Abstract
Current models for in vitro studies of tissue function and physiology, including responses to hypoxia or environmental toxins, are limited and rely heavily on standard 2-dimensional (2-D) cultures with immortalized murine or human cell lines. To develop a new more powerful model system, we have pursued methods to establish and expand cultures of primary lung cell types and reconstituted tissues from marine mammals. What little is known about the physiology of the deep-sea diving pygmy sperm whale (PSW), Kogia breviceps, comes primarily from stranding events that occur along the coast of the southeastern United States. Thus, development of a method for preserving live tissues and retrieving live cells from deceased stranded individuals was initiated. This report documents successful cryopreservation of PSW lung tissue. We established in vitro cultures of primary lung cell types from tissue fragments that had been cryopreserved several months earlier at the stranding event. Dissociation of cryopreserved lung tissues readily provides a variety of primary cell types that, to varying degrees, can be expanded and further studied/manipulated in cell culture. In addition, PSW-specific molecular markers have been developed that permitted the monitoring of fibroblast, alveolar type II, and vascular endothelial cell types. Reconstitution of 3-D cultures of lung tissues with these cell types is now underway. This novel system may facilitate the development of rare or disease-specific lung tissue models (e.g., to test causes of PSW stranding events and lead to improved treatments for pulmonary hypertension or reperfusion injury in humans). Also, the establishment of a “living” tissue bank biorepository for rare/endangered species could serve multiple purposes as surrogates for freshly isolated samples.
INTRODUCTION
Biorepositories continue to increase in number and size throughout the world, and their contents are essential for pathologic, toxicologic and molecular analyses (Ayers, 2011; Cortes et al., 2010; Moritz and Labbe, 2008; Troyer, 2008). Techniques such as laser capture microdissection and gene expression analysis on formalin fixed paraffin-imbedded tissues have also considerably improved the value of available biospecimens. However, among the vertebrate biological materials available, the banking of viable primary cells and tissues is very limited compared to the much greater number of immortalized cell lines. For example, the American Type Culture Collection (ATCC), established in 1914, has available only a dozen primary cell types, most of which are human, and no model tissues (www.atcc.org). Here, we describe a straightforward approach to the cryopreservation of viable lung tissue from both rare and common organisms. Such an approach will be highly valuable for the establishment of primary cells from unique model strains (e.g., transgenic mouse lines) and unusual or poorly studied organisms. Among the numerous advantages of viable tissue specimens is that multiple tissue-specific cell types could be retrieved and reconstituted for ex vivo organ studies, which is especially important in regard to rare species where tissue acquisition is limited.
The pygmy sperm whale (PSW), Kogia breviceps, inhabits all temperate and tropical waters and is the second most commonly stranded cetacean in the southeastern United States (Odell, 2004; Scott, 2001). Due to its deep water pelagic lifestyle (including limited periods at sea level), most information about the PSW has been obtained from stranded animals and very little is known about these cetaceans in the wild, including behavior and accurate population estimates (Odell, 2004; Santos et al., 2006). Based on stomach contents of stranded animals, we know that this species feeds in deep water, primarily on cephalopods and, less often, on deep-sea fishes and shrimp (McAlpine et al., 1997). Toothed whales (order Cetacea, suborder Odontoceti) are the deepest diving marine mammals known and include beaked whales and sperm whales, which are believed to reach depths of up to 2000 m (Watwood et al., 2006). These deep diving marine mammals are inspired divers, inhaling air then collapsing their lungs during dives to reduce nitrogen intake and decrease oxygen consumption (Kooyman and Ponganis, 1998; Tyack et al., 2006). This process of resurfacing, lung expansion, exhalation and inhalation is highly efficient, with approximately 90% of lung volume gas exchanged (compared to 15–20% exchange volume for humans) (Tyack et al., 2006).
The isolation and analysis of different cell types will provide a wealth of information about the health status of stranded animals. Additionally, the data obtained may help better understand the adaptations of marine mammals to extreme conditions as well as environmental involvement in stranding events. Here, we present a method to cryopreserve viable lung tissue from a stranded pygmy sperm whale, we show that this method can be applied to other model organisms, and that multiple viable cell types can be retrieved from long-term frozen samples. This method has direct applications in studies on normal lung function and responses to environmental stressors in rare and/or endangered species and may have direct relevance to human lung health.
MATERIALS AND METHODS
Tissue origin
Lung tissue was isolated from a female pygmy sperm whale (Kogia breviceps; ID no. SC0934) stranded on Myrtle Beach, SC, USA, on July 24, 2009 as part of the Southeastern US Marine Mammal Stranding Network. After examination and poor prognosis at the stranding location, the whale was subjected to humane sacrifice according to the American Veterinary Medical Association (AVMA) standards and the general guidelines from the National Marine Fisheries Service (NMFS). The whale was then transported by NOAA/NOS personnel of the stranding network group to the NOAA Center for Coastal Environmental Health and Biomolecular Research (CCEHBR), in Charleston, SC, USA, where a complete necropsy, including lung excision, was performed (approximately 12 h after discovery of the stranded individual). A primary clinical assessment described the animal with cardiomyopathy, heavy parasite load and a thin blubber layer. Lung tissue was also isolated from an adult (8 week old) female mouse (FVB/N strain; AAALAC certified protocol).
