Abstract
Objective
We sought to determine the association between time to initial antibiotics and mortality of septic shock patients treated with an emergency department (ED) based early resuscitation protocol.
Design
Pre-planned analysis of a multicenter randomized controlled trial of early sepsis resuscitation.
Setting
3 urban US EDs.
Patients
Adult septic shock patients.
Interventions
A quantitative resuscitation protocol in the ED targeting 3 physiological variables: central venous pressure, mean arterial pressure and either central venous oxygen saturation or lactate clearance. The study protocol was continued until all endpoints were achieved or a maximum of 6 hours.
Measurements
Data on patients who received an initial dose of antibiotics after presentation to the ED were categorized based on both time from triage and time from shock recognition to initiation of antibiotics. The primary outcome was in-hospital mortality.
Main Results
Of 291 included patients, mortality did not change with hourly delays in antibiotic administration up to 6 hours after triage: 1 hour (OR 1.2, 0.6–2.5), 2 hours (OR 0.71, 0.4–1.3), 3 hours (OR 0.59, 0.3–1.3). Mortality was significantly increased patients who received initial antibiotics after shock recognition (N=172, 59%) compared with before shock recognition (OR 2.4, 1.1–4.5); however, among patients who received antibiotics after shock recognition, mortality did not change with hourly delays in antibiotic administration.
Conclusion
In this large, prospective study of ED patients with septic shock, we found no increase in mortality with each hour delay to administration of antibiotics after triage. However, delay in antibiotics until after shock recognition was associated with increased mortality.
Keywords: sepsis, antibiotics, septic shock, emergency medicine
Introduction
Severe sepsis hospitalizations have doubled over the last decade resulting in at least 750,000 persons affected annually in the United States (US).(1;2) Estimates suggest that 500,000 patients with severe sepsis are treated annually in US emergency departments (ED).(3) The Surviving Sepsis Campaign (SSC) international consensus guidelines recommend initiating broad-spectrum antibiotic coverage within the first hour of recognizing severe sepsis and septic shock.(4) These recommendations are based largely upon one large retrospective study(5) and expert consensus. Despite these guidelines, a recent large cohort of 165 hospitals treating over 15,000 patients with septic shock from the SSC registry demonstrated that only 68% of patients received broad-spectrum antibiotics within 3 hours of ED presentation,(6) demonstrating the difficulty of achieving antibiotic administration within current guidelines in routine clinical practice.
To date no prospective study has examined the timing of antibiotic administration and its association with mortality in ED sepsis patients treated with an early quantitative resuscitation protocol. Thus, the optimal timing of antibiotic administration and its impact on outcome remains unclear in the early treatment of severe sepsis and septic shock. The aim of this study was to evaluate if the timing of antibiotic administration in relation to both triage time and time of shock recognition was associated with in-hospital mortality among a group of consecutive, prospectively enrolled patients presenting to three US EDs with septic shock and treated with an early quantitative resuscitation protocol.
Methods
Study Design
We conducted a preplanned analysis of a recently completed prospective, parallel group, non-blinded randomized clinical trial designed to assess the non-inferiority of lactate clearance versus central venous oxygen saturation (ScvO2) as the protocol endpoint that evaluated the adequacy of oxygen delivery during ED based early quantitative resuscitation of sepsis.(7)
The trial took place from January 2007 to January 2009 at Carolinas Medical Center, Charlotte, NC, Beth Israel Deaconess Medical Center, Boston, MA, and Cooper University Hospital, Camden, NJ, all of which are large, urban, tertiary care hospitals staffed by emergency medicine resident physicians supervised by board certified emergency medicine attending physicians. The study was approved by the Institutional Review Board at each institution and all participants or their surrogate provided written informed consent for participation. The trial was registered on Clinicatrials.gov identifier NCT00372502.
