Abstract
Several noncoding microRNAs (miR or miRNA) have been shown to regulate the expression of drug-metabolizing enzymes and transporters. Xenobiotic drug-induced changes in enzyme and transporter expression may be associated with the alteration of miRNA expression. Therefore, this study investigated the impact of 19 xenobiotic drugs (e.g., dexamethasone, vinblastine, bilobalide and cocaine) on the expression of 10 miRNAs (miR-18a, -27a, -27b, -124a, -148a, -324-3p, -328, -451, -519c and -1291) in MCF-7, Caco-2, SH-SY5Y and BE(2)-M17 cell systems. Our data revealed that miRNAs were differentially expressed in human cell lines and the change in miRNA expression was dependent on the drug, as well as the type of cells investigated. Notably, treatment with bilobalide led to a 10-fold increase of miR-27a and a 2-fold decrease of miR-148a in Caco-2 cells, whereas no change of miR-27a and a 2-fold increase of miR-148a in MCF-7 cells. Neuronal miR-124a was generally down-regulated by psychoactive drugs (e.g., cocaine, methadone and fluoxetine) in BE(2)-M17 and SH-SY5Y cells. Dexamethasone and vinblastine, inducers of drug-metabolizing enzymes and transporters, suppressed the expression of miR-27b, -148a and -451 that down-regulate the enzymes and transporters. These findings should provide increased understanding of the altered gene expression underlying drug disposition, multidrug resistance, drug-drug interactions and neuroplasticity.
Keywords: miRNA; drug disposition; metabolism; regulation, xenobiotic
Introduction
Absorption, distribution, metabolism and excretion processes determine the levels of drugs that eventually reach the target sites, exerting pharmacological or toxicological responses. Drug-metabolizing enzymes are responsible for metabolic elimination and transporters affect absorption, distribution and excretion. Different levels of expression of drug-metabolizing enzymes and/or transporters, controlled by genetic or epigenetic factors [1–6], may significantly alter the pharmacokinetic properties of a drug, leading to therapeutic failure or toxicity.
MicroRNAs (miRNAs) are short (~21 nt long), noncoding RNAs that control the posttranscriptional expression of target genes [7]. They act on a partially complementary segment within the 3'-untranslated region (3'UTR) of target transcript, leading to translation inhibition and/or mRNA cleavage. miRNAs have been shown to play an important role in development and metabolism as well as cell differentiation, proliferation and apoptosis. Recent studies have also demonstrated the importance of miRNAs in xenobiotic metabolism and disposition through the regulation of drug-metabolism enzymes, transporters and/or nuclear receptors [1–3]. Examples include the regulation of cytochrome P450 1B1 (CYP1B1) and CYP3A4 by miR-27b [8, 9], CYP2E1 by miR-378 [10], P-glycoprotein (MDR1/ABCB1) by miR-451 and -27a [11, 12], breast cancer resistance protein (MCRP/ABCG2) by miR-519c and -328 [13–15], multidrug resistance associated protein 1 (MRP1/ABCC1) by miR-326 [16], MRP2/ABCC2 by miR-379 [17], pregnane X receptor (PXR/NR1I2) by miR-148a [18], and vitamin D receptor (VDR/NR1I1) by miR-27b [9] and miR-125b [19, 20]. As a result, these enzyme and transporter regulatory miRNAs may modulate the capacity of drug metabolism and disposition, and affect the response of cells to xenobiotic drugs.
On the other hand, medications, drugs of abuse, toxins or hormones readily alter the expression of miRNAs in cells or tissues (reviewed in [1, 21]). Modulation of miRNA expression by a xenobiotic drug, which regulates drug-metabolizing enzymes or transporters, might lead to considerable change in pharmacokinetic and pharmacodynamic properties of a concomitant agent or the drug itself. Thus it is unclear how drug-metabolizing enzyme and transporter regulatory miRNAs are affected by xenobiotic drugs. Therefore, this study investigated the impact of xenobiotic drugs on expression of drug metabolism and disposition regulatory miRNAs (e.g., miR-27a, -27b, -148a, -328, -451 and -519c) in different cell lines. Caco-2, a human intestine cancer cell model widely used in drug absorption studies and MCF-7, a model for human breast cancer research, were used to examine possible effects of anticancer drugs (e.g., gemcitabine, taxol, doxorubicin, vinblastine, imatinib and mitoxantrone) and natural products (e.g., daidzein, tanshinone IIA and bilobalide) on miRNA expression. In addition, two neuroblastoma cell models, SH-SY5Y and BE(2)-M17, were employed to explore potential influence of psychoactive drugs (e.g., desipramine, DMT, 5-MeO-DMT, harmaline, fluoxetine and methadone) on expression of neuronal miRNAs (e.g., miR-124a, -18a and -328), as well as enzyme and transporter regulatory miR-27b. Our data revealed that xenobiotic drugs could have distinct effects on miRNA expression in different type of cells.