Vacuum (expansion) perfusion and cryopreservation of lung tissues
PSW lung tissue was excised, placed in a sterile Petri dish and cut into 2–3 cm3 cubes using a sterile scalpel and sterile forceps. Cubes were washed in 1X Dulbecco’s Phosphate-Buffered Saline (DPBS; Invitrogen, Carlsbad, CA, USA) and transferred into a sterile Luer-lock syringe (BD Biosciences, San Jose, CA, USA) containing freshly prepared cryopreserving medium (CM; Dulbecco’s Modified Eagle Medium-DMEM [Invitrogen], supplemented with 10% high quality dimethyl sulfoxide [DMSO; Sigma Aldrich, St. Louis, MO, USA] and 10% fetal bovine serum [FBS; Sigma Aldrich]). The Luer lock syringe was equipped with a BD Connecta™ Plus stopcock (BD Biosciences). This device was used to create a partial vacuum in the syringe chamber to perfuse the lung tissue with CM. Briefly, after all of the air was removed from the CM/tissue mix, the stopcock was closed and the cubes were perfused with CM by moving the syringe plunger slowly backwards to increase chamber volume (partial vacuum/tissue expansion) and forwards to reduce chamber volume (return to atmospheric pressure; 1 atm). The partial vacuum was achieved with 1.4cc plunger expansion per gram of tissue. Gas released from tissue airways was observed after return to original volume (1 atm) in the first few cycles. Visible gas was removed from the apparatus by opening the stopcock. This process was repeated a total of 7 times to insure complete perfusion.
For comparison, mouse lung tissue was similarly perfused. Briefly, lung tissue was perfused with sterile DPBS (via right ventricle), separated into individual lobes and subjected to expansion-perfusion with 1.7 mL of plunger expansion/g tissue. After expansion perfusion with CM, PSW cubes and mouse lung lobes were transferred individually into sterile cryovials (Thermo Scientific, Rochester, NY, USA) containing CM, and subjected to slow freezing (~1 °C/min) controlled by an isopropanol bath container (Nalgene/Thermo Scientific) in a −80 °C freezer.
Thawing and dissociation of cryopreserved lung tissue
Cryovials containing lung tissue samples were stored in a −80°C freezer for a period ranging from 1 week to several months. For thawing, cryovials were transferred from −80 °C freezer storage into a dry ice container, brought to a 37 °C water bath, and rapidly thawed with agitation at 37 °C. Once the CM medium thawed, the CM and tissue were transferred into a sterile 60mm dish containing RPMI-1640 (Invitrogen) supplemented with 2% FBS (RPMI/FBS). The tissue was divided into smaller pieces using sterile scalpel and forceps and washed 3 times in RPMI/FBS. Tissue was then dissociated enzymatically for 5–6 h at 37 °C with gentle rocking in DMEM-F12 (Invitrogen) containing a mixture of collagenase I and II and low activity dispase/neutral protease (300 U/mL; Liberase DL, Roche Applied Science, Mannheim, Germany) and hyaluronidase (100 U/mL; AppliChem GmbH, Darmstadt, Germany), according to the manufacturer’s specifications. After enzymatic digestion, tissue was triturated with a P1000 Pipetman to break up clumps, pelleted at 450 g for 7 min, and resuspended in a 4:1 (v/v) mixture of NH4Cl lysis buffer (StemCell Technologies Inc, Vancouver, BC, Canada) and HF (Hanks’ balanced Salt Solution, Invitrogen, supplemented with 2% FBS) to lyse red blood cells. The sample was then pelleted and resuspended with pipetting for 1 min in prewarmed 0.25% Trypsin-EDTA (Invitrogen, Carlsbad, CA, USA). The sample was diluted 5-fold in HF, pelleted, and resuspended with pipetting for 1 min in prewarmed Dispase (5mg/mL; BD Biosciences) and DNase I (1 mg/mL; StemCell Technologies Inc) to generate a cell suspension. The suspension was diluted 5-fold in HF, filtered through a 40 μm cell strainer (BD Biosciences), pelleted, and resuspended in various specialized media for culture (see below).
Culture of primary lung cell types
Cell count assessment of disaggregated cells from cryopreserved lung tissues was performed using a hemocytometer on an inverted light microscope with Trypan blue exclusion as the viability assay. Cells were plated on tissue culture-treated plastic freshly coated with 0.1% gelatin (Sigma Aldrich) in phenol red-free, HEPES-buffered DMEM-F12, supplemented with 10% FBS, GlutaMAX™ (L-alanyl-L-glutamine; Invitrogen), 2-mercaptoethanol (Invitrogen), non-essential amino acids (Invitrogen) and antibiotic-antimycotic solution (Invitrogen) and incubated at 37 °C in 5% CO2, 21% O2.
All thawed cells were cultured for two passages in DMEM-F medium prior to plating and expansion in selective media. Primary vascular endothelial (VE)-like cells were expanded on tissue culture-treated plastic coated with 0.1% gelatin in EGM-2 medium (CC-3162; Lonza, Walkersville, MD, USA) and pneumocytes/alveolar type II (ATII)-like cells were expanded on tissue culturetreated plastic coated with 0.1% gelatin in either HITES medium [RPMI 1640 medium supplemented with antibiotics, hydrocortisone (10 nM), insulin (5 pug/mL), transferrin (100 Ag/mL), 17β-estradiol (10 nM), and sodium selenite (30 nM), supplemented with 5% FBS] or small airway epithelial cell medium (SAEC, PromoCell, Heidelberg, Germany) at 37 °C in 5% CO2, 21% O2. Fibroblast-like cells were expanded in DMEM-F. For growth curves, cells were plated at 2 × 104 cells/35mm dish and expanded to 80–90% confluence prior to counting and passage at the same plating density (2 × 104 cells/35mm dish; n=3 per condition).
