Abstract
Objective
Levels of acute phase reactants are impacted by age. To what extent cardiovascular risk associated with aging is due to an increase in the inflammatory burden is not known. We assessed the relationship with age of inflammatory markers, representing a) systemic (C-reactive protein [CRP], fibrinogen and serum amyloid-A [SAA]) and b) vascular (lipoprotein-associated phospholipase A2 [Lp-PLA2] and pentraxin-3 [PTX-3]) inflammation.
Methods and Results
We determined Lp-PLA2 mass and activity, CRP, fibrinogen, SAA, and PTX-3 levels and other CVD risk factors in 336 Caucasians and 224 African Americans. Levels of systemic inflammatory markers increased significantly with age in both ethnic groups (P<0.05 for all), while trend patterns of vascular inflammatory markers did not change significantly with age for either group. In multivariate regression models adjusting for confounding variables, age remained independently associated with a composite z-score for systemic, but not vascular inflammation (β=0.250, P<0.001 and (β=0.276, P<0.001, for Caucasians and African Americans respectively).
Conclusions
We report an increase in the systemic, but not vascular, inflammatory burden over age. Levels of both categories of inflammatory markers over age were similar across ethnicity after adjustment for confounders. Our results underscore the importance of age in evaluating inflammatory markers to assess cardiovascular risk.
Keywords: Inflammation, aging, cardiovascular disease, epidemiology
Over the past decade there has been a growing appreciation that inflammation plays a critical role at every stage of the atherosclerotic process from lesion initiation to the rupture of atherosclerotic plaque 1. In support of this concept, many prospective studies demonstrate a positive association between risk for cardiovascular disease (CVD) and plasma levels of C-reactive protein (CRP) 2, 3 or fibrinogen 4, 5. More recently, other markers such as lipoprotein-associated phospholipase A2 (Lp-PLA2) were considered informative with regard to vascular inflammation and have shown to be associated with CVD 6–8. Notably, the weak correlation between Lp-PLA2 and CRP suggests that these inflammatory markers may reflect differences in underlying mechanisms 9. Further, ethnic differences in levels of circulating inflammatory markers have been reported, potentially related to demographic or lifestyle factors 10.
Although traditional cardiovascular risk factors are useful for predicting cardiovascular events in younger populations, their predictive value decreases with age 11. Therefore, one could speculate that presence of a low-grade inflammation may play a more substantial role in the pathogenesis of CVD in older compared to younger adults. However, information about the distribution pattern of inflammatory markers over the age spectrum is limited, and previous studies have yielded conflicting results. Thus, studies among healthy subjects failed to detect any significant difference in the production of interleukin-1 (IL-1) and IL-6 between young, middle-aged, and older participants 12. Other studies among healthy volunteers found an increase in circulating levels of IL-6, CRP, serum amyloid-A (SAA) and other inflammatory markers with age 13, 14. Further, as we have demonstrated previously, compared to Caucasians, African Americans had higher levels of systemic inflammatory markers, but lower levels of vascular inflammatory markers 15. To address the question to what extent inflammatory markers are affected by aging, we investigated two sets of inflammatory markers, one set representing general, systemic inflammation (CRP, fibrinogen and SAA) and another set representing vascular inflammation (Lp-PLA2 mass, activity and pentraxin-3 [PTX-3]) across the age spectrum in African Americans and Caucasians. Specifically, we hypothesized that the presence of heterogeneity in the relationship of systemic and vascular inflammatory markers with age will aid our understanding of their predictive ability in the assessment of cardiovascular risk.
MATERIALS AND METHODS
Subjects
Subjects were recruited from a patient population scheduled for diagnostic coronary arteriography either at Harlem Hospital Center in New York City or at the Mary Imogene Bassett Hospital in Cooperstown, NY. The study design including exclusion and inclusion criteria has been described previously 16, 17. Briefly, a total of 648 patients, self-identified as Caucasians (n=344), African American (n=232) or Other (n=72) were enrolled. Exclusion criteria for this study included the use of lipid-lowering drugs, as well as hormone replacement therapies. The present report is based on findings in 560 subjects (336 Caucasians, 224 African Americans); 16 subjects were excluded due to incomplete data. The study was approved by the Institutional Review Boards at Harlem Hospital, the Mary Imogene Bassett Hospital, Columbia University College of Physicians and Surgeons, and University of California Davis, and informed consent was obtained from all subjects.