The detailed methods of the study have been described.(7) In brief, consecutive patients presenting to one of the participating EDs were eligible for enrollment if they were older than 17 years, had confirmed or suspected infection, two or more systemic inflammatory response criteria(8), and hypoperfusion evidenced by hypotension after fluid challenge or a blood lactate concentration of at least 4 mmol/L. After enrollment patients were randomly assigned to 1 of 2 groups. Each group received structured quantitative resuscitation while in the ED. The study protocol was continued until all endpoints were achieved or a maximum of 6 hours. The published results of this study showed a 6% (95% confidence intervals −3 to 14%) in-hospital mortality difference between the two study groups, confirming the primary hypothesis of non-inferiority between the two resuscitation endpoints.(7)
As a part of the protocol, all patients received broad spectrum antibiotic coverage according to local hospital guidelines. The Online Supplement provides an example of one of the antibiotic guidelines. The only requirement for antibiotic administration was that they be administered as early as possible after recognition of sepsis.
Data Analysis and Outcomes
The primary outcome was in-hospital mortality. We compared the outcomes of subjects who received an initial dose of antibiotics after compared to before each hourly increment up to a maximum of 6 hours after ED triage. We also compared outcomes of patients receiving initial antibiotics after compared to before each hourly increment after shock recognition. Shock recognition was defined as the time that the patient developed 2 or more SIRS criteria and either a systolic blood pressure (SBP) lower than 90 mmHg after a minimum of 20 mL/kg rapid volume challenge or a blood lactate concentration of at least 4 mmol/L. Recognizing that some patients would receive antibiotics before shock recognition, we analyzed outcomes of patients that received antibiotics prior compared to after recognition of shock; however, if patients received antibiotics prior to shock recognition they were excluded from the hourly incremental analysis.
One infectious disease specialist reviewed the blood culture and clinical data from all subjects. We followed our previously published criteria for determining positive blood cultures.(9) A positive blood culture required that a bacterial or fungal pathogen be isolated by routine culture in the blood. Staphylococcus epidermidis was uniformly considered a contaminate and other coagulase negative staphylococci were similarly considered to be unlikely to cause septic shock and were considered contaminates unless the patient had a preexisting indwelling venous catheter. Antibiotic administration was considered appropriate if the patient received an initial antimicrobial to which the cultured bacteria had in vitro sensitivity. In the case of negative cultures, antibiotics were considered appropriate if they were given in accordance with local guidelines and were extended spectrum antibiotics.
Categorical data are presented as proportions with 95% confidence intervals (CI). Continuous data are presented as means and standard deviations or medians and interquartile ranges. Results were compared using chi-squared, Fisher exact, Mann-Whitney, or Krussel-Wallis tests as appropriate. In order to attempt to control for potential confounders, we constructed a multivariate logistic regression model using inhospital death as the dependent variable. Candidate variables were compared using the Krussel-Wallis test to assess for differences in hourly intervals versus the entire cohort, and were added to the multivariate model if p<0.10 in order to maintain the event (death) per independent variable ratio of approximately 8–10:1 that is necessary for multivariate modeling.(10) The model was refined using reverse stepwise elimination. Model fit was determined with Hosmer and Lemeshow’s goodness of fit test. All statistical tests were two sided with p<0.05 considered significant. Data were analyzed using commercially available statistical software (StatsDirect 2.7.7, Cheshire, England and STATA 10.0, College Station, TX).
Results
Of 300 patients enrolled in study 291 received a first dose of antibiotics after presentation to the hospital. The remaining 9 patients had received antibiotics prior to hospital arrival (7 from another outpatient facility, 2 from rehabilitation/chronic nursing facility) and were excluded from subsequent analysis. Fifty nine percent (172/291) of patients received the initial dose of antibiotics after recognition of shock. Baseline characteristics of the entire cohort are shown in Table 1 and the various sources of infection are shown in Table 2. Overall mortality was 55/291 (18.9%).
Table 1.