Western Blot Analysis
Protein concentrations of medium from cell culture were determined using a Micro BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). To rid of albumin from FCS in medium, 250 μl of isolated medium samples, including translucent particulate (see Results section), were applied to 1 mL C8 solid phase extraction (SPE) columns (Alltech, Deerfield, IL, USA) pre-conditioned with successive washes of methanol, 1:1 (v/v) methanol/water and 18 M-Ohm/cm water (1 mL each). Bound protein was then washed with 1mL H2O, and eluted by passage of a) 1 mL H2O:methanol (1:1 v/v) and b) 1 mL 100% methanol. Eluted protein fractions (100 μL each) were dried via centrifugal vacuum and separated by SDS-PAGE (4–12% NuPAGE gels, Invitrogen), transferred onto nitrocellulose, and probed with rabbit polyclonal antibody produced in house using purified bovine mature SP-B (secreted form) as the antigen (Baatz et al., 2001). Peroxidase-conjugated secondary antibodies and SuperSignal West Dura chemiluminescence kit (both Pierce Biotechnology) were used for visual detection with a FluorChem 8900 Imager (Alpha Innotech, San Leandro, CA, USA).
RT-PCR and qPCR
RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA, USA), and cDNA generated using 1 μg of total RNA and the Omniscript RT Kit (Qiagen) according to the manufacturer’s instructions. When sample size was limiting, RNA was extracted using the Arcturus Picopure kit (Invitrogen) and cDNA generated using Superscript III reverse transcriptase (Invitrogen).
The primers used to amplify actin (ACT), surfactant protein B (SP-B), surfactant protein C (SP-C), and procollagen (COL) were designed based on conserved regions of alignments generated using ClustalW and sequences from closely related species. When available, sequences from dolphin (Tursiops truncatus), cow (Bos taurus), sheep (Ovis aries), pig (Sus scrofa), and human (Homo sapiens) were used. The sequences and primers used are shown in Table 1.
Table 1.
List of primers used in the PCR amplifications described in the results section.
| GENE ID | Primer couples | SEQUENCE (5′->3′) | Amplicon size (bp) | Species | NCBI Acc.no. |
|---|---|---|---|---|---|
| ACT | forward | GCACCACACCTTCTACAACGAGCTG | 330 | T.truncatus | AY744138 |
| reverse | AGCCAGGTCCAGACGCAGGATGG | ||||
| SP-B | forward | CCGAGTTCTGGTGCCAAAGCCTG | 480 | Sus scrofa | NM_001102679 |
| reverse | GCATGGGAATGGATAACTGCTGCTC | ||||
| SP-C | forward | TCA AAC GCC TTC TCA TCG TGG TCG TG | 260 | Sus scrofa | NM_001044533 |
| reverse | AGG TTC CCG GGG CTG GCT TGT AG | ||||
| COL | forward | ACATCCCACCAGTCACCTGCGTAC | 250 | Bos taurus | NM_001034039 |
| reverse | GTC TCC TTT CGG TCC CTC GAC TC |
Primers used in RT-PCR and qPCR were for actin (ACT); surfactant protein B (SP-B); surfactant protein C (SP-C); procollagen (COL). The species listed is the one used to design the primers after a conserved region for each gene was identified aligning nucleotide sequences from closely related species using ClustalW. The sequences used, when possible, were from dolphin (Tursiops truncatus), cow (Bos taurus), sheep (Ovis aries), pig (Sus scrofa) and human (Homo sapiens), publicly available at http://www.ncbi.nlm.nih.gov.
Standard RT-PCR was performed using 100 ng of cDNA and Advantage cDNA PCR Kit (Clontech, Mountain View, CA), following the manufacturer’s instructions using the following conditions: 95 °C for 4 min, 2 cycles with 95 °C for 20 s, 56°C for 30 s and 68 °C for 1 min, 2 cycles with 95 °C for 20 s, 54°C for 30 s and 6 8°C for 1 min, 2 cycles with 95 °C for 20 s, 52 °C for 30 s and 68 °C for 1 min and 30 cycles with 95 °C for 20 s, 50 °C for 30 s and 68 °C for 1 min. Following image acquisition of 1% agarose gels using a FluorChem 8900 Imager (Alpha Innotech), relative molecular weights and expression levels of SP-B and SP-C PCR products were quantified using ImageJ software with normalization to actin expression.
Quantitative real time PCR (qPCR) was performed using 10ng of cDNA, iQ SYBR Green Supermix (BIO-RAD, Hercules, CA, USA) and 5 μM primer concentration of using an Eppendorf Mastercycler® RealPlex2 (Eppendorf, Hamburg, Germany) and touchdown PCR protocol: 1 cycle of 95 °C for 20 s, 1 cycle from 66 °C to 63 °C for 25 s each, 2 cycles from 62–59 °C for 25 s each, then 35 cycles at 58 °C for 25 s, followed by 72 °C for 20 s. After the final cycle, PCR products were subjected to melt curve analysis (from 55 °C to 95 °C over 20 min) to confirm the presence of a single amplicon. Actin was used as internal control for relative quantification and the analysis was carried out using the mean Ct values from 3 replicate samples. Briefly, the averaged Ct values were normalized to the corresponding actin mRNA Ct value and the comparative Ct method of analysis (2−ΔΔCt) was used to determine quantitative differences in gene expression between cultured cells and whole tissue samples.