Clinical and Biochemical Assessment
Blood pressure was measured with a random-zero mercury sphygmomanometer. Waist circumference was calculated as the average of 2 measurements taken after inspiration and expiration at the midpoint between the lowest rib and iliac crest. Participants were asked to fast for 12 hours, and blood samples were drawn approximately 2 to 4 hours before the catheterization procedure. Serum and plasma samples were separated and stored at −80°C prior to analysis. Concentrations of total and HDL cholesterol and glucose (Roche, Sommerville, NJ) were determined using standard enzymatic procedures 18, 19. HDL cholesterol levels were measured after precipitation of apoB-containing lipoproteins with dextran sulfate 20. The LDL cholesterol levels were calculated with the formula of Friedewald et al 21. High-sensitivity CRP levels were measured using an enzyme-linked immunoabsorbent assay, standardized according to the World Health Organization First International Reference Standard 22, 23; CV 8.9%. Fibrinogen levels were measured by the clot-rate method of Clauss 24; CV 3.0%. Insulin levels were assessed using a Coat-A-Count RIA kit (DPC Diagnostic Products Co, Los Angeles, CA). Homeostasis model assessment – insulin resistance (HOMA-IR) was calculated using the updated model available from the Oxford Centre for Endocrinology and Diabetes 25. PTX-3 was measured by PTX-3 (human) Detection Set from Alexis Biochemicals (Axxora, LLC); CV 10.2%. Plasma SAA concentrations were measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Invitrogen, Inc., Carlsbad, CA) 15; CV 5.4–10.3%. Lp-PLA2 mass was assayed using a microplate-based enzyme linked immunosorbent assay; CV 4.8–7.2%, and Lp-PLA2 activity was measured with a colorimetric activity method (diaDexus, Inc., South San Francisco, CA) 26–28; CV 6.5–7.8%. All biochemical assessments were made in duplicate.
Statistics
Analysis of data was done with SPSS statistical analysis software (SPSS Inc, Chicago, IL). Results were expressed as means ± SE. Levels of triglycerides, insulin, HOMA-IR, Lp-PLA2 mass and activity and the cardiovascular score were logarithmically transformed to achieve normality for statistical analysis. Proportions were compared between groups using χ2 test or Fisher’s exact test as appropriate. Group means were compared using Student's t-test. Gender-adjusted Pearson’s partial correlation coefficients were calculated for age and inflammatory markers across ethnicities. To construct a composite score of multiple markers, we first calculated a z-score for each inflammatory marker [z = (x – x̄) / SD, where x is an individual marker value, x̄ is the mean marker value, and SD is the standard deviation of marker values]. Using the individual z-scores, we next calculated a composite z-score for systemic inflammation [i.e., z-score (systemic) = Average (z-CRP, z-fibrinogen, z-SAA)], and for vascular inflammation [z-score (vascular) = Average (0.5 z-Lp-PLA2 mass, 0.5 z-Lp-PLA2 activity, z-PTX-3)]. As we used Lp-PLA2 mass and activity to calculate a composite z-score for vascular inflammation, these two parameters were given a coefficient of 0.5 in the formula. Multivariate linear regression analysis was performed to explore the independent association of age with the composite z-scores, adjusted for confounding variables. Two-tailed P-values less than 0.05 were considered statistically significant.
RESULTS
Caucasians were slightly older and more obese as compared to African Americans (Table 1). There was no difference in the gender distribution, while a higher proportion of Caucasian women were postmenopausal. Levels of systemic inflammatory markers were higher in African Americans compared to Caucasians, although the difference for SAA did not reach statistical significance. The opposite pattern was observed for markers of vascular inflammation: Lp-PLA2 mass, activity and PTX-3 levels were significantly lower in African Americans compared to Caucasians. Next we tested the correlations between markers of systemic and vascular inflammation within each ethnic group (Supplementary Table 1). Among systemic inflammatory markers, CRP, SAA and fibrinogen were strongly correlated to each other, while these markers showed only a weak correlation with markers of vascular inflammation. For markers of vascular inflammation, Lp-PLA2 mass showed a moderate correlation with Lp-PLA2 activity, but not with any other inflammatory marker. PTX-3 showed a weak or no correlation with other markers. The association of each marker of systemic and vascular inflammation to the presence of CAD is shown in Supplementary Table 2. For both ethnic groups, fibrinogen levels and Lp-PLA2 activity were associated with the presence of CAD. Additionally, for African Americans, the presence of CAD was associated with higher CRP or SAA levels.