Material and Methods
Chemicals and Reagents
Gemcitabine, tanshinone IIA, vinblastine and taxol were purchased from LKT Laboratories, Inc. (St. Paul, MN). Daidzen and doxorubicin were bought from LC Laboratories (Woburn, MA). Dexamethasone, bilobalide and imatinib were purchased from VWR International, LLC (Bridgeport, NJ). Mitoxantrone, desipramine, fluoxetine, harmaline, disulfiram, cocaine, 5-methoxy-N,N-dimethyltryptamine (5-MeODMT), methadone and methylphenidate were bought from Sigma-Aldrich (St. Louis, MO). N,N-dimethyltryptamine (DMT) was supplied by RTI International (Research Triangle Park, NC). RPMI1640, MEM, F12:MEM and fetal bovine serum (FBS) were bought from Hyclone (Waltham, MA). Oligonucleotide primers were synthesized by Integrated DNA Technologies (Skokie, IL). SyBR Green was purchased from Invitrogen (Carlsbad, CA), and GoTaq master mix, RNAsin, M-MLV RT enzyme, and dNTPs were from Promega (Madison, WI). Cell lines were bought from ATCC (Manassas, VA).
Cell Culture
Human breast cancer (MCF-7), human colorectal adenocarcinoma (Caco-2), and human neuroblastoma SH-SY5Y and BE(2)-M17 cells were cultured in RPMI 1640, MEM medium, and F12:MEM (1:1) medium supplemented with 10% of fetal bovine serum, respectively. The cells were cultured at 37°C in a humidified atmosphere with 5% CO2/95% air. Cell culture media consisted of 10,000 units/mL of penicillin and 10,000 units/mL of streptomycin. Media were replaced three times (MCF-7 and Caco-2 cells) or twice (SH-SY5Y and BE(2)-M17) a week, and cells were trypsinized and subcultured every 7 days.
Drug Treatment
MCF-7, Caco-2, and SH-SY5Y/ BE(2)-M17 cells were seeded on a 24-well plate at a density of 1.0 × 105, 2.0 × 105 and 3.0 × 105 cells per well, respectively, 24 h prior to treatment. The final concentration of methanol or dimethyl sulfoxide (DMSO) in the culture medium was less than 0.1%. Preliminary experiments showed that methanol and DMSO at these concentrations did not show any cytotoxicity. The concentration, drug vehicle and time of treatment are shown in Table 1. A number of drugs (e.g., desipramine, DMT, 5-MeO-DMT, harmaline, fluoxetine and methadone) acting on the central nervous system were chosen to treat the neuroblastoma cell lines. Several anticancer drugs (e.g., gemcitabine, taxol, doxorubicin, vinblastine, imatinib and mitoxantrone) and natural products (e.g., daidzein, tanshinone IIA and bilobalide) were used to treat MCF-7 and Caco-2 cells. Drug concentrations were chosen according to those reported previously [22–34], which did not cause cytotoxicity to the cells.
Table 1.
Drugs used to treat different human cell lines.
| Drug | Concentration (μM) | Vehicle | Cell lines | Time of exposure (h) |
|---|---|---|---|---|
| Cocaine | 10 | Water | SH-SY5Y/BE(2)-M17 | 24* |
| Desipramine | 10 | Methanol | SH-SY5Y/BE(2)-M17 | 24 |
| DMT | 100 | Water | SH-SY5Y/BE(2)-M17 | 24* |
| 5-MeO-DMT | 10 | Water | SH-SY5Y/BE(2)-M17 | 24* |
| Harmaline | 10 | Water | SH-SY5Y/BE(2)-M17 | 24* |
| Disulfiram | 1 | DMSO | SH-SY5Y/BE(2)-M17 | 24 |
| Fluoxetine | 10 | Water | SH-SY5Y/BE(2)-M17 | 24 |
| Methadone | 10 | Water | SH-SY5Y/BE(2)-M17 | 24 |
| Methylphenidate | 10 | Water | SH-SY5Y/BE(2)-M17 | 24* |
| Bilobalide | 100 | DMSO | MCF-7/ Caco-2 | 48 |
| Daidzein | 30 | DMSO | MCF-7/ Caco-2 | 48 |
| Dexamethasone | 5 | DMSO | MCF-7/ Caco-2 | 48 |
| Doxorubicin | 10 | Water | MCF-7/ Caco-2 | 48 |
| Gemcitabine | 10 | Water | MCF-7/ Caco-2 | 48 |
| Imatinib | 10 | Water | MCF-7/ Caco-2 | 48 |
| Mitoxantrone | 5 | Water | MCF-7/ Caco-2 | 48 |
| Tanshinone IIA | 1 | DMSO | MCF-7/ Caco-2 | 48 |
| Taxol | 5 | DMSO | MCF-7/ Caco-2 | 48 |
| Vinblastine | 1 | Water | MCF-7/ Caco-2 | 48 |
Multiple doses; cells treated every 8 h during a 24-h period.
Stem Loop Reverse Transcription
Total RNA was extracted from cells with Trizol (Invitrogen, Carlsbad, CA). Stem-loop reverse transcription (RT) was conducted as described [15], using miRNA-selective primers (Supplemental Table S1). Specifically, each RT reaction contained 450 ng of total RNA, 2 μL of 5× RT buffer (Promega), 0.5 μL of 10 mM dNTP mix, 0.3 μL of RNAsin inhibitor (40U/μL), 0.4 μL of M-MLV RT enzyme (Promega) and 0.4 μL of primer (1 μM). The reactions were carried out on a 96-well plate at 16°C for 30 min, 42°C for 30 min, 85°C for 5 min and then maintained at 4°C.