RESULTS
Selective Growth of Lung Cells Derived from Cryopreserved Lung Tissues
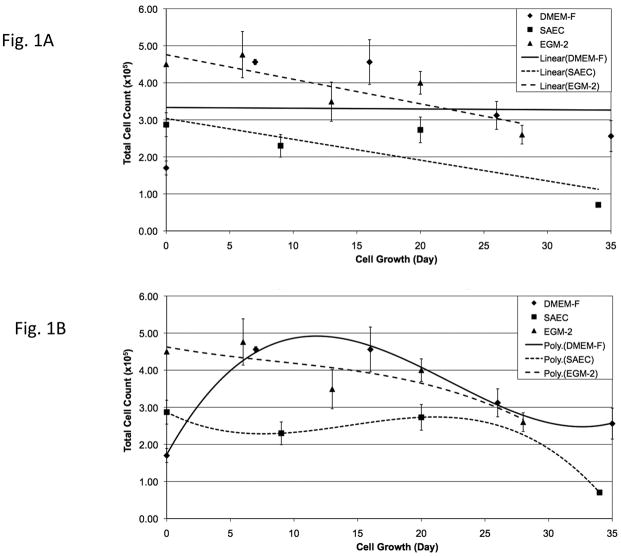
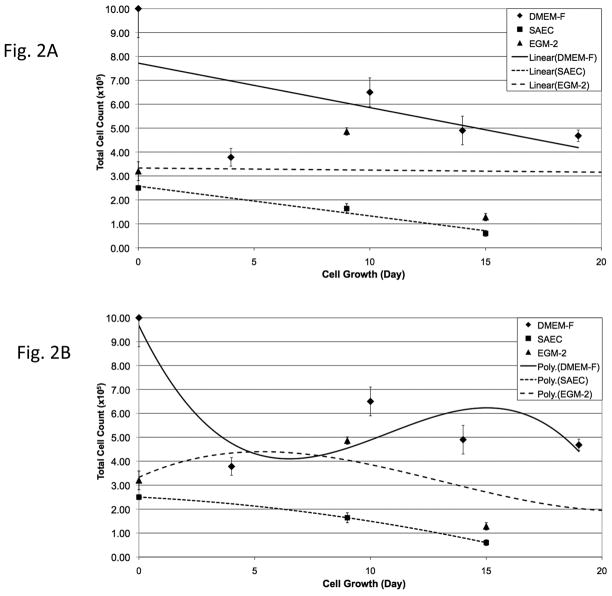
Cryopreserved PSW and mouse lung tissues were thawed and dissociated as described in Materials & Methods. After initial expansion in DMEM-F medium, aliquots of cells were plated in either alveolar type II cell (SAEC) or vascular endothelial cell (EGM-2) specialized media and monitored for selective cell growth and morphology. Qualitative PSW lung cell growth curves (Fig. 1) demonstrated a stable growth trend for cells cultured in DMEM-F medium and overall diminished growth rates for cells cultured in SAEC or EGM-2 media (Fig. 1A). Following cell counts using 3rd order polynomial plots to illustrate growth trends, growth rates in all media appeared optimal at 10–20 days in culture (passages 2–3) and thereafter declined (Fig. 1B). Qualitative mouse lung cell growth curves (Fig. 2) demonstrated overall stable growth for cells cultured in EGM-2 and overall diminished growth rates for cells cultured in DMEM-F and SAEC (Fig. 2A). Following cell counts using 3rd order polynomial trend plots, growth rates of mouse lung cells in DMEM-F or SAEC media appeared optimal for only ~10 days in culture (passages 1–2) and thereafter declined (Fig. 1B). In contrast, mouse lung cells maintained in EGM-2 showed a distinct initial drop in growth rate, followed by stable growth (Fig. 2B). This result may indicate greater selectivity of EGM-2 medium for vascular endothelial (VE) cells, thereby producing an early loss of non-VE cells from the population. Because these are primary cells grown in vitro, overall diminishing growth rates were anticipated for both PSW and mouse cells. Not anticipated, however, was the higher yield, but poorer persistence of thawed mouse versus PSW lung cells (Figs. 1 & 2). The higher yield of cells from the mouse may reflect differences in exposure of cells to cryoprotectant prior to freezing, the extended time for PSW lung cryopreservation after stranding event (over 12 hrs), and/or efficiency of enzymatic release of cells from tissue after thawing due to differences in lung tissue size and structure.
Figure 1. Maintenance and expansion of PSW cells from cryopreserved tissue in selective media.
Thawed and dissociated pygmy sperm whale (PSW) lung cells were passed twice in DMEM-F medium and plated at 2 × 104 cells/35mm dishes in DMEM-F, SAEC or EGM-2 media. At 80–90% confluence, cells were counted and replated at the same starting density. Panels A and B are linear and 3rd order polynomial plots of the same data sets. Day 0 represents the day in which disaggregated cells were plated on culture dishes.
Figure 2. Maintenance and expansion of mouse cells from cryopreserved tissue in selective media.
Thawed and dissociated mouse lung cells were passed twice in DMEM-F medium and plated at 2 × 104 cells/35mm dishes in DMEM-F, SAEC or EGM-2 media. At 80–90% confluence, cells were counted and replated at the same starting density. Panels A and B are linear and 3rd order polynomial plots of the same data sets. Day 0 represents the day in which disaggregated cells were plated on culture dishes.