Table 1.
Levels of inflammatory markers across ethnicity.
| Characteristics | Caucasians (n=336) |
African Americans (n=224) |
P |
|---|---|---|---|
| Men/Women | 217/119 | 126/98 | 0.052 |
| Age, yrs | 56.8±0.6 | 54.8±0.6 | 0.025 |
| BMI, kg/m2 | 29.7±0.3 | 28.6±0.4 | 0.043 |
| Systolic blood pressure, mm Hg | 125±1 | 129±2 | 0.058 |
| Diastolic blood pressure, mm Hg | 75±1 | 78±1 | 0.004 |
| Postmenopausal (%) | 92 (79%) | 65 (66%) | 0.032 |
| Inflammatory markers | |||
| CRP, mg/L | 7.0±0.6 | 9.8±1.0 | 0.004 |
| SAA, mg/L | 82±8 | 111±13 | 0.284 |
| Fibrinogen, mg/dL | 328±5 | 383±7 | <0.001 |
| Lp-PLA2 mass, ng/mL | 293±4 | 232±5 | <0.001 |
| Lp-PLA2 activity, nmol/min/mL | 173±2 | 141±3 | <0.001 |
| PTX-3, ng/mL | 2.33±0.15 | 1.87±0.23 | <0.001 |
Data are expressed as mean ± SEM. Data for CRP, SAA, Lp-PLA2 mass, Lp-PLA2 activity and PTX-3 were logarithmically transformed to normalize the distribution of marker values before statistical analyses.
Pearson’s partial correlation coefficients between each inflammatory marker and age across ethnicity adjusted for gender are listed in Table 2. In both ethnic groups, systemic inflammatory markers were significantly and positively associated with age. In Caucasians, among markers of vascular inflammation, Lp-PLA2 mass was positively associated with age while no significant associations with age were seen for Lp-PLA2 activity or PTX-3. We did not observe any associations between markers of vascular inflammation and age among African Americans.
Table 2.
Pearson’s partial (adjusted for gender) correlation coefficients between age and each of inflammatory markers across ethnicity
| Inflammatory markers | Caucasians | African Americans | ||
|---|---|---|---|---|
| r | P | r | P | |
| CRP, mg/L | 0.141 | 0.012 | 0.136 | 0.045 |
| SAA, mg/L | 0.171 | 0.002 | 0.152 | 0.025 |
| Fibrinogen, mg/dL | 0.282 | <0.001 | 0.226 | 0.001 |
| Lp-PLA2 mass, ng/mL | 0.163 | 0.003 | 0.075 | 0.912 |
| Lp-PLA2 activity, nmol/min/mL | 0.016 | 0.773 | 0.043 | 0.521 |
| PTX-3, ng/mL | 0.075 | 0.182 | 0.049 | 0.468 |