Real-Time PCR
SYBR Green Real-time quantitative PCR (qPCR) reactions were conducted on a MyIQ real-time PCR system (Bio-Rad, Hercules, CA), as previously described [9, 15]. In general, each reaction included 10 μL of RT product 10× diluted, 1× GoTaq Master Mix (containing 0.15× SYBR Green), 0.5 μM miRNA specific forward primer and 0.25 μM universal reverse primer (Supplemental Table S1). The reactions were conducted on a 96-well plate at 95°C for 5 min, followed by 45 cycles of 95°C for 4 sec, 65°C for 18 sec, and 72°C for 5 sec. All qPCR reactions were performed in duplicate. The cycle number (CT) at which the amplicon crossed a defined threshold was defined for each individual miRNA. The relative level of each miRNA over internal standard was calculated using the formula 2−ΔCT, where ΔCT was the difference in CT values between miRNA and internal standard, U74 small nucleolar RNA.
Statistical analyses
Drug treatment was performed in triplicate, and each experiment was repeated once with cells pertaining to different passages. All values were expressed as mean ± SD. Different treatments were compared by unpaired Student's t test or One-Way ANOVA. Statistical analyses were carried out using GraphPad Prism version 5.00 (GraphPad Software Inc., San Diego, CA). Difference was considered statistically significant when probability was less than 0.05 (P < 0.05).
Results
MicroRNA profiling in human cell lines
First, we determined the relative abundance of individual miRNAs in Caco-2, MCF-7, SH-SY5Y and BE(2)-M17 cell model systems which may contribute to regulation of drug metabolism and disposition or neuronal actions. Our data (Table 2) showed that miR-27a/b, -324-3p and -148a were highly expressed (CT values below 25) in Caco-2 cells, whereas they were moderately (CT values between 25 and 30) expressed in MCF-7 cells. In contrast, miR-1291, -519c and -451 expression levels were relatively low (CT values higher than 30) in both Caco-2 and MCF-7 cells. miR-328 was expressed at a moderate level in Caco-2 and MCF-7 cells, in contrast to a higher level in neuroblastoma SH-SY5Y and BE(2)-M17 cell lines. In addition, miR-27b and -18a were highly expressed and miR-124a was moderately expressed in SH-SY5Y and BE(2)-M17 cells. Acquisition of miRNA profiles is essential for the following studies aiming to explore the impact of xenobiotic drugs on miRNA expression. As an example, because miR-1291 and miR-519c levels were revealed to be too low in MCF-7 and Caco-2 cells, respectively, miR-1291 and miR-519c were not studied in MCF-7 and Caco-2 cells, respectively, following the treatment with different drugs.
Table 2.
Abundance of individual miRNAs in different human cell lines.
| miRNA | Caco-2 cells | MCF-7 cells | SH-SY5Y | BE(2)-M17 | ||||
|---|---|---|---|---|---|---|---|---|
| CT value | Abundance* | CT value | Abundance* | CT value | Abundance* | CT value | Abundance* | |
| miR-18a | - | - | - | - | 18.3 | High | 20.3 | High |
| miR-27a | 24.6 | High | 28.1 | Moderate | - | - | - | - |
| miR-27b | 22.9 | High | 27.6 | Moderate | 24.1 | High | 23.3 | High |
| miR-124a | - | - | - | - | 28.9 | Moderate | 28.3 | Moderate |
| miR-148a | 24.5 | High | 27.1 | Moderate | - | - | - | - |
| miR-324-3p | 22.7 | High | 27.9 | Moderate | - | - | - | - |
| miR-328 | 27.4 | Moderate | 29.6 | Moderate | 24.9 | High | 24.1 | High |
| miR-451 | 30.2 | Low | 32.1 | Low | ||||
| miR-519c | - | - | 36.2 | Low | - | - | - | - |
| miR-1291 | 30.2 | Low | - | - | - | - | - | - |
Arbitrary miRNA abundance was classified according to CT values: high (CT below 25), moderate (CT between 25 and 30), and low (CT above 30).“-” not measured.