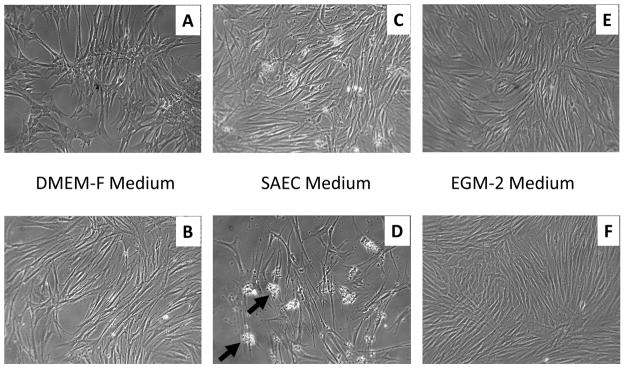
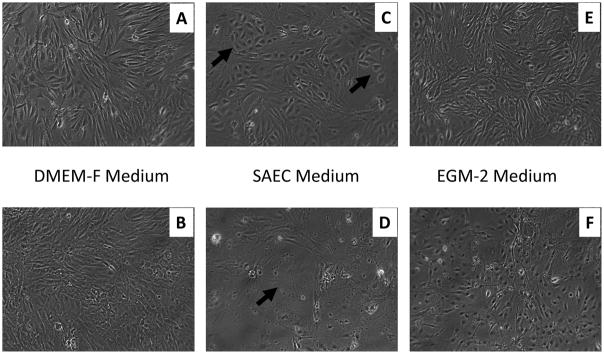
Morphologically, the mouse lung cells appeared more characteristic of the classic cuboidal shape of ATII cells than the PSW lung cells at both lower and higher passages in the SAEC culture media (Figs. 3 & 4). Thawed mouse lung cells cultured in SAEC demonstrated cells with an ATII-like morphology at lower passage (Fig. 4C) and either a senescent or alveolar Type I (ATI)-like thin and elongated morphology at higher passage (Fig. 4D). Nevertheless, the PSW lung cells grown in SAEC medium did produce an abundant amount of translucent particulate that became more apparent with passage (compare Figs. 3C,D; Arrows, Fig. 3D). Secretion of this particulate by PSW lung cells was readily apparent after low passage (two days in culture) and remained for several passages (at least 10 days in culture) without diminution (Fig. 3C,D). This material was identified by Western Blot to contain mature surfactant protein B (SP-B; Figure 5), a marker of alveolar ATII epithelial cells (Vorbroker et al., 1995). Though translucent particulate was not readily observed in medium from mouse lung cells (using standard light microscopy), western blotting revealed SP-B was present in medium indicating that mouse lung cells mouse lung cells grown in the SAEC medium also secreted SP-B (Figure 5). Cells grown in HITES medium, which is commonly used for ATII-like cell culture (Grek et al., 2010), did not result in production of this SP-B-containing particulate, nor was the media enriched for any measurable surfactant protein, thus indicating that HITES medium did not promote maintenance of ATII cell characteristic marker (data not shown).
Figure 3. Bright field images of PSW lung cell types grown in selective media.
Passage 2 (panels A, C, E) and passage 4 (panels B, D, F) PSW cells grown in DMEM-F (panels A, B), SAEC (panels C, D) or EGM-2 (panels E, F) media and imaged. Arrows in panel D point to insoluble cell associated material found in SAEC medium (image width = 1mm).
Figure 4. Bright field images of mouse lung cell types grown in selective media.
Passage 2 (panels A, C, E) and passage 4 (panels B, D, F) PSW cells grown in DMEM-F (panels A, B), SAEC (panels C, D) or EGM-2 (panels E, F) media and imaged. Arrows in panel C point to cells with a typical ATII cell morphology. Arrow in D points to a cell with a typical senescent and/or ATI cell morphology (image width = 1mm).
Figure 5. Surfactant protein B (SP-B) is present in the supernatant of PSW cells cultured in SAEC medium.
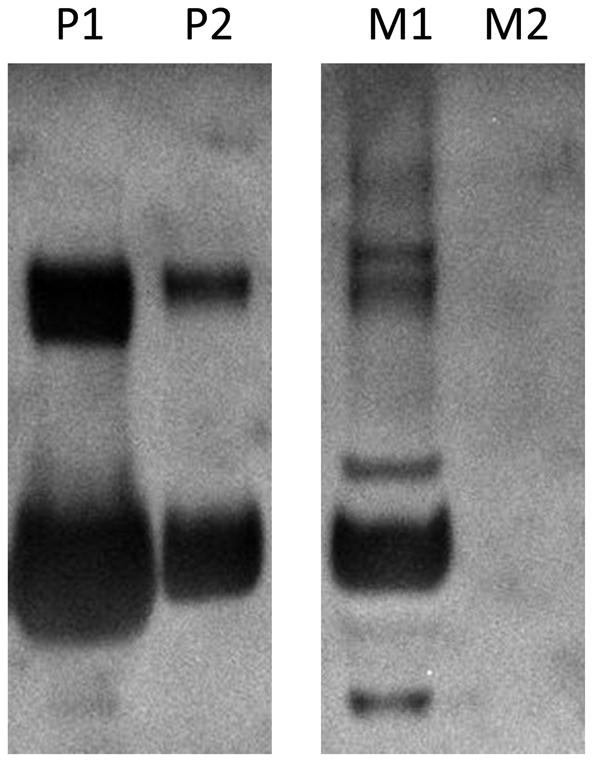
SP-B oligomer was identified via Western Blot to be abundantly secreted by PSW. Lanes P1, P2: C8 solid phase extraction (SPE) Methanol/water (1:1 v/v) and Methanol fractions, respectively, of Pygmy sperm whale lung cell media. Lanes M1 and M2: C8 SPE methanol/water (1:1 v/v) and methanol fractions, respectively, of mouse lung cells media. Media from both mouse and PSW cell cultures were isolated with the same protocol (described in Material and Methods) and cultured under the same conditions.
