Abstract
Background and Aims
Metabolic syndrome (MetS) is a complex condition characterized by different phenotypes, according to combinations of risk factors and is associated with cardiovascular abnormalities. Whether control of MetS components by treatment produces improvement in the associated cardiovascular abnormalities is unknown. We investigated whether partial control of components of MetS was associated with less echocardiographic abnormalities than the complete presentation of MetS based on measured components.
Methods and Results
We evaluated markers of echocardiographic preclinical cardiovascular disease in MetS (ATPIII) defined by measured components or by history of treatment, in 1,421 African- American and 1,195 Caucasian non-diabetic HyperGEN participants, without prevalent cardiovascular disease or serum creatinine>2 mg/dL. Of 2,616 subjects, 512 subjects had MetS by measured components and 328 by history. Hypertension was found in 16% of participants without MetS, 6% of those with MetS by history and 42% of those with MetS by measured components. Obesity and central fat distribution had similar prevalence in both MetS groups (both p<0.0001 vs No-MetS). Blood pressure was similar in MetS by history and No-MetS, and lower than in MetS by measured components (p<0.0001). LV mass and midwall shortening, left atrial (LA) dimension and LA systolic force were similarly abnormal in both MetS groups (all p<0.0001 vs. No-MetS) without difference between them.
Conclusions
There is little impact of control by treatment of single components of MetS (namely hypertension) on echocardiographic abnormalities. Lower blood pressure in participants with MetS by history was not associated with substantially reduced alterations in cardiac geometry and function.
INTRODUCTION
The metabolic syndrome (MetS) is a complex condition that can be characterized by different phenotypes, according to different clustering of risk factors characterizing the syndrome. MetS may increase CV risk beyond what might be predicted by the additive effect of single components (1). Part of the MetS-related high CV risk is due to the frequent coexistence of what is called “preclinical CV disease” (2), an effect especially evident in the absence of diabetes. In epidemiological studies, MetS may be detected by documented abnormality of metabolic and/or hemodynamic components or by ongoing treatment for them. For instance, therapy for arterial hypertension means that high blood pressure is considered present for diagnosis of MetS, independently of the level of blood pressure (BP) at the time of evaluation. This approach, however, blunts understanding of whether control of components of MetS by treatment is associated with less preclinical CV disease in individuals who meet criteria for the MetS. Thus, it is unknown whether therapy-modified single components of the MetS have the same impact on the CV system as the presentation of the full spectrum of MetS components.
Accordingly, this cross-sectional study was designed to understand whether partial correction of some components of the MetS is associated with reduced echocardiographic abnormalities, compared to the full presentation requiring the presence of all altered components. To achieve this goal, we analyzed the distribution of components of the MetS based on either history of MetS components or direct evidence of abnormalities, to assess whether, and to what extent, therapeutic control of one or more components contributing to MetS diagnosis is associated with less abnormal CV phenotype, assessed with echocardiography (3).
METHODS
Study sample
The HyperGEN Study is part of the NHLBI Family Blood Pressure Program, designed to assess the genetic basis of hypertension in population-based samples (4). The HyperGEN study is a cross-sectional survey based on a sib-pair design that recruited persons with onset of hypertension before age 60, and at least one additional hypertensive sibling who could be enrolled in the study. Unmedicated adult offspring of hypertensive siblings and a random sample of age-matched subjects from the same source population were also recruited, including normotensive controls. Further details about recruitments and characteristics have been previously reported (4).
For the purpose of this study, we excluded participants with diabetes or prevalent CV disease (including coronary heart disease, history of stroke and heart failure) or more than mild renal failure, all conditions that could primarily alter cardiac morphology and function (5). Therefore this analysis included 1,421 African-American (886 women) and 1,195 Caucasian (623 women) non-diabetic (i.e. fasting blood glucose <126 mg/dl and no anti-diabetic therapy) HyperGEN participants, without prevalent CV disease or moderate-to-severe renal failure (i.e. serum creatinine>2 mg/dL). 6. IRB approval was obtained and written informed consent collected from all participants.
Definitions
MetS was defined according to the ATPIII criteria (6), using direct measurements of the MetS components at the time of survey, without considering whether or not participants were taking medications. This criterion was labeled as “Direct Diagnosis” (DD). Participant who met criteria for diagnosis of MetS by DD were compared with a second group of participants who had the three components allowing the diagnosis of MetS, but in whom at least one of these MetS components was normalized by the therapy; these participants, therefore, could not be included in the DD group because one or more treated components of the MetS were normal at the time of the survey. This criterion was labeled as “Historical Diagnosis” (HD). Participants without either DD or HD MetS were labeled as “No-MetS”.
Obesity and central fat distribution (by waist girth) were categorized according to the NIH guidelines (7): measured waist girth>88 cm in women or >102 cm in men constituted the obesity criterion of MetS for both DD and HD groups (table 1), without reference to historical information. Similarly, because diabetic participants were excluded, and no therapy was given for impaired fasting glucose, this variable was similarly included in the definition of MetS in both DD and HD groups. The other components of MetS could differ in the two groups (table 1). Thus two components of the syndrome could be modified by management: high blood pressure and dyslipidemia.
Table 1.