Effects of different xenobiotic drugs on miRNA expression in Caco-2 and MCF-7 cells
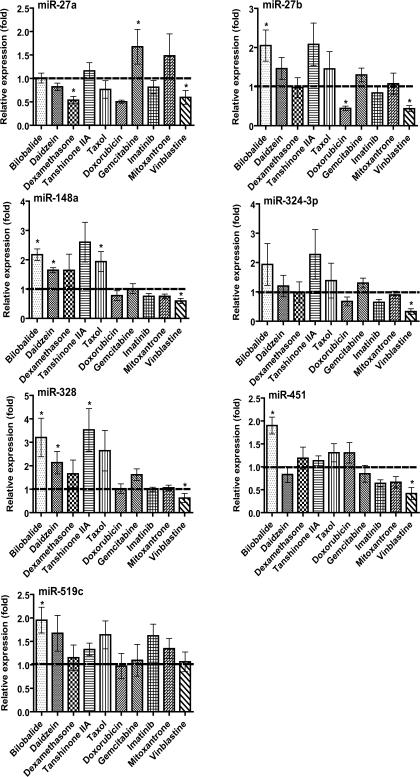
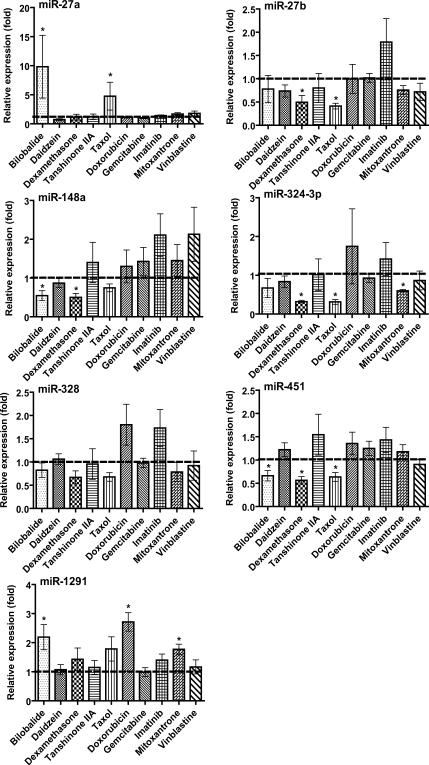
After a 24- or 48-h exposure to individual drugs, expression of some miRNAs was altered significantly, whereas others remained unchanged in MCF-7 and Caco-2 cells (Fig. 1 and 2). Mitoxantrone did not alter the expression of any tested miRNAs in MCF-7 cells (Fig. 1), and vinblastine did not affect the expression of any tested miRNAs in Caco-2 cells (Fig. 2). In contrast, mitoxantrone had significant influence on the expression of miR-27a, -324-3p and -1291 in Caco-2 cells (Fig. 2), and vinblastine exhibited considerable impact on the expression of miR-27a, -27b, 324-3p, 328, -148a and -451 in MCF-7 cells (Fig. 1). The cell type-specific change in miRNA expression was also true for other xenobiotic drugs such as daidzein, doxorubicin and imatinib. Furthermore, one drug may have distinct effects on the expression of different miRNAs in the same type of cells. For example, treatment with bilobalide led to a 10-fold increase of miR-27a and a 2-fold increase of miR-1291 but a 2-fold decrease of miR-148a and -451 in Caco-2 cells (Fig. 2). Taxol resulted in a 5-fold increase of miR-27a but a 2-to 3-fold decrease of miR-27b, -324-3p and -451 in Caco-2 cells (Fig. 2). In addition, none of the tested miRNAs was affected by gemcitabine in either MCF-7 or Caco-2 cells. The results indicate that different xenobiotic drugs could have distinct effects on miRNA expression, dependent upon the cell system chosen.
Fig. 1.
Effect of different xenobiotic drugs on miRNA expression in human breast cancer (MCF-7) cells. Cells were treated with different xenobiotic drugs (Table 1). Total RNA was isolated with Trizol reagent and reverse transcribed using specific primers (Supplemental Table S1). Individual miRNAs were profiled by qPCR analyses. miRNA level was normalized to small RNA U74 and compared to that (set as 1-fold) of vehicle control. Values represent mean ± SD of triplicate cultures repeated twice (N = 6). *P < 0.05 as compared to vehicle control.
Fig. 2.
Effect of different xenobiotic drugs on miRNA expression in human colon carcinoma (Caco-2) cells. Cells were treated with individual drugs (Table 1). All other details are as Figure 1. *P < 0.05 as compared to vehicle control.
A separate study was conducted to evaluate the effects of drugs on miRNA expression over time (0–72 h). Expression of miRNA-519c was elevated more than 5-fold (5.51 ± 1.21) at 24 h and 2-fold (1.95 ± 0.28) at 48 h, and then back to normal (1.00 ± 0.30) at 72 h in MCF-7 cells following the treatment with bilobalide. Another interesting finding is that, after 72 h of treatment with doxorubicin or mitoxantrone, miR-27a and -1291 levels were still increased more than 2-fold (2.20 ± 0.37) and 5-fold (5.44 ± 1.27), respectively, in Caco-2 cells. The results demonstrate a time-dependent change of miRNA expression in cells when exposed to xenobiotic drugs.
Effects of psychoactive drugs on miRNA expression in SH-SY5Y and BE(2)-M17 cells
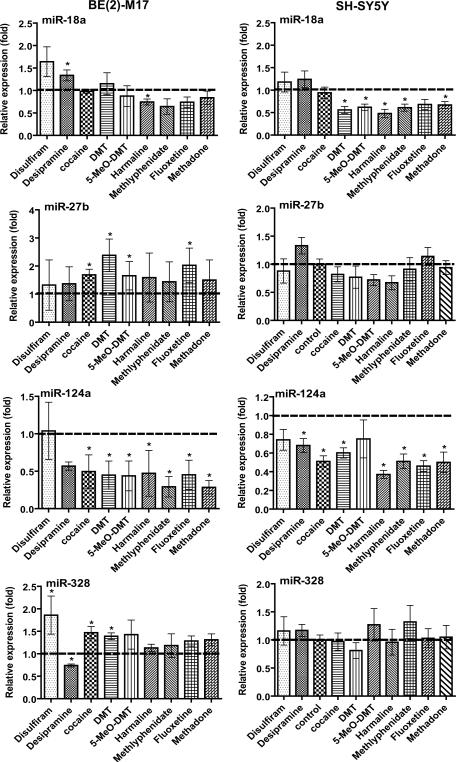
We further employed human neuroblastoma SH-SY5Y and BE(2)-M17 cell model systems to examine the impact of a number of therapeutic and abused drugs on the expression of miR-124a, -18a, -27b and -328. Similar to the findings from Caco-2 and MCF-7 cells (Fig. 1 and 2), some miRNAs were not affected by any of the drugs tested whereas others were changed significantly (Fig. 3). For example, none of the tested drugs altered the expression of miR-27b and -328 in SH-SY5Y cells. In contrast, miR-27b and -328 were both elevated significantly by cocaine and DMT in BE(2)-M17 cells. Most interestingly, the neuron-specific miR-124a was markedly down-regulated by all tested drugs except disulfiram in SHSY5Y and BE(2)-M17 cells (Fig. 3). miR-18a seemed to be generally down-regulated by these psychoactive drugs including DMT, 5-MeO-DMT, harmaline, methylphenidate and methadone. The results indicate that psychoactive agents might significantly alter the expression of neuronal miRNAs.