Identification of Lung Cell Types Expanded from Cryopreserved Lung Tissues
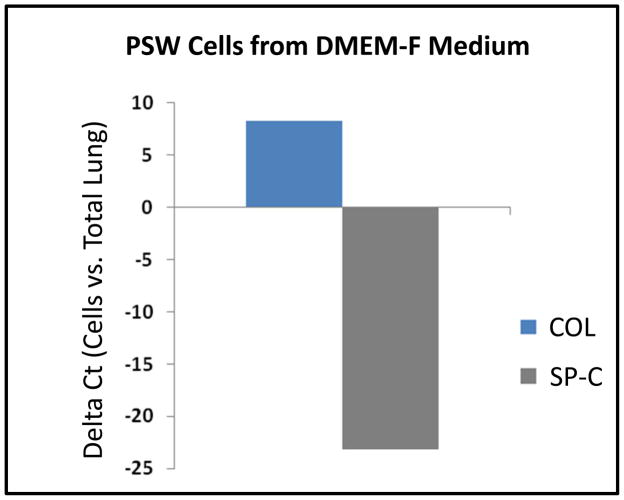
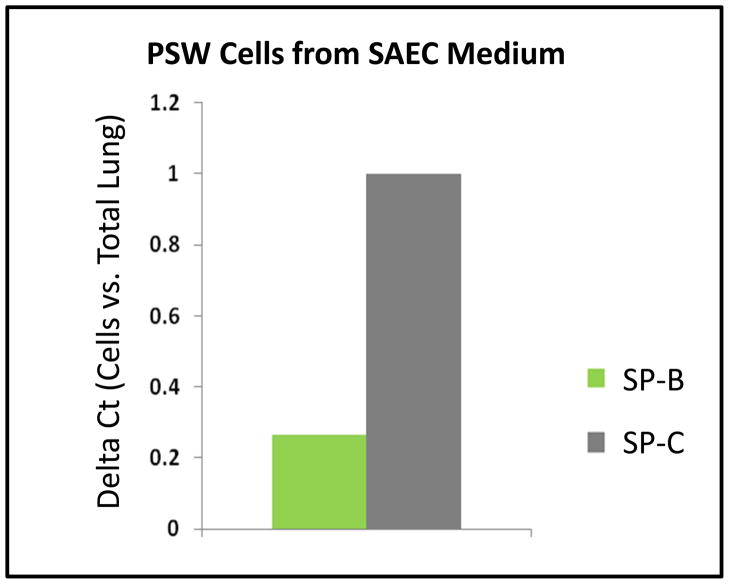
Since characteristic cell morphology was significantly less pronounced in cultures of thawed PSW lung cells compared to mouse cells, the expression of cell-specific molecular markers was analyzed. Dolphin/PSW-specific primers were designed using conserved sequences corresponding to the fibroblast-specific marker procollagen (COL) (Krustrup et al., 2006) and the pneumocyte-specific markers surfactant protein-B (SP-B) and -C (SP-C) (Vorbroker et al., 1995). To determine if PSW cells cultured in DMEM-F medium were enriched for populations of fibroblast and depleted in other lung cell types (e.g., ATII cells), we performed PCR on PSW cDNA after 4 passages in DMEM-F medium. As shown in Figure 6, COL is highly expressed and SP-C is barely detectable in mRNA from PSW cells cultured in DMEM-F medium (relative to values quantified in total PSW lung tissue mRNA), indicating a significant enrichment of fibroblasts. Similarly, to determine if PSW cells cultured in SAEC medium were enriched for populations of pneumocytes and depleted in other lung cell types (e.g., fibroblasts), we performed qPCR on PSW cDNA after 4 passages in SAEC medium. As shown in Figure 7, pneumocyte-specific markers SP-B and SP-C were highly expressed in SAEC cultures. As expected, both mRNAs were more highly expressed in these cultures than in total PSW lung tissue mRNA and SP-C is approximately 3-fold more abundant than SP-B mRNA, indicating an enrichment in surfactant-producing cells when cultured in SAEC medium (Fig. 7).
Figure 6. PSW cells derived from cryopreserved lung tissue and cultured in DMEM-F medium are enriched for fibroblast-specific marker expression.
Quantitative real-time PCR was used to measure relative, steady-state expression of procollagen (COL, a fibroblast marker) and surfactant protein C (SP-C, an alveolar Type II cell, ATII, marker) mRNAs extracted from PSW primary cell cultures grown in DMEM-F medium. Relative level of expression is given as compared to that of mRNA extracted from PSW whole lung tissue (ΔCt values, normalized to actin).
Figure 7. PSW cells derived from cryopreserved lung tissue and cultured in SAEC medium are enriched for pneumocyte-specific marker expression.
