was experimentally-induced; although this represents a strength from a causal inference standpoint, whether the results generalize to an actual (non-programmed) lapse is not known. Finally, although we observed significant levels of relapse, we only followed participants for 14 days after the experimentally manipulated lapse. In general, a majority of relapses occur within a two week window of quitting (Shiffman et al., 1996a). Nonetheless, a longer follow-up period would have provided a richer source of data.
These limitations notwithstanding, this study fills an important gap in the smoking literature by illuminating the relationship between lapse and relapse and specifying a new, mediating role for craving in the relapse process. The study is distinguished by its use of an experimental design to examine these questions in a way that enhanced the clinical utility and applicability of the model and findings. As such, these results have implications for modifying the treatment protocols for existing cognitive-behavioral and pharmacological treatments designed to help smokers prevent lapses from becoming relapses.
Acknowledgments
This research was supported by R01CA127491.
Special thanks are due to Rachel Burns, James Coley, Sarah Frith, Justin Greenfield, Jill Schaeffer, and Michelle Horner for their invaluable assistance in executing the procedures of this research.
Footnotes
Copies of the informed consent document are available by request to the first author.
In 95% of cases, participants’ first instance of smoking was the beginning of the 7-day relapse; there were essentially no second lapses for anyone in the study. For all intents and purposes, days to first smoking and days to relapse are indistinguishable in our data.
Contributor Information
William G. Shadel, RAND Corporation
Steven C. Martino, RAND Corporation
Claude Setodji, RAND Corporation.
Daniel Cervone, Department of Psychology, University of Illinois at Chicago.
Katie Witkiewitz, Department of Psychology, Washington State University.
Ellen Burke Beckjord, RAND Corporation.
Deborah Scharf, RAND Corporation.
Regina Shih, RAND Corporation.
References
- Abrams D, Emmons KM, Niaura R, Goldsten M, Sherman CE. Tobacco dependence: Integrating individual and public health perspectives. In: Nathan P, Langenbacher J, McGrady B, Frankenstein W, editors. Annual Review of Addictions: Treatment and Research. New York: Pergamon Press; 1991. pp. 391–436. [Google Scholar]
- Baker T, Morse E, Sherman C. The motivation to use drugs: A psychobiological analysis of urges. In: Rivers C, editor. The Nebraska Symposium on Motivation: Alcohol use and abuse. Lincoln: University of Nebraska Press; 1987. pp. 257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, Fiore MC, McCarthy DE, Majeskie MR. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The Moderator-Mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addition. Clinical Pharmacology & Therapeutics. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Brandon T, Tiffany S, Obremski, Baker T. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brandon T, Irvin Vidrine J, Litvin EB. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brown R. Intensive behavioral treatment. In: Abrams DB, Niaura R, Brown R, Emmons K, Goldstein MG, Monti PM, editors. The tobacco dependence treatment handbook. New York: Guilford; 2003. pp. 178–229. [Google Scholar]
- Chandra S, Scharf D, Shiffman S. Within-day temporal patterns of smoking, withdrawal symptoms, and craving. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2010.12.027. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology. 1996;127:102–107. doi: 10.1007/BF02805981. [DOI] [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology. 1992;108:495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- Chow S. In defense of experimental data in relativistic milieu. New Ideas in Psychology. 1995;15:259–279. [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the Normative Aging Study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gwaltney C, Shiffman S, Balabanis M, Paty J. Dynamic self-efficacy and outcome expectancies: Prediction of smoking lapse and relapse. Journal of Abnormal Psychology. 2005;114:661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied survival analysis: Regression modeling of time to event data. New York: John Wiley & Sons; 1999. [Google Scholar]
- Hughes J, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond R, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–26. [PubMed] [Google Scholar]
- Juliano L, Donny E, Houtsmuller EJ, Stitzer M. Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology. 2006;115:166–175. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Killen J, Fortman S. Craving is associated with smoking relapse: Evidence from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kraemer H, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur Approaches. Health Psychology. 2008;27(No. 2) Suppl.:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt G, Donovan D. Relapse prevention. 2nd ed. New York: Guilford; 2005. [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams D. Smoking cessation: progress, priorities, and prospectus. Journal of Consulting and Clinical Psychology. 2002;70:494–509. doi: 10.1037//0022-006x.70.3.494. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow D, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:3–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: mediating roles of nicotine dependence and duration of deprivation. Addictive Behaviors. 1996;21:139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Perkins K, Lerman C, Stitzer M. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Piasecki T, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Selvin S. Statistical analysis of epidemiological data. 3rd Edition. New York: Oxford University Press; 2004. [Google Scholar]
- Shadel WG, Niaura R. Brief behavioral interventions. In: Abrams DB, Niaura R, Brown R, Emmons K, Goldstein MG, Monti PM, editors. Treating nicotine addiction: An evidence based practice guide. New York: Guilford Press; 2003. pp. 101–117. [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology, and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Comment on craving. Addiction. 2000;95 Suppl. 2:S171–S175. doi: 10.1080/09652140050111744. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Reflections on smoking relapse research. Drug and Alcohol Review. 2006;25:15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg J, Paty J, Perz W, Gnys M, Kassel J, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106:133–152. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: Prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology. 1996a;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kassel J, Gwaltney C, McChargue D. Relapse prevention with smokers. In: Marlatt GA, Donovan DM, editors. Relapse prevention. 2nd ed. New York: Guilford; 2005. pp. 92–129. [Google Scholar]
- Shiffman S, Paty JA. Smoking patterns of non-dependent smokers: contrasting chippers and dependent smokers. Journal of Abnormal Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel J, Hickox M. First lapses to smoking: within subjects analysis of real time reports. Journal of Consulting and Clinical Psychology. 1996b;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, Khayrallah M, Jorenby D, Ryan C, Ferguson C. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology. 2003;166:343–350. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Aubin H. Craving’s place in addiction theory: Contributions of the major models. Neuroscience and Biobehavioral Reviews. 2010;34:606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tiffany S, Warthen MW, Goedeker KC. The functional significance of craving in nicotine dependence. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer; 2009. pp. 171–197. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]