Fig. 3.
Effects of different psychoactive drugs on miRNA expression in SH-SY5Y and BE(2)-M17 human neuroblastoma cells. Cells were treated with different drugs (Table 1). All other details are as Figure 1. *P < 0.05 as compared to vehicle control.
Additive effect of drugs on miRNA expression
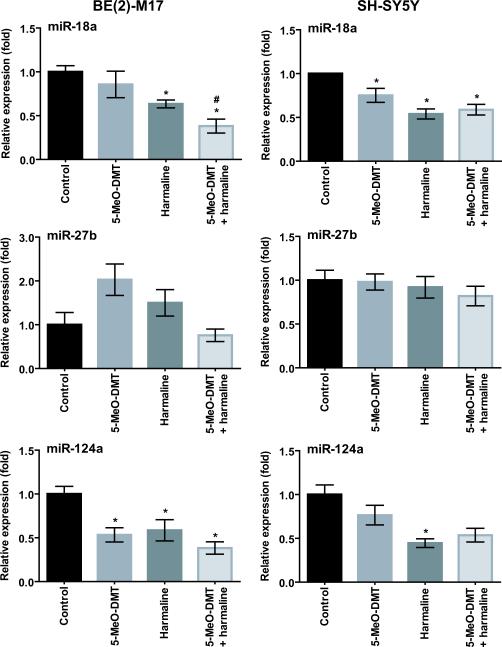
5-MeO-DMT is often used with a monoamine oxidase-A (MAO-A) inhibitor such as harmaline. Coadministration of harmaline results in an increased and prolonged exposure to 5-MeO-DMT [35, 36]. To test if concurrent harmaline potentiates 5-MeO-DMT drug effects, we compared miRNA expression in human neuroblastoma cells treated with 5-MeO-DMT alone and combined with harmaline. Consistent with the above findings (Fig. 3), harmaline and 5-MeO-DMT decreased the expression of miR-18a and -124a, whereas it had none or only a minor impact on miR-27b in SH-SY5Y and BE(2)-M17 cells. A significant additive effect of harmaline and 5-MeO-DMT was only observed for miR-18a in BE(2)-M17 cells (0.85 ± 0.37 for 5-MeO-DMT alone versus 0.38 ± 0.19 for 5-MeO-DMT plus harmaline) (Fig. 4).
Fig. 4.
Concurrent harmaline may potentiate the impact of 5-MeO-DMT on miRNA expression. SH-SY5Y and BE(2)-M17 cells were treated with 5-MeO-DMT and harmaline alone or together for 24 h. The expression of individual miRNAs was determined by stem loop RT-qPCR analyses and normalized to U74. Values represent mean ± SD of triplicate cultures repeated twice (N = 6). *P < 0.05 as compared to vehicle control; #P < 0.05 as compared to 5-MeO-DMT alone.
Discussion
There is accumulating evidence that noncoding miRNAs may contribute to epigenetic regulation of drug metabolism and disposition [1–3]. Because miRNAs can modulate the expression of drug-metabolizing enzymes and/or transporter, change of miRNA expression might alter miRNA signaling in cells. The present study clearly demonstrates that xenobiotic drugs may significantly affect miRNA expression in different type of cell model systems. The change of miRNA expression may be dependent upon the type of cells, as well as the drugs that cells are exposed to.
A number of miRNAs have been shown to control posttranscriptional gene regulation of CYP1B1 (miR-27b), CYP3A4 (miR-27b), ABCB1/P-gp (miR-451), ABCG2/BCRP (miR-519c and -328), NR1I2/PXR (miR-148a) or NR1I1/VDR (miR-27b) [8, 9, 11–14, 18] critical for drug disposition. Suppression of miRNA expression in control of drug metabolism and disposition may help explain the change in expression of efflux transporters or enzymes underlying potential drug-drug interaction as well as multidrug resistance [1]. For instance, dexamethasone is a well-known inducer of CYP3A4 and ABCB1, at least partially through the activation of PXR/NR1I2 [37]. Given our finding that dexamethasone lowers the expression of miR-27b, -451 and -148a in Caco-2 cells (Fig. 2), induction of CYP3A4 and ABCB1 presumably involves the actions of dexamethasone on miR-27b, -148a and -451. Another example is vinblastine, which has been shown to up-regulate ABCC1 expression in MCF-7 cells [38], ABCB1 in LS180 cells [39] and CYP3A4 in HepG2 cells [40]. Our data reveal that vinblastine reduces the expression of miR-27a/b, -324-3p, -328, -148a and -451 in MCF-7 cells (Fig. 1). Therefore, dysfunction of miRNA signaling likely contributes to vinblastine-caused overexpression of CYP3A4, ABCB1 and ABCC1 underlying multidrug resistance. Nevertheless, whether the change of miRNAs will lead to a significant alternation of miRNA targets and their biological functions awaits further investigation.