Data for CRP, SAA, Lp-PLA2 and PTX-3 were logarithmically transformed to normalize the distribution of marker values before statistical analyses.
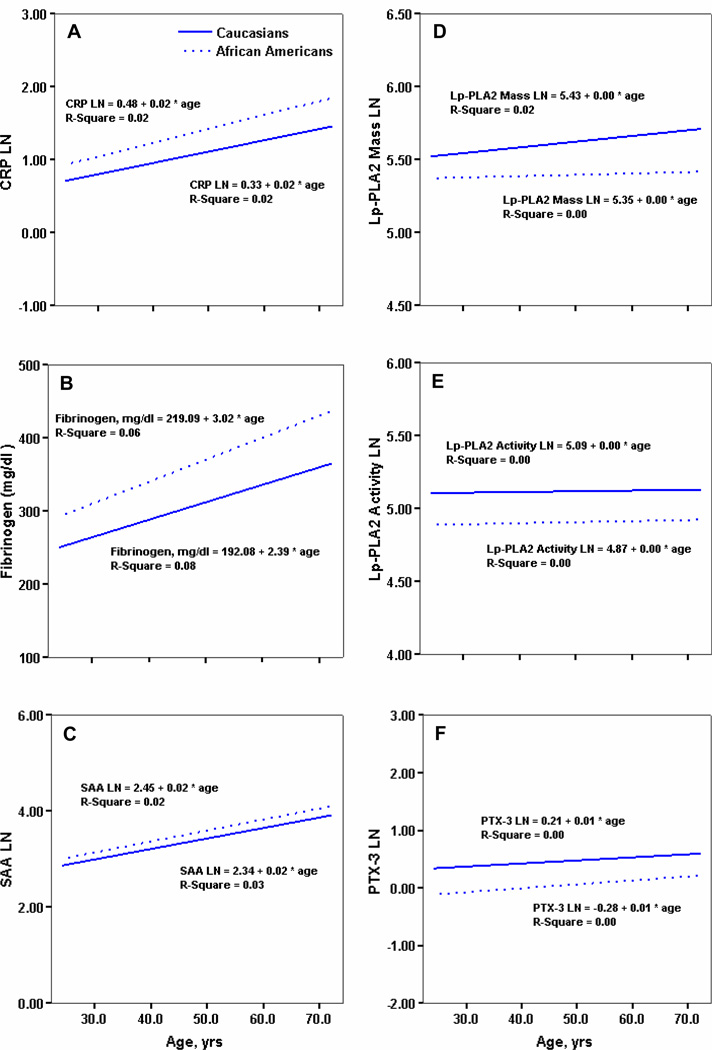
The trend pattern of each individual marker with age is shown in Figure 1. Among markers of systemic inflammation, CRP levels increased significantly (Figure 1A) with age in both ethnic groups (P=0.012 and P=0.047, for African Americans and Caucasians, respectively). Similarly, in both groups, levels of fibrinogen and SAA increased significantly (Figure 1B and C) over age (P<0.001 and P=0.003 for African Americans, and P<0.001 and P=0.001 Caucasians, respectively). In contrast, the trend patterns for vascular inflammatory markers (Lp-PLA2 activity and PTX-3) did not change with age for either ethnic group (Figure 1D and E), except a slight, but significant increase of Lp-PLA2 mass with age among Caucasians (Figure 1F).
Figure 1.
Relation between levels of inflammatory markers (panel A: CRP, B: fibrinogen, C:SAA, D: Lp-PLA2 mass, E: Lp-PLA2 activity, F: PTX-3) and age in Caucasians and African Americans. Straight line represents Caucasians, dotted line represents African Americans.
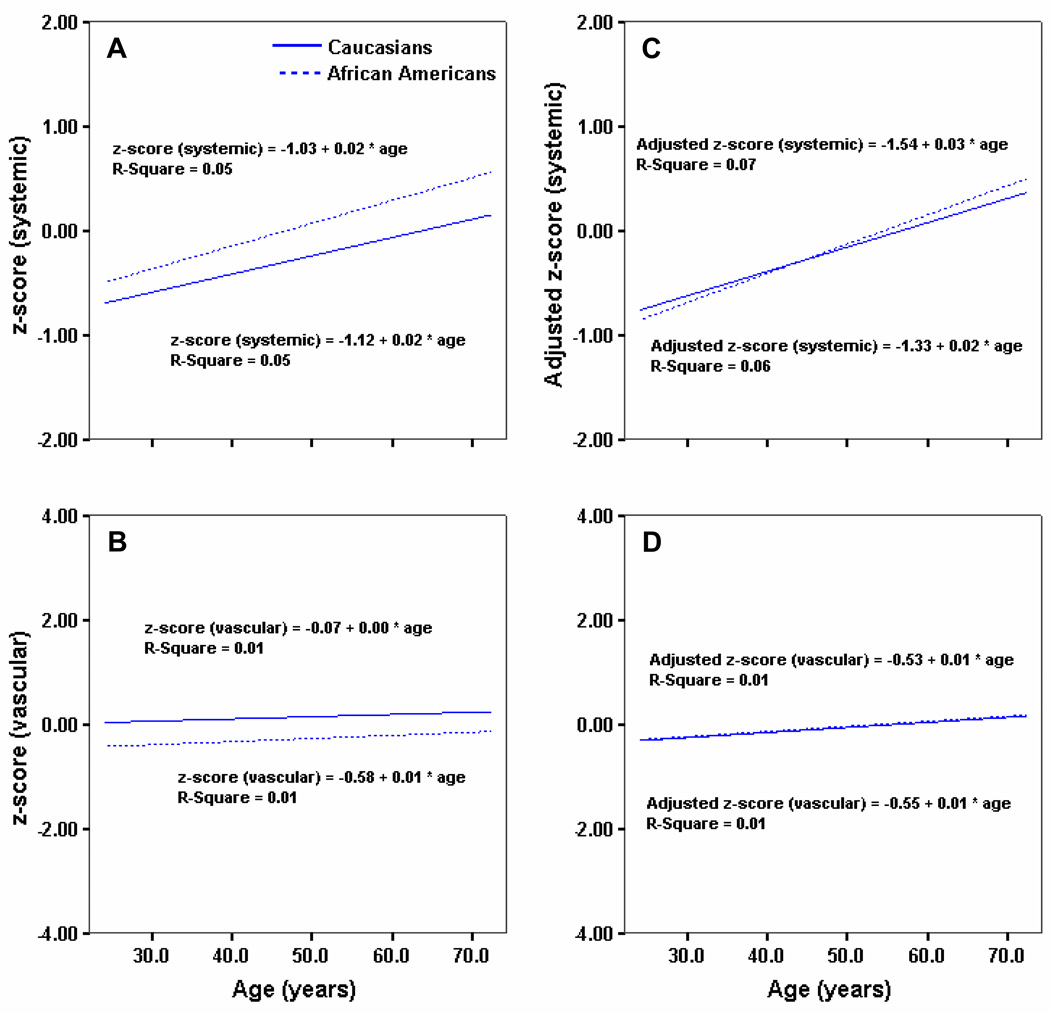
To collectively compare systemic and vascular inflammatory markers, we developed a composite z-score based on the three respective markers in each category, as outlined in Methods. The relationship between age and the unadjusted composite z-score for systemic and vascular inflammation is shown in Figure 2A and 2B, respectively. Reflecting the findings for the individual markers, the composite z-score for systemic inflammation increased significantly with age in both ethnic groups (P=0.001 and P<0.001 for African Americans and Caucasians, respectively), and the score levels were significantly higher in African Americans than in Caucasians across the age spectrum (Figure 2A). In contrast, we did not observe any significant change over age for the composite vascular inflammation z-score for either group (Figure 2B). Further, across age, the composite vascular inflammation z-score was significantly higher in Caucasians compared to African Americans.
Figure 2.