Patient demographics and clinical characteristics.
| Variable (n=291) | Value |
|---|---|
| Age (IQR) | 62 (50, 73) |
| Race (%) | |
| Caucasian | 158 (54) |
| Black American | 101 (34) |
| Hispanic | 27 (9) |
| Other | 5 (2) |
| Sex (%) | |
| Male | 156 (53) |
| Female | 135 (46) |
| Eligibility Criteria (IQR) | |
| Temperature (°F) | 99 (97, 101) |
| Heart rate (beats per minute) | 102 (85, 112) |
| Respiratory rate (breaths per minute) | 22 (18, 27) |
| White blood count (cells per mm3) | 12.4 (7.7, 17.5) |
| Systolic Blood Pressure (mmHg) | 86 (77, 98) |
| Lactate (mmol/L) | 3.3 (1.8, 5.8) |
| Baseline Laboratory Values (IQR) | |
| Platelets (per mm3) | 214 (135, 294) |
| Hemoglobin (mg/dL) | 11.4 (9.8, 13.4) |
| Creatinine (mg/dl) | 1.7 (1.1, 3.0) |
| Total Bilirubin (mg/dL) | 1.0 (0.6, 1.6) |
| HCO3 (mg/dL) | 21 (17, 24) |
| International normalized ratio | 1.3 (1.1, 1.7) |
| Disease severity (IQR) * | |
| SAPS II score | 42 (30, 55) |
| SOFA score | 6 (4,9) |
| MEDS score | 11 (8, 14) |
Abbreviations: IQR – interquartile range; SAPS - simple acute physiology score; SOFA - Sequential Organ Failure Assessment; MEDS - mortality in emergency department sepsis; F – Fahrenheit; mm – millimeter; Hg – mercury; mg – milligram; dL - deciliter
At 0 hours
Table 2.
Source of infection.
| Source | N (%) |
|---|---|
| Pneumonia | 99 (34.0) |
| Urinary tract infection | 71 (24.4) |
| Intraabdominal | 49 (16.8) |
| Skin and soft tissue | 23 (7.9) |
| Indwelling intravascular catheter | 11 (3.8) |
| Surgical Wound | 7 (2.4) |
| Endocarditis | 4 (1.4) |
| Meningitis | 3 (1.0) |
| Septic arthritis | 2 (0.7) |
| Tuberculosis | 1 (0.3) |
| Ear, nose, thorat | 1 (0.3) |
| Toxic shock syndrome | 1 (0.3) |
| Unknown | 40 (13.8) |
| Two or more sources | 21 (7.2) |
N – number of patients
Positive blood cultures for pathological organisms were obtained in 100 of 291 (34.4%) patients. The organisms isolated from the blood and their frequencies of occurrence are summarized in Table 3. The mortality rate for blood culture positive septic shock was 26/100 (26.0%) versus 29/191 (15.2%), for blood culture negative septic shock; p=0.03. Of the 100 patients with positive blood cultures, 91 received antibiotics in the ED to which the causative organism was susceptible. Of the 9 patients who failed to receive appropriate antibiotics, 7/9 received broad spectrum antibiotics to which the causative organism was resistant (multidrug resistant gram-negative rods in 6/9, and multidrug resistant enterococci in1/9), and 2/9 patients had fungemia that was untreated in the ED. The mortality for patients treated with appropriate antibiotics for blood culture positive sepsis in the emergency department was 23/91 (25.3%) vs 3/9 (33.3%), for those treated with inappropriate antibiotics; p = 0.69.
Table 3.
Organisms isolated from the blood.