Treatment with natural compounds can also modulate miRNA expression in cells. The most prominent effect found in the present study is the increase of miR-27a/b, -451, -328 and -148a expression by bilobalide (Fig. 1 and 2). It is noteworthy that bilobalide is a major constituent of Ginkgo Biloba, an herbal medication used worldwide. Some in vivo and in vitro studies suggest that the extract of Ginkgo biloba leaves and some of the constituents show anticancer activity [41], whereas regular use of Ginkgo biloba does not reduce the risk of cancer in humans [42]. Alteration of miRNA expression may be associated with the action of bilobalide in modulation of drug metabolism and disposition [43]. Another interesting observation is that bilobalide has distinct effects on different miRNAs (miR-27a is increased 10-fold but miR-148a is decreased 2-fold) in the same type of cells (e.g., Caco-2; Fig. 2). Moreover, the same miRNA (e.g., miR-148a) may be altered by bilobalide in the opposite way in different type of cells, e.g., a 2-fold increase in MCF-7 cells but a 2-fold decrease in Caco-2 cells (Fig. 1 and 2). The differential effects of a drug on miRNAs expression in different type of cells are likely related to cell-specific biogenesis of miRNAs [7].
In agreement with the previous finding that neuronal miR-124a is suppressed in cocaine-treated rats [44], cocaine reduces miR-124a expression in both BE(2)-M17 and SH-SY5Y human neuroblastoma cell lines (Fig. 3). Brain-specific miR-124a is a major regulator in control of neuronal identity [45, 46]. A general suppression of miR-124a by psychoactive drugs (Fig. 3) may indicate neuroplasticity in response to xenobiotic agents. An equally important finding is the reduction of miR-18a expression by many psychoactive drugs (e.g., DMT and 5-MeO-DMT) in SH-SY5Y cells. miR-18a has been shown to control posttranscriptional gene regulation of glucocorticoid receptor (GR/NR3C1) [47] and estrogen receptor-alpha (ERα/NR3A1) [48, 49], ligand-inducible transcription factors controlling development, metabolism, immune response or neuronal differentiation. Interestingly, miR-18a is elevated by desipramine, a tricyclic antidepressant. A prolonged treatment with desipramine decreases the expression of GR/NR3C1 in Wistar-Kyoto rats with depressive behavior [50], which might be related to the elevation of miR-18a by desipramine (Fig. 3).
miR-328 is ubiquitously expressed in human tissues including brain [51]. miR-328 level has been shown to be elevated in schizophrenia [52]. As schizophrenic patients have a lifetime prevalence rate for cocaine abuse between 15 and 50%, adjuvant treatment with desipramine decreases cocaine usage and improves psychiatric symptoms [53]. The opposite effects of desipramine and cocaine on miR-328 expression in BE(2)-M17 cells suggest that desipramine may attenuate the impact of cocaine on miR-328 expression.
One limitation of the present study is that the mechanisms of drug-caused change of miRNA expression were not investigated. Since miRNAs are derived from miRNA precursors that are directly transcribed from the human genome or through mRNA splicing pathways, change of miRNA expression may be related to the actions of drugs on transcription factors of miRNA precursors. For instance, ERα/NR3A1 has been shown to regulate the maturation of a large number of miRNAs [54]. Meanwhile, xenobiotic drugs may act on other proteins within miRNA processing machineries, leading to an altered expression of mature miRNAs. As an example, enoxacin improves RNA interference and promotes miRNA processing through enhancing the RNA-binding protein [55, 56]. Identification of the underlying mechanisms would ultimately provide increased understanding of drug actions and cellular defense against xenobiotic agents.
Conclusion
This study has demonstrated that noncoding miRNAs are differentially expressed in human cell lines and the expression of some miRNAs may be significantly altered by specific xenobiotic drugs. A change in miRNA expression is not only dependent on the drug that cells are exposed to but also the type of cells. Delineation of the impact of xenobiotic drugs on miRNA processing would advance mechanistic understanding of altered gene expression underlying drug disposition, multidrug resistance, drug-drug interaction and neuroplasticity. Rather, further studies are warranted to identify the mechanisms for drug-induced change of miRNA expression and to define the specific impact of altered miRNA function on target gene expression.
Supplementary Material
Acknowledgements
This project was supported by Interdisciplinary Research Development Fund, University at Buffalo, The State University of New York, and in part by grant (R01DA021172) from National Institutes of Health. Rodrigues, A.C. was a recipient of fellowship from Fundação do Amparo à Pesquisa do Estado de São Paulo (FAPESP), Sao Paulo, Brazil (2008/014065-9).