Semi-quantitative RT-PCR and gel densitometry were used to compare relative levels of SP-B and SP-C mRNA expression in PSW primary cultures grown in SAEC medium to the expression observed in PSW whole lung tissue (values normalized to actin).
DISCUSSION
Since strandings of PSWs This parallel approach permitted us to demonstrate the broad capabilities and efficaciousness of our cryopreservation/rederivation method. Thus, the well-characterized mouse model system was used as a control to validate the procedures employed and to demonstrate ‘proof of principle’ for potential utility of this cryopreservation method for different mammalian species. We have demonstrated a method for the cryopreservation of lung tissue from a stranded marine mammal and the subsequent retrieval of viable lung cell types from the tissue after thawing. Differential efficiencies of viable cell retrieval and growth rates were found between PSW and mouse lung tissues. These results indicate that conditions can be further optimized with different sources of lung tissues. PSW lungs, as with other toothed whales, are cartilaginous and are likely to have increased amounts and altered proportions of collagens, glycosaminoglycans, and other extracellular molecules. Such differences with the mouse lung may reduce exposure of cells to cryoprotectant during perfusion and are likely to reduce the efficiency of release of cells during enzymatic digestion after thawing. A better understanding of extracellular distribution in the PSW would be a first step in optimizing the latter process.
Perhaps one of the most convincing evidence for successful isolation and culture of distinct populations of cells from the cryopreserved lung tissue is the abundant secretion of surfactant protein SP-B by PSW cells cultured in SAEC medium. This finding is highly significant -not only because it is a characteristic marker of ATII cells-but also because SP-B is normally secreted by ATII cells into the airway and is required to maintain alveolar integrity to avoid collapse (Whitsett and Baatz, 1992). In addition, evidence of in vitro secretion of SP-B by cultured primary cells in literature is limited and usually requires highly specific medium and approximately 5 to 7 days prior to evident SP-B secretion (Rice et al., 2002).
Limited expansion of primary cells in culture is another area of focus for optimization. Chemical and genetic means for the inhibition of replicative senescence and/or generation of induced pluripotent stem cells (iPSCs) may be considered for the maintenance of lung cell types present and for the differentiation of a variety of cell types from the PSW fibroblasts described here. Which approaches are optimal for improving lung cell type expansion and for iPSC generation from fibroblasts (and the differentiative capacity of PSW iPSCs generated) remain to be determined, but are well worth pursuing. Such approaches could at least in part circumvent problems associated with the cryopreservation of other tissues that are less easily perfused.
Cell culture models for the lung cell types described here could be used in vitro and in reconstituted 3-dimensional (3D) lung tissues to better understand the lung physiology of deep diving mammals, such as their response to hypoxia and for testing the response to stressors, such as biotoxins (Grek et al., 2009). An understanding of PSW lung physiology, which is adapted to withstand extremes in hypoxia and pressure, may be useful for the treatment of pulmonary disorders in humans. Conversely, deep diving marine mammals may be more susceptible to injury from toxins at the water’s surface, associated with increases in mortality, unusual mortality events (UMEs), and previously unseen pathologies. The primary lung cell types described could be used as surrogates for the intact animal to test these toxins. According to the NOAA Office of Protected Species, the underlying causes of marine mammal UMEs have been increasingly associated with harmful algal blooms containing biotoxins, such as brevetoxin and domoic acid (http://www.nmfs.noaa.gov/pr/health/mmume/), the latter of which has been implicated as a primary cause of biotoxin-related PSW strandings in the southeast United States (Fire et al., 2009). The impact of biotoxin exposure to PSW lung cell types could be investigated with the cells derived from our cryopreserved samples. Also, in the case of mass stranding events and UMEs, there would be great utility for our method for cryopreserving large numbers of samples.
This report provides a method for the preparation of viable cell types that would otherwise be unavailable for study. A potential limitation of this approach is that the yield of sufficient numbers of cells for various subsequent studies may be limiting. However, induced pluripotent stem cell technology could be used to both 1) expand populations of cells and 2) produce other non-lung cell types that may not be easily maintained through cryopreservation. Also, there is tremendous potential to use this variety of differentiated cell types from fibroblast-derived PSW iPSCs to study the cause of the stranding event itself. For example, hepatocytes can be used to test heavy metal exposure, and cardiomyocytes can be used to better understand causes of hypertrophic heart disease, both of which are common in stranded pygmy sperm whales (Bossart et al., 1985). This method could also be used for feasible tissue banking of lung tissues from a variety of marine mammalian species for toxicological, mechanistic and pathological studies.
Acknowledgments
The authors would like to thank Deirdre D. Walker for technical assistance with the counting of cells and maintenance of records. This work was conducted with support from the National Institutes of Health, Grant Number HL085738.
Abbreviations
- ATI cells
alveolar type I pneumocytes
- ATII cells
alveolar type II pneumocytes
- ACT
actin
- CM
cryopreserving medium
- COL
procollagen
- DMEM-F
Dulbecco’s modified Eagle medium-F12
- EGM-2
vascular endothelial cell specialized media
- SAEC
small airway epithelial cell
- SP-B
pulmonary surfactant protein B
- SP-C
pulmonary surfactant protein C
- SPE
solid phase extraction
- PSW
pygmy sperm whale
- VE cells
vascular endothelial cells
Footnotes
This paper is based on a presentation given at the 5th Aquatic Annual Models of Human Disease conference: hosted by Oregon State University and Texas State University-San Marcos, and convened at Corvallis, OR, USA September 20–22, 2010.