| N | |
|---|---|
| Gram positive organisms | |
| Staphylococcus aureus | 21 |
| Methicillin sensitive | 11 |
| Methicillin resistant | 10 |
| Coagulase negative staphylococcus | 1 |
| Streptococcus pneumoniae | 7 |
| Other streptococcus species | 9 |
| Enterococcus species | 8 |
| Peptostreptococcus | 1 |
| Bacillus cereus | 1 |
| Clostridium perfringens | 2 |
| Diptherioids | 2 |
| Micrococcus | 1 |
| Lactobacillus | 1 |
| Gram negative organisms | |
| Escherichia coli | 17 |
| Klebsiella species. | 7 |
| Proteus species | 7 |
| Serretia marcescens | 4 |
| Pseudomonas species | 2 |
| Enterobacter species | 2 |
| Vibrio vulnificus | 1 |
| Acinetobacter species | 1 |
| Morgonella species | 1 |
| Citrobacter species | 1 |
| Yeast/Fungi | |
| Candida species | 3 |
| Positive Blood Cultures | 100 |
N – number of patients
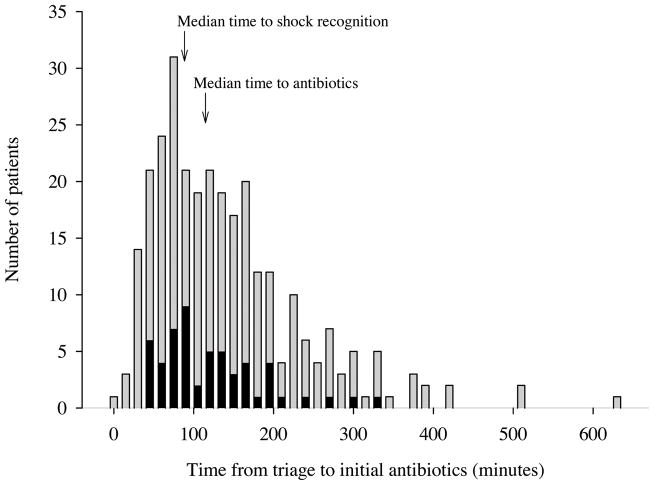
The median time from triage to initial antibiotic administration was 115 minutes (IQR 65, 175). Table 4 summarizes the relative mortality and odds ratios for death associated with hourly intervals from ED triage to antibiotic administration among all 291 subjects. Figure 1 depicts the time from triage to initial antibiotics in the entire cohort, stratified by final hospital outcome (alive versus dead). We found no association between in-hospital mortality and the time from ED triage to administration of antibiotics during the first 6 hours of resuscitation.
Table 4.
In-hospital mortality: Triage to initial antibiotics
| Time to antibiotics | N | Mortality (%) | Difference (%) | OR* | 95% CI | Adjusted OR* | 95% CI |
|---|---|---|---|---|---|---|---|
| ≤ 1 hour | 65 | 16.9 | 2.6 | 1.18 | 0.57–2.46 | 1.81 | 0.74–4.44 |
| >1 hour | 226 | 19.5 | |||||
| ≤ 2 hours | 155 | 21.3 | −5.1 | 0.71 | 0.39–1.30 | 1.07 | 0.54–2.16 |
| >2 hours | 136 | 16.2 | |||||
| ≤ 3 hours | 223 | 20.6 | −7.4 | 0.59 | 0.27–1.27 | 0.66 | 0.27–1.63 |
| >3 hours | 68 | 13.2 | |||||
| ≤ 4 hours | 255 | 20.4 | −12.1 | 0.35 | 0.10–1.20 | 0.39 | 0.08–1.90 |
| >4 hours | 36 | 8.3 | |||||
| ≤ 5 hours | 274 | 19.7 | −13.8 | 0.25 | 0.03–1.96 | 0.69 | 0.07–6.86 |
| >5 hours | 17 | 5.9 | |||||
| ≤ 6 hours | 281 | 19.6 | −19.6 | --- | --- | --- | --- |
| >6 hours | 10 | 0 |
N – number of patients; OR – odds ratio; CI – confidence interval
Odds of death with increasing delays in antibiotic administration
Figure 1.
Graphical depiction of the time from triage to initial antibiotics in the entire cohort, stratified by final hospital outcome. Grey bars represent patients who survived the hospitalization and black bars represent patients who died in the hospital.