Abbreviations
- miRNA/miR
microRNA
- DMT
dimethyltryptamine
- 5-MeO-DMT
5-methoxy-N,N-dimethyltryptamine
- DMSO
dimethyl sulfoxide
- CYP3A4
cytochrome P450 3A4
- MDR1/ABCB1
P-glycoprotein
- BCRP/ABCG2
breast cancer resistance protein
- MRP1/ABCC1
multidrug resistance associated protein 1
- PXR/NR1I2
pregnane X receptor
- VDR/NR1I1
vitamin D receptor
- RT
reverse transcriptase
- qPCR
real-time quantitative PCR
References
- 1.Yu AM. Role of microRNAs in the regulation of drug metabolism and disposition. Expert Opin Drug Metab Toxicol. 2009;5:1513–28. doi: 10.1517/17425250903307448. [DOI] [PubMed] [Google Scholar]
- 2.Gomez A, Ingelman-Sundberg M. Epigenetic and microRNA-dependent control of cytochrome P450 expression: a gap between DNA and protein. Pharmacogenomics. 2009;10:1067–76. doi: 10.2217/pgs.09.56. [DOI] [PubMed] [Google Scholar]
- 3.Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol Appl Pharmacol. 2010;245:378–93. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen G, Kong AN. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–55. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shomron N. MicroRNAs and pharmacogenomics. Pharmacogenomics. 2010;11:629–32. doi: 10.2217/pgs.10.26. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Dolan ME. The emerging role of microRNAs in drug responses. Curr Opin Mol Ther. 2010;12:695–702. [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–8. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 9.Pan YZ, Gao W, Yu AM. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab Dispos. 2009;37:2112–7. doi: 10.1124/dmd.109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohri T, Nakajima M, Fukami T, Takamiya M, Aoki Y, Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol. 2010;79:1045–52. doi: 10.1016/j.bcp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–8. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–9. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 13.To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3' untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28:5147–61. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75:1374–9. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, -519c and -520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81:783–92. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S, Shim H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–24. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, Remmler C, Cascorbi I. Down-regulation of ABCC2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Mol Pharmacol. 2011 doi: 10.1124/mol.110.070714. [DOI] [PubMed] [Google Scholar]
- 18.Takagi S, Nakajima M, Mohri T, Yokoi T. Post-transcriptional regulation of human pregnane × receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem. 2008;283:9674–80. doi: 10.1074/jbc.M709382200. [DOI] [PubMed] [Google Scholar]
- 19.Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol. 2009;76:702–9. doi: 10.1124/mol.109.056986. [DOI] [PubMed] [Google Scholar]
- 20.Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int J Cancer. 2009;125:1328–33. doi: 10.1002/ijc.24459. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Kong D, Wang Z, Sarkar FH. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res. 2010;27:1027–41. doi: 10.1007/s11095-010-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, de Boeck G, Sparreboom A, Verweij J, Nooter K. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4:747–52. doi: 10.4161/cbt.4.7.1826. [DOI] [PubMed] [Google Scholar]
- 23.Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural Transm. 2009;116:1591–9. doi: 10.1007/s00702-009-0308-8. [DOI] [PubMed] [Google Scholar]
- 24.Donnici L, Tiraboschi E, Tardito D, Musazzi L, Racagni G, Popoli M. Time-dependent biphasic modulation of human BDNF by antidepressants in neuroblastoma cells. BMC Neurosci. 2008;9:61. doi: 10.1186/1471-2202-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber BM, Bubko I, Krzyszton-Russjan J, Anuszewska EL. Synergistic action of doxorubicin and sulindac in human cervix carcinoma cells - studies on possible mechanisms. Med Sci Monit. 2010;16:BR45–51. [PubMed] [Google Scholar]
- 26.Honorat M, Mesnier A, Di Pietro A, Lin V, Cohen P, Dumontet C, Payen L. Dexamethasone down-regulates ABCG2 expression levels in breast cancer cells. Biochem Biophys Res Commun. 2008;375:308–14. doi: 10.1016/j.bbrc.2008.07.149. [DOI] [PubMed] [Google Scholar]
- 27.Iljin K, Ketola K, Vainio P, Halonen P, Kohonen P, Fey V, Grafstrom RC, Perala M, Kallioniemi O. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res. 2009;15:6070–8. doi: 10.1158/1078-0432.CCR-09-1035. [DOI] [PubMed] [Google Scholar]
- 28.Joyce PI, Atcheson R, Marcus RJ, Heffernan AM, Rowbotham DJ, Lambert DG. Interaction of local anaesthetic agents with the endogenous norepinephrine transporter in SH-SY5Y human neuroblastoma cells. Neurosci Lett. 2001;305:161–4. doi: 10.1016/s0304-3940(01)01822-5. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane × receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res. 2009;26:872–82. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, VandenBoom TG, 2nd, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pakkanen JS, Nousiainen H, Yli-Kauhaluoma J, Kylanlahti I, Moykkynen T, Korpi ER, Peng JH, Lukas RJ, Ahtee L, Tuominen RK. Methadone increases intracellular calcium in SH-SY5Y and SH-EP1-halpha7 cells by activating neuronal nicotinic acetylcholine receptors. J Neurochem. 2005;94:1329–41. doi: 10.1111/j.1471-4159.2005.03279.x. [DOI] [PubMed] [Google Scholar]