Relation between age and unadjusted composite z-score for systemic (A) and vascular (B) inflammation in Caucasians and African Americans. Relation between age and adjusted (gender, BMI, HOMA-IR, HDL-C, LDL-C and smoking) composite z-score for systemic (C) and vascular (D) inflammation in Caucasians and African Americans. Straight line represents Caucasians, dotted line represents African Americans.
To address the impact of possible confounders on the relation between age and composite z-scores (systemic and vascular), we included the following variables in a multivariate regression model as covariates: gender, obesity (BMI), insulin resistance (HOMA-IR), lipid levels (LDL and HDL cholesterol) and smoking. As seen in Figure 2C and 2D, irrespective of ethnicity, the adjusted z-score for systemic inflammation increased significantly with age. No apparent increase was seen for the vascular inflammation z-score. Further, even after adjusting for the confounders, the trajectories of trend were not significantly different for the two ethnic groups (Figure 2C and 2D). In support of the trend pattern data, age remained independently associated with the composite z-score for systemic inflammation in both ethnic groups in multivariate linear regression models (Table 3, model 1). Age, gender, BMI, HOMA-IR, LDL cholesterol, HDL cholesterol, and smoking all together explained about 12% of the variability of the composite z-score for systemic inflammation in Caucasians (Table 3, model 1). For African Americans, age, gender, BMI, HOMA-IR, LDL cholesterol, HDL cholesterol and smoking explained about 18% of the corresponding variability (Table 3, model 1). Again, we did not observe any significant association of age with the composite z-score for vascular inflammation in either ethnic group (Table 3, model 2). In Caucasians, of the factors included in the model, only LDL cholesterol was significantly associated with the composite z-score for vascular inflammation (Table 3, model 2). In African Americans, none of the variables considered in the model significantly related to the composite vascular inflammation z-score.
Table 3.