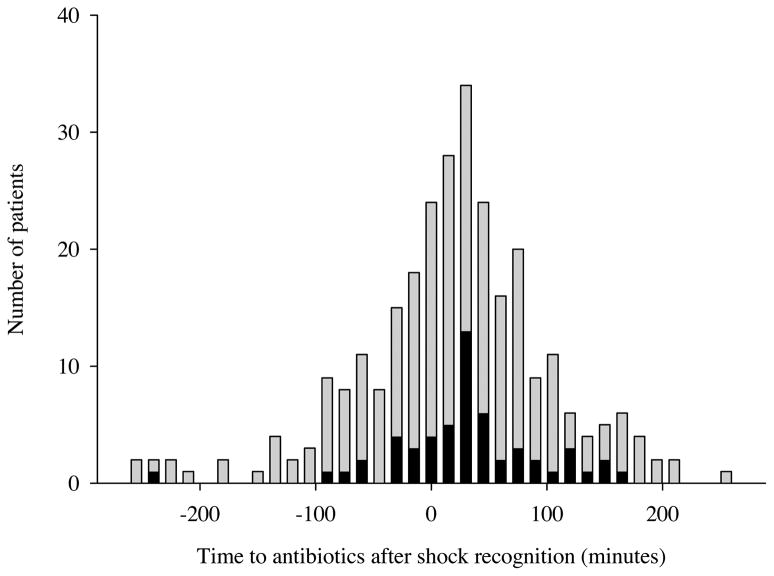
The median time to shock recognition among all subjects was 89 minutes (IQR 48, 180). A total of 172 (59%) patients received antibiotics after shock recognition. When compared with patients who received antibiotics before shock recognition (n=119), patients receiving antibiotics after shock recognition had a significant increase in the odds of death (OR 2.4, 95% CI 1.1 to 4.5). Figure 2 depicts the time from shock recognition to initial antibiotics in the entire cohort, stratified by final hospital outcome (alive versus dead).
Figure 2.
Graphical depiction of the time from shock recognition to initial antibiotics in the entire cohort, stratified by final hospital outcome. Shock recognition is indicated by Time 0. Subjects with negative times received antibiotics before shock recognition. Grey bars represent patients who survived the hospitalization and black bars represent patients who died in the hospital.
Table 5 summarizes the relative mortality and odds ratios for death associated with hourly intervals from shock recognition to antibiotic administration among the 172 subjects who received antibiotics after shock recognition. We found no increase in mortality associated with delay to administration of antibiotics during the first 3 hours after shock recognition. Only 8 patients received antibiotics after 3 hours of shock recognition
Table 5.
In-hospital mortality: Shock recognition to initial antibiotics
| Time to antibiotics | N | Mortality (%) | Difference (%) | OR* | 95% CI | Adjusted OR* | 95% CI |
|---|---|---|---|---|---|---|---|
| Prior to shock recognition | 119 | 11.8 | 12 | 2.35 | 1.12–4.53 | 2.59 | 1.17–5.74 |
| After shock recognition | 172 | 23.8 | |||||
| ≤ 1 hour | 101 | 25.8 | −4.7 | 1.29 | 0.63–2.67 | 0.93 | 0.41–2.12 |
| >1 hour | 71 | 21.1 | |||||
| ≤ 2 hours | 145 | 24.1 | −1.9 | 1.11 | 0.42–2.98 | 0.69 | 0.21–2.22 |
| >2 hours | 27 | 22.2 | |||||
| ≤ 3 hours | 164 | 23.8 | 1.2 | 0.94 | 0.18–4.82 | 0.84 | 0.13–5.52 |
| >3 hours | 8 | 25.0 |
N – number of patients; OR – odds ratio; CI – confidence interval
Odds of death with increasing delays in antibiotic administration
In order to attempt to control for potential confounders, we constructed a multivariate logistic regression model using in-hospital death as the dependent variable. The final model included age, total SOFA score at enrollment, initial lactate, race, and achievement of lactate clearance goal. As the independent variables of most interest, time to antibiotics at each time point cutoff and appropriate antibiotics were forced into the final models. The final model demonstrated goodness-of-fit by Hosmer and Lemeshow’s (p=0.27). The adjusted odds ratios revealed no significant changes from the unadjusted odds ratios and are shown in Tables 4 and 5.
Discussion
In this report, we document the association between timing of initial antibiotic treatment and mortality in emergency department patients undergoing a quantitative resuscitation protocol for septic shock. Our results indicate no association between time from triage to initial antibiotic administration and hospital mortality. However, our data suggest an increased risk of death if antibiotics are delayed until after the recognition of shock. Once a patient meets consensus definition for shock, our data showed no association between subsequent hourly delays in antibiotic administration and mortality.