- 32.Raje S, Cao J, Newman AH, Gao H, Eddington ND. Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther. 2003;307:801–8. doi: 10.1124/jpet.103.053504. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt AJ, Krieg JC, Clement HW, Gebhardt S, Schulz E, Heiser P. Impact of drugs approved for treating ADHD on the cell survival and energy metabolism: an in-vitro study in human neuronal and immune cells. J Psychopharmacol. 2009;24:1829–33. doi: 10.1177/0269881109105563. [DOI] [PubMed] [Google Scholar]
- 34.Shi SL, Li QF, Liu QR, Xu DH, Tang J, Liang Y, Zhao ZL, Yang LM. Nuclear matrix protein, prohibitin, was down-regulated and translocated from nucleus to cytoplasm during the differentiation of osteosarcoma MG-63 cells induced by ginsenoside Rg1, cinnamic acid, and tanshinone IIA (RCT) J Cell Biochem. 2009;108:926–34. doi: 10.1002/jcb.22324. [DOI] [PubMed] [Google Scholar]
- 35.Shen HW, Jiang XL, Winter JC, Yu AM. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metab. 2010;11:659–66. doi: 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen HW, Wu C, Jiang XL, Yu AM. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochem Pharmacol. 2010;80:122–8. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–90. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 38.Schrenk D, Baus PR, Ermel N, Klein C, Vorderstemann B, Kauffmann HM. Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol Lett. 2001;120:51–7. doi: 10.1016/s0378-4274(01)00306-x. [DOI] [PubMed] [Google Scholar]
- 39.Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2010;66:765–71. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith NF, Mani S, Schuetz EG, Yasuda K, Sissung TM, Bates SE, Figg WD, Sparreboom A. Induction of CYP3A4 by vinblastine: Role of the nuclear receptor NR1I2. Ann Pharmacother. 2010;44:1709–17. doi: 10.1345/aph.1P354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeFeudis FV, Papadopoulos V, Drieu K. Ginkgo biloba extracts and cancer: a research area in its infancy. Fundam Clin Pharmacol. 2003;17:405–17. doi: 10.1046/j.1472-8206.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 42.Biggs ML, Sorkin BC, Nahin RL, Kuller LH, Fitzpatrick AL. Ginkgo biloba and risk of cancer: secondary analysis of the Ginkgo Evaluation of Memory (GEM) Study. Pharmacoepidemiol Drug Saf. 2010;19:694–8. doi: 10.1002/pds.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng Y, Bi HC, Zhao LZ, He F, Liu YQ, Yu JJ, Ou ZM, Ding L, Chen X, Huang ZY, Huang M, Zhou SF. Induction of cytochrome P450s by terpene trilactones and flavonoids of the Ginkgo biloba extract EGb 761 in rats. Xenobiotica. 2008;38:465–81. doi: 10.1080/00498250701883233. [DOI] [PubMed] [Google Scholar]
- 44.Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci. 2009;42:350–62. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maisel M, Habisch HJ, Royer L, Herr A, Milosevic J, Hermann A, Liebau S, Brenner R, Schwarz J, Schroeder M, Storch A. Genome-wide expression profiling and functional network analysis upon neuroectodermal conversion of human mesenchymal stem cells suggest HIF-1 and miR-124a as important regulators. Exp Cell Res. 2010;316:2760–78. doi: 10.1016/j.yexcr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T, Meijer OC, de Kloet ER, Fitzsimons CP. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–8. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- 48.Liu WH, Yeh SH, Lu CC, Yu SL, Chen HY, Lin CY, Chen DS, Chen PJ. MicroRNA-18a prevents estrogen receptor-alpha expression, promoting proliferation of hepatocellular carcinoma cells. Gastroenterology. 2009;136:683–93. doi: 10.1053/j.gastro.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Loven J, Zinin N, Wahlstrom T, Muller I, Brodin P, Fredlund E, Ribacke U, Pivarcsi A, Pahlman S, Henriksson M. MYCN-regulated microRNAs repress estrogen receptor-alpha (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc Natl Acad Sci U S A. 2010;107:1553–8. doi: 10.1073/pnas.0913517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaffer DJ, Tunc-Ozcan E, Shukla PK, Volenec A, Redei EE. Nuclear orphan receptor Nor-1 contributes to depressive behavior in the Wistar-Kyoto rat model of depression. Brain Res. 2010;1362:32–9. doi: 10.1016/j.brainres.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 51.Lee EJ, Baek M, Gusev Y, Brackett DJ, Nuovo GJ, Schmittgen TD. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. Rna. 2008;14:35–42. doi: 10.1261/rna.804508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2010;69:180–7. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 53.Ziedonis D, Richardson T, Lee E, Petrakis I, Kosten T. Adjunctive desipramine in the treatment of cocaine abusing schizophrenics. Psychopharmacol Bull. 1992;28:309–14. [PubMed] [Google Scholar]
- 54.Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, Takeyama K, Minami Y, O'Malley BW, Kato S. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell. 2009;36:340–7. doi: 10.1016/j.molcel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Shan G, Li Y, Zhang J, Li W, Szulwach KE, Duan R, Faghihi MA, Khalil AM, Lu L, Paroo Z, Chan AW, Shi Z, Liu Q, Wahlestedt C, He C, Jin P. A small molecule enhances RNA interference and promotes microRNA processing. Nat Biotechnol. 2008;26:933–40. doi: 10.1038/nbt.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melo S, Villanueva A, Moutinho C, Davalos V, Spizzo R, Ivan C, Rossi S, Setien F, Casanovas O, Simo-Riudalbas L, Carmona J, Carrere J, Vidal A, Aytes A, Puertas S, Ropero S, Kalluri R, Croce CM, Calin GA, Esteller M. Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1014720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.