Multivariate regression analysis of the composite z-scores for systemic and vascular inflammation with confounding variables as covariates in Caucasians and African Americans.
| Model | Independent | ||||
|---|---|---|---|---|---|
| Ethnicity | Variables | R2 | F | β | P |
| Model 1 (systemic inflammation) | |||||
| Caucasians | 0.124 | 5.8 | <0.001 | ||
| Age, years | 0.250 | <0.001 | |||
| Gender, M/F | 0.121 | 0.039 | |||
| BMI, kg/m2 | 0.178 | 0.002 | |||
| HOMA-IR | 0.097 | 0.100 | |||
| LDL-C, mg/dL | −0.030 | 0.610 | |||
| HDL-C, mg/dL | 0.033 | 0.575 | |||
| Smoking, Y/N | 0.144 | 0.014 | |||
| African Americans | 0.177 | 6.7 | <0.001 | ||
| Age, years | 0.276 | <0.001 | |||
| Gender, M/F | −0.029 | 0.670 | |||
| BMI, kg/m2 | 0.214 | 0.001 | |||
| HOMA-IR | −0.009 | 0.902 | |||
| LDL-C, mg/dl | 0.171 | 0.011 | |||
| HDL-C, mg/dl | −0.168 | 0.012 | |||
| Smoking, Y/N | 0.146 | 0.030 | |||
| Model 2 (vascular inflammation) | |||||
| Caucasians | 0.062 | 2.7 | 0.010 | ||
| Age, years | 0.104 | 0.078 | |||
| Gender, M/F | −0.063 | 0.286 | |||
| BMI, kg/m2 | 0.004 | 0.945 | |||
| HOMA-IR | 0.090 | 0.127 | |||
| LDL-C, mg/dL | 0.175 | 0.003 | |||
| HDL-C, mg/dL | −0.064 | 0.284 | |||
| Smoking, Y/N | −0.013 | 0.825 | |||
| African Americans | 0.031 | 0.9 | 0.450 | ||
| Age, years | 0.096 | 0.158 | |||
| Gender, M/F | −0.044 | 0.520 | |||
| BMI, kg/m2 | −0.015 | 0.827 | |||
| HOMA-IR | 0.081 | 0.231 | |||
| LDL-C, mg/dL | 0.084 | 0.217 | |||
| HDL-C, mg/dL | −0.046 | 0.499 | |||
| Smoking, Y/N | 0.000 | 0.996 | |||
DISCUSSION
Age is an established cardiovascular risk factor and has quantitatively a strong impact on the Framingham risk score 29. While the overall effect of age as a classic risk factor is well recognized, contribution of age to increasing cardiovascular risk remains unclear yet. Risk associated with age could possibly reflect an accumulating burden of a number of contributing factors- thereby it is tempting to suggest that inflammation may contribute to this burden. In the present study we addressed the issue to what extent inflammatory markers are associated with age taking other established risk factors into account. Further, we attempted to assess differences between markers representing a more general, systemic inflammation versus a vascular-focused inflammatory process. The main novel finding in our study was a constant increase of the systemic, but not the vascular, inflammatory burden over age and that this pattern was seen irrespective of ethnic background. Our findings underscore the importance of age in elevating inflammatory burden when assessing cardiovascular risk.
Although the notion that elevated inflammatory markers increase the risk of CVD has been increasingly recognized 30, underlying mechanisms and pathways remain to be elucidated. Notably, high levels of inflammatory markers are known to be associated with many factors contributing to an increased CVD risk such as smoking, visceral obesity, sedentary life style and excessive alcohol consumption, suggesting a considerable degree of complexity. Further, an increase of inflammatory cytokines can potentially contribute to the progression of many chronic and degenerative diseases, such as atherosclerosis, cancer, obesity, diabetes, and congestive heart failure. Thus, presence of these risk factors and CVD itself may in turn further stimulate the inflammatory process, resulting in a vicious cycle. In support of this, Ferrucci et al have demonstrated that increases in inflammatory markers with age are due, at least partly, to a progressive increase in the burden of cardiovascular risk factor and morbidity 31. On the other hand, inflammation is an integral and necessary part of the response to pathogens and constitutes an important part of the immune defense system. In view of these complex relationships, it is important to characterize the role of individual markers of inflammation in predicting risk of disease development.
Many prospective studies demonstrate a positive association between risk for CVD and markers of systemic inflammation, i.e., acute phase reactants such as CRP and fibrinogen 2–5. Further, recently more specific vascular inflammatory markers, such as Lp-PLA2 and PTX-3, have been shown to be associated with CVD 6–8. However, the correlation between Lp-PLA2 and CRP is weak, suggesting that markers of systemic and vascular inflammation may reflect different underlying mechanisms. We have previously studied the associations of CRP, fibrinogen and Lp-PLA2 with CAD risk across African Americans and Caucasians 32–34, and our findings indicated an independent impact of vascular inflammation as contributory to CAD risk, suggesting that different sets of markers could provide complementary information as risk predictors.