The Surviving Sepsis Campaign (SSC) international consensus guidelines recommends always administering broad spectrum antibiotics within the first hour of recognizing severe sepsis and septic shock.(4) This recommendation is largely based on one large retrospective study published in 2006.(5) In that study Kumar et al reported that administration of antimicrobials within the first hour of documented hypotension was associated with a survival rate of 79.9%. Each hour of delay in antimicrobial administration over the ensuing 6 hrs was associated with an average decrease in survival of 7.6%. These findings were not confirmed by our data presented in this report. There are several considerations that may explain these differences in findings. First, the Kumar et al study included all intensive care unit (ICU) patients diagnosed with septic shock, and the overall reported mortality rate was 56%. Thus it appears that this cohort of subjects had a higher severity of illness than the present study. Furthermore, we studied cohort of only ED patients, rather than patients presenting from a variety of settings and likely receiving various methods of initial resuscitation. Our overall mortality rate was 19%, consistent with other studies of ED patients receiving early aggressive resuscitation.(11–13) While Kumar et al attempted to control for the variability of resuscitation, it is likely that the initial resuscitative efforts across such a wide spectrum of patients in various care settings were considerably different. As such, the extrapolation of this retrospective data from an ICU patient population to the earliest hours of resuscitation in ED patients receiving standardized resuscitation may be inappropriate. All of the patients in the current study underwent the same early recognition and aggressive treatment protocol, likely resulting in more uniform screening and initial resuscitative efforts, and may provide additional explanation for the differences in findings. That is, when sepsis recognition and resuscitation are early, aggressive and prescribed, the administration of antibiotic measured in hourly increments of time is less important than is just administering the antibiotics during the initial resuscitative phase.
Our data are consistent with the findings of Gaieski et al, who published a retrospective analysis evaluating the timing of antibiotics and association with mortality in ED patients treated with an early goal-directed therapy (EGDT) protocol.(14) However, similar to Kumar et al, the Gaieski report emphasizes appropriateness of antibiotic administration in their conclusions. In our report, we made an a priori decision to evaluate only initial antibiotic administration, and not put major emphasis on if the antibiotic coverage was considered appropriate, as has been proposed by both Kumar and Gaieski.(5;14) We had two important rationales for our decision. First, while it makes intuitive sense to only measure the effect of an antibiotic with activity against the causative organism, the reality of accurately performing this measurement in a clinical setting, particularly the ED, is nearly impossible. Appropriateness of antibiotics is based on culture data not available for 24 to 96 hours or longer after initial antibiotic administration and therefore it is impossible for a clinician to know if a prescribed antibiotic is appropriate. Thus it seems inappropriate to require this standard when determining the effect of antibiotic timing on outcome. Our rationale is similar to that for determination of appropriateness of cardiac catheterization laboratory activation for ST segment myocardial infarction being based on the initial evaluation and EKG, not the presence or absence of a culprit lesion.(15) Second, despite the best attempt of authors to standardize the evaluation of appropriateness of antibiotics, the measure is performed retrospectively and is extremely complex and subject to interpretation. Interestingly this issue is not just semantics, particularly given the high rate of culture-negative septic shock, 30–43% in the aforementioned studies, leading to a dual standard of appropriateness based upon the presence or absence of a positive culture. Appropriateness in these cases of culture negative sepsis is relegated to ‘broad spectrum’, which can be argued to be less restrictive than the evaluation of culture positive subjects. Furthermore, all studies of septic shock require a subjective analysis of the causative organism. Such a judgment is particularly difficult in cases where more than one culture is positive with different organisms, and subjective decisions as to the causative organism must be made, even if they are made with predefined decision rules. Factors such as presence of a indwelling urinary or venous catheter, presence of an immunocompromised state that allows “contaminants” to become virulent sources of infection, and nosomcosial exposures all confound these analyses and are extraordinarily difficult to standardize and control.