NOAA Disclaimer
This publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, to any advertising or sales promotion which would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has as its purpose an interest to cause the advertised product to be used or purchased because of this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayers LW. Methods in molecular biology. Clifton, NJ: 2011. The AIDS and Cancer Specimen Resource; p. 193. [DOI] [PubMed] [Google Scholar]
- Baatz JE, Zou Y, Cox JT, Wang Z, Notter RH. High-yield purification of lung surfactant proteins SP-B and SP-C and the effects on surface activity. Protein Expr Purif. 2001;23:180–190. doi: 10.1006/prep.2001.1492. [DOI] [PubMed] [Google Scholar]
- Bossart GD, Odell DK, Altman NH. Cardiomyopathy in stranded pygmy and dwarf sperm whales. J Am Vet Med Assoc. 1985;187:1137–1140. [PubMed] [Google Scholar]
- Cortes B, Schiffman M, Herrero R, Hildesheim A, Jimenez S, Shea K, Gonzalez P, Porras C, Fallas G, Rodriguez AC. Establishment and operation of a biorepository for molecular epidemiologic studies in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2010;19:916–922. doi: 10.1158/1055-9965.EPI-10-0066. [DOI] [PubMed] [Google Scholar]
- Fire SE, Wang Z, Leighfield TA, Morton SL, McFee WE, McLellan WA, Litaker RW, Tester PA, Hohn AA, Lovewell G, Harms C, Rotstein DS, Barco SG, Costidis A, Sheppard B, Bossart GD, Stolen M, Durden WN, Van Dolah FM. Domoic acid exposure in pygmy and dwarf sperm whales (Kogia spp.) from southeastern and mid-Atlantic U.S. waters. Harmful Algae. 2009;8:658–664. [Google Scholar]
- Grek CL, Newton DA, Qiu Y, Wen X, Spyropoulos DD, Baatz JE. Characterization of alveolar epithelial cells cultured in semipermeable hollow fibers. Exp Lung Res. 2009;35:155–174. doi: 10.1080/01902140802495870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grek CL, Newton DA, Spyropoulos DD, Baatz JE. Hypoxia Upregulates Expression of Hemoglobin in Alveolar Epithelial Cells. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman GL, Ponganis PJ. The physiological basis of diving to depth: birds and mammals. Annu Rev Physiol. 1998;60:19–32. doi: 10.1146/annurev.physiol.60.1.19. [DOI] [PubMed] [Google Scholar]
- Krustrup D, Rossen K, Thomsen HK. Procollagen 1 - a marker of fibroblastic and fibrohistiocytic skin tumors. J Cutan Pathol. 2006;33:614–618. doi: 10.1111/j.1600-0560.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- McAlpine DF, Murison LD, Hoberg EP. New records for the pygmy sperm whale, Kogia breviceps (Physeteridae) from Atlantic Canada with notes on diet and parasites. Mar Mammal Sci. 1997;13:701–704. [Google Scholar]
- Moritz C, Labbe C. Cryopreservation of goldfish fins and optimization for field scale cryobanking. Cryobiology. 2008;56:181–188. doi: 10.1016/j.cryobiol.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Odell DK, Barros NB, Stolen MK. Dwarft and pygmy sperm whale (genus kogia) stranding patterns in the southeastern United States. 84th Annual Meeting of the American Society of Mammologists; Arcata, CA. 2004. [Google Scholar]
- Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;283:L256–264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- Santos MB, Pierce GJ, pez A, Reid RJ, Ridoux V, Mente E. Pygmy sperm whales kogia breviceps in the northeast atlantic: New information on stomach contents and strandings. Mar Mammal Sci. 2006;22:600–616. [Google Scholar]
- Scott MD, Hohn AA, Westgate AJ, Nicolas JR, Whitaker BR, Campbell WB. A note on the release and tracking of a rehabilitated pygmy sperm whale (Kogia breviceps) J Cetacean Res Manage. 2001;3:87–94. [Google Scholar]
- Troyer D. Biorepository standards and protocols for collecting, processing, and storing human tissues. Meths Mol Biol. 2008;441:193–220. doi: 10.1007/978-1-60327-047-2_13. [DOI] [PubMed] [Google Scholar]
- Tyack PL, Johnson M, Soto NA, Sturlese A, Madsen PT. Extreme diving of beaked whales. J Exp Biol. 2006;209:4238–4253. doi: 10.1242/jeb.02505. [DOI] [PubMed] [Google Scholar]
- Vorbroker DK, Profitt SA, Nogee LM, Whitsett JA. Aberrant processing of surfactant protein C in hereditary SP-B deficiency. Am J Physiol. 1995;268:L647–656. doi: 10.1152/ajplung.1995.268.4.L647. [DOI] [PubMed] [Google Scholar]
- Watwood SL, Miller PJ, Johnson M, Madsen PT, Tyack PL. Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus) J Anim Ecol. 2006;75:814–825. doi: 10.1111/j.1365-2656.2006.01101.x. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Baatz JE. Hydrophobic surfactant proteins SP-B and SP-C: molecular biology, strycture and function. In: Robertson B, Batenburg JJ, editors. Pulmonary Surfactant: from molecular biology to clinical science. Elsevier Science publishers; Amsterdam: 1992. in: LMGVG. [Google Scholar]