In assessing the relation of systemic and vascular inflammatory biomarkers with age, we observed that all markers of systemic inflammation increased significantly over age, both for African Americans and Caucasians. In contrast, we did not observe any change in trend patterns of vascular inflammatory markers with age in either ethnic group. Further, in univariate analysis, we noted ethnic differences in the relationship between age and some markers of systemic and vascular inflammation. To collectively test the overall effect and distribution of systemic and vascular inflammation over age, we constructed composite scores by combining z-scores of the three respective markers in each category. In agreement with the findings for the separate markers, we observed a significant increase of the composite z-score for systemic, but not vascular inflammation with age in both ethnic groups. Age still remained independently associated with the composite z-score for systemic, but not vascular inflammation in both ethnic groups, even after adjusting for confounding variables that potentially could affect the relationship between levels of inflammatory markers and age. Interestingly, the differences between African Americans and Caucasians disappeared after adjustment for the confounders, suggesting that differences seen in the univariate analyses were likely due to differences in confounding factors between the ethnic groups rather than an inherent ethnic difference. Our approach of using composite z-scores has several advantages: a) it enables a comparison of individual inflammatory markers within each category at the same scale, b) it provides a standardized way to assess the relative contribution of individual inflammatory markers to age, and c) makes it possible to collectively assess effects of systemic and vascular risk factors as a group over age. Further, use of the score system allowed us to document that an increase of systemic inflammation with age persisted even after adjusting for confounding factors. Notably, the confounding factors taken into account, together with age, explained only a minor fraction of the overall variability in systemic inflammatory scores (12% for Caucasians and 18% for African Americans), suggesting that other factors beyond traditional cardiovascular risk factors, for example, environmental or genetic factors or both, may play a larger role. Overall, our findings suggest that systemic inflammatory markers may reflect overall age-related inflammatory burden to a greater extent than vascular markers. One implication of this finding is that vascular inflammation may reflect more specific to a localized atherogenic process but less to a more widespread inflammatory process.
We acknowledge some of the limitations of this study. In view of the complexity of the inflammatory process and the considerable arsenal of markers available, it is well recognized that a classification of systemic vs. vascular inflammatory markers likely represent a relatively narrow and perhaps somewhat simplistic view. However, we nevertheless adopted this approach as an initial attempt to address the relationship of individual inflammatory markers with other cardiovascular risk factors. Further, the cross-sectional study design does not allow us to evaluate the causative and longitudinal effect of age and other factors that might influence on levels of inflammatory markers. Subjects in our study were recruited from patients scheduled for coronary angiography and are likely more typical of a high-risk patient group than the general population at large. However, utility of high-risk population is particularly valuable in assessing inflammatory factors that might be enriched in this population and ultimately their effects on risk of cardiovascular disease. We did study two race/ethnicity groups but expanded studies are needed to investigate other ethnic background groups. Although our findings support the notion that systemic inflammatory burden measured as increased levels of inflammatory markers is persistently increased by age, additional studies are warranted to verify these results in more general populations as well as in prospective studies.
In conclusion, the findings suggest an increase in the systemic, but not vascular inflammatory burden over age. This observation has several potential implications: 1) use of systemic inflammatory markers in assessment of cardiovascular risk may be more influenced by age-related processes than is the case for vascular markers; 2) we did not observe any differences in levels of inflammatory markers over age between the two ethnic groups when adjusting for confounding variables, including traditional risk factors; 3) age is an independent predictor of systemic inflammation, even after adjustment for other risk factors, and traditional cardiovascular risk factors may only explain a small fraction of the variability of inflammatory markers. Our results underscore the important influence of age on inflammatory markers in identification of a high cardiovascular risk phenotype across ethnicity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank diaDexus, Inc. for assistance with Lp-PLA2 measurements. We thank Dr. Laurel Beckett, Division of Biostatistics, Department of Public Health Sciences, UC Davis for advice in statistical analysis.