With the aforementioned rational taken into consideration, appropriateness of antibiotics could have been an important confounder of our findings. Thus we incorporated appropriateness of antibiotics in our multivariate model. These adjusted results were nearly identical as our unadjusted results. Namely, there appears to be no association between hourly delays in antibiotic administration after triage and mortality, even when controlling for appropriateness. We interpret these results to suggest that when all other parts of early resuscitation are sufficiently refined, the importance of timeliness of antibiotics appears to recede.
The strength of this study is that we prospectively studied the timing of antibiotic administration to ED patients with septic shock. All patients received a standardized, prescribed early recognition and resuscitation protocol, removing much of the variability in both patient population and early treatment present in other studies. In general, patients received antibiotics early in their hospital course, with 75% of patients receiving initial antibiotics within 3 hours and 97% within 6 hours of triage.
This study has several weaknesses which deserve consideration. First, all three of the hospital systems have considerable experience with early quantitative resuscitation protocols, and our results may not be generalizable to hospitals without such protocols. Second, the vast majority of patients received antibiotics within 3 hours of triage, and the relatively small numbers of patients in subsequent time points leads to wide confidence intervals, and makes it more difficult to draw definitive conclusions regarding associations as time points become progressively longer. Third, while we did not observe significant associations in our study it is possible that a larger study would be able to detect a difference. Given our confidence intervals, however, we would expect such an effect size to be small, and significantly less than those previously reported.(5;14) Fourth, while our mortality rate is similar to previous reports(11–13), it is lower than reports in other septic shock populations.(16;17) Fifth, it is impossible in most cases to identify the exact time of onset of septic shock and thus the timing of antibiotics in relation to onset of shock can often not be ascertained. This is an inherent limitation to the nature of sepsis research. Finally, given the design of our study, we are only able to draw conclusions regarding associations and not causation.
Conclusion
In this large prospective study of ED septic shock patients who received standardized early recognition and aggressive resuscitation at 3 experienced institutions, we failed to demonstrate an association between timing of antibiotic administration from ED triage and hospital mortality. A delay in antibiotics until after shock recognition, as compared to before, was associated with increased mortality; however if antibiotics are administered after shock recognition there is no increase in mortality with hourly delays.
Supplementary Material
Acknowledgments
This work was supported by grant K23GM076652 (Jones) from the National Institute of General Medical Sciences/National Institutes of Health. Dr Puskarich is supported by grant 10POST3560001 from the American Heart Association. Dr. Trzeciak is supported by grant GM083211 from the National Institute of General Medical Sciences/National Institutes of Health. Dr Shapiro was supported by grants HL091757 and GM076659 from the NIH.
Dr. Horton has participated in HIV trials with Gilead and GlaxoSmithKline, and has received support from the Cannon and Silverman Foundations. Dr. Kline holds stock ownership in CP Diagnostics and received US patents.
This study was funded, in part, by the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Horton’s clinic has conducted research studies on HIV with Gilead, GlaxoSmithKline and Tibotec. Dr. Kline holds stock ownership in CP Diagnostics and received US patents.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
Reference List
- 1.Angus D, Linde-Zwirble W, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Dellinger R, Townsend S, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 7.Jones AE, Shapiro N, Trzeciak S, et al. Lactate Clearance vs Central Venous Oxygen Saturation as Goals of Early Sepsis Therapy: A Randomized Clinical Trial. JAMA. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. Ref Type: Generic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bone R, Balk R, Cerra F, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 9.Heffner AC, Horton JM, Marchick MR, et al. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis. 2010;50(6):814–820. doi: 10.1086/650580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 11.Jones AE, Focht A, Horton JM, et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trzeciak S, Dellinger RP, Abata NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129(2):225–235. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34(4):1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 14.Gaieski D, Mikkelsen M, Bank RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergecy department. Crit Care Med. 2010;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 15.Kontos MC, Kurz MC, Roberts CS, et al. An evaluation of the accuracy of emergency physician activation of the cardiac catheterization laboratory for patients with suspected ST-segment elevation myocardial infarction. Annals of Emergency Medicine. 2010;55(5):423–430. doi: 10.1016/j.annemergmed.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 17.Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.