SOURCES OF FUNDING
This research project was supported by grant HL 62705 (PI: L Berglund) from the National Heart, Lung, and Blood Institute and by the UC Davis Clinical and Translational Research Center (RR024146). Dr Anuurad is a recipient of an UC Davis Clinical and Translational Science Center K12 Award (RR024144).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Castelli WP, D'Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–1186. [PubMed] [Google Scholar]
- 5.Cantin B, Despres JP, Lamarche B, Moorjani S, Lupien PJ, Bogaty P, Bergeron J, Dagenais GR. Association of fibrinogen and lipoprotein(a) as a coronary heart disease risk factor in men (The Quebec Cardiovascular Study) Am J Cardiol. 2002;89:662–666. doi: 10.1016/s0002-9149(01)02336-0. [DOI] [PubMed] [Google Scholar]
- 6.Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol. 2003;14:347–352. doi: 10.1097/00041433-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 8.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 9.Santos S, Rooke TW, Bailey KR, McConnell JP, Kullo IJ. Relation of markers of inflammation (C-reactive protein, white blood cell count, and lipoprotein-associated phospholipase A2) to the ankle-brachial index. Vasc Med. 2004;9:171–176. doi: 10.1191/1358863x04vm543oa. [DOI] [PubMed] [Google Scholar]
- 10.Miller MA, Cappuccio FP. Ethnicity and inflammatory pathways - implications for vascular disease, vascular risk and therapeutic intervention. Curr Med Chem. 2007;14:1409–1425. doi: 10.2174/092986707780831131. [DOI] [PubMed] [Google Scholar]
- 11.Beckett N, Nunes M, Bulpitt C. Is it advantageous to lower cholesterol in the elderly hypertensive? Cardiovasc Drugs Ther. 2000;14:397–405. doi: 10.1023/a:1007812232328. [DOI] [PubMed] [Google Scholar]
- 12.Ahluwalia N, Mastro AM, Ball R, Miles MP, Rajendra R, Handte G. Cytokine production by stimulated mononuclear cells did not change with aging in apparently healthy, well-nourished women. Mech Ageing Dev. 2001;122:1269–1279. doi: 10.1016/s0047-6374(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 13.Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25:224–230. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- 14.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 15.Enkhmaa B, Anuurad E, Ozturk Z, Zhang W, Pearson T, Berglund L. Differential associations of serum amyloid A and pentraxin-3 with allele-specific lipoprotein(a) levels in African Americans and Caucasians. Transl Res. 2011:1–7. doi: 10.1016/j.trsl.2011.01.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, Berglund L. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–2624. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 17.Anuurad E, Rubin J, Lu G, Pearson TA, Holleran S, Ramakrishnan R, Berglund L. Protective effect of apolipoprotein E2 on coronary artery disease in African Americans is mediated through lipoprotein cholesterol. J Lipid Res. 2006;47:2475–2481. doi: 10.1194/jlr.M600288-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538–542. [PubMed] [Google Scholar]
- 19.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 20.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 23.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 24.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 25.Adler AI, Levy JC, Matthews DR, Stratton IM, Hines G, Holman RR. Insulin sensitivity at diagnosis of Type 2 diabetes is not associated with subsequent cardiovascular disease (UKPDS 67) Diabet Med. 2005;22:306–311. doi: 10.1111/j.1464-5491.2004.01418.x. [DOI] [PubMed] [Google Scholar]
- 26.Dada N, Kim NW, Wolfert RL. Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn. 2002;2:17–22. doi: 10.1586/14737159.2.1.17. [DOI] [PubMed] [Google Scholar]
- 27.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586–1593. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 28.Kosaka T, Yamaguchi M, Soda Y, Kishimoto T, Tago A, Toyosato M, Mizuno K. Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin Chim Acta. 2000;296:151–161. doi: 10.1016/s0009-8981(00)00216-3. [DOI] [PubMed] [Google Scholar]
- 29.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 30.Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 31.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anuurad E, Ozturk Z, Enkhmaa B, Pearson TA, Berglund L. Association of lipoprotein-associated phospholipase A2 with coronary artery disease in African-Americans and Caucasians. J Clin Endocrinol Metab. 2010;95:2376–2383. doi: 10.1210/jc.2009-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anuurad E, Tracy RP, Pearson TA, Kim K, Berglund L. Synergistic role of inflammation and insulin resistance as coronary artery disease risk factors in African Americans and Caucasians. Atherosclerosis. 2009;205:290–295. doi: 10.1016/j.atherosclerosis.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anuurad E, Tracy RP, Pearson TA, Beckett L, Berglund L. Comparison of C-reactive protein and metabolic syndrome as cardiovascular risk factors in African-Americans and European-Americans. Am J Cardiol. 2009;103:523–527. doi: 10.1016/j.amjcard.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.