Definition of metabolic syndrome according NCEP-ATPIII (7), by direct assessment of abnormality or ongoing therapy.
| Factor | ATPIII components in DD | ATPIII components in HD |
|---|---|---|
| Abdominal obesity | >102/88 cm waist (M/W) | >102/88 cm waist (M/W) |
| Triglycerides | ≥150 mg/dL | ≥150 mg/dL or lipid lowering therapy |
| HDL-cholesterol | <40/50 mg/dL (M/W) | <40/50 mg/dL (M/W) or lipid lowering therapy |
| Blood pressure | ≥130/≥85 mmHg | ≥130/>85 mmHg or antihypertensive therapy |
| Fasting glucose | ≥110 mg/dL | ≥110 mg/dL |
DD=diagnosis of MetS based on direct measurements of the components.
HD=diagnosis of MetS based on historical exposition, with one or more treated components of the MetS normal at the time of survey.
HOMA index was also calculated as an estimate of insulin resistance (HOMAr), using fasting glucose and insulin levels (8).
Echocardiographic Methods
Imaging and Doppler echocardiograms were obtained using standardized acquisition methods employed in multiple studies and evaluated in the Echocardiography Reading Center at Weill-Cornell Medical Center (9,10), and measured according to the recommendations of the American Society of Echocardiography (11,12).
According to wide clinical and epidemiological evidence, we used three anatomical and three functional indicators of echocardiographic preclinical CV disease (3):
LV mass by an anatomically validated formula [r=0.90 vs. necropsy LV mass (13)], and normalized for height in m2.7, which has been shown to maximize population risk attributable to LV hypertrophy in populations with high prevalence of obesity (14). Values of > 49.2 g/m2.7 for men and > 46.7 g/m2.7 for women were used to identify LV hypertrophy. LV hypertrophy is the most potent predictor of CV events after age (15). LV diastolic diameter was normalized for the linear measure of height.
Relative wall thickness, calculated as posterior wall thickness/LV internal radius, as a measure of LV geometry. LV concentric geometry was identified when relative wall thickness was ≥ 0.43. LV concentric geometry has been shown to predict CV risk independently of LV mass index (16).
Left atrial (LA) systolic dimension, measured from the parasternal long-axis view and normalized for the linear measure of height. Left atrial dimension is another powerful and independent marker of adverse outcome (17,18).
Ejection fraction, measured from Doppler-assessed transaortic stroke volume (19) normalized to LV end-diastolic volume, calculated from linear measurements according to a validated method (20). Ejection fraction is the most commonly used measure of LV systolic function at the chamber level.
Midwall shortening, measured from linear measures at the midwall level, as previously reported (21). Midwall shortening is a measure of LV myocardial function, independent of LV geometry and less sensitive to modification of myocardial afterload than ejection fraction (21). Midwall shortening is more associated with CV events than measures of LV chamber function in patients with initially uncomplicated hypertension (22).
LA systolic force, a measure of LA function, assessed at the mitral annulus level, as previously reported (23). LA systolic force has been shown to be associated with CV events, independently of LV hypertrophy (24).
Statistical Analysis
Data were analyzed using SPSS 12.0 software (SPSS, Chicago, IL). Mean ± one standard deviation are shown for continuous variables. The Kolmogorov-Smirnov statistic, with a Lilliefors significance level were used to test for normal distribution. Continuous variables with non-normal distribution were log transformed for parametric statistics. Differences among the three groups of participants were assessed using ANOVA and the REGW F post-hoc test. To assess dependence among categories a χ2 distribution test was used. Spearman correlation was used to explore trends among the groups defined by MetS and the control group without MetS.
Anatomic and functional measures were compared among the three groups, using ANCOVA, adjusting for age, sex and ethnicity. Specific differences between the HD group and the other groups were also examined after Sidak’s adjustment for multiple comparisons.
The null hypothesis was rejected at two-tailed p≤0.05.
RESULTS
Characteristics of the study population
A total of 1 776 participants (978 women or 55%) were free of MetS (No-MetS). MetS was identified in 512 subjects (295 women or 58%) by the DD criterion, and in 328 additional individuals (235 women or 72%) by the HD criterion (p<0.0001 for sex distribution vs. the other groups).
In the HD group, MetS prevalence was 13% in Caucasians, 12% in African Americans, and 25% and 15% respectively in the DD group. Anti-hypertensive therapy was given to 636 of 1 776 No-MetS (or 36%), 344 DD (67%) and 277 HD participants (or 84%; p<0.0001). Lipid-lowering therapy was used in 2% of No-MetS, 10% of DD and in 27% of HD (p<0.0001), paralleling the same distribution as anti-hypertensive treatment.
Table 2 shows the frequency of single components of MetS in the three groups of participants. The frequency of both high BP values (by ATP III definition) and high triglycerides levels in the DD group was nearly twice and the prevalence of high fasting glucose more than 3-fold greater than in the HD group (table 2, all p<0.0001).
Table 2.
Single components of ATPIII MetS on the basis of diagnostic criteria
| No- MetS | DD | HD | p< | ||
|---|---|---|---|---|---|
| MetS vs No-Mets | DD vs HD | ||||
| High BP | 27% | 61% | 34% | 0.0001 | 0.0001 |
| Central fat | 42% | 89% | 88% | 0.0001 | ns |
| High glucose | 1% | 36% | 10% | 0.0001 | 0.001 |
| High tryglycerides | 9% | 75% | 34% | 0.0001 | 0.0001 |
| Low HDL-cholesterol | 24% | 81% | 75% | 0.0001 | 0.05 |
DD=diagnosis of MetS based on direct measurements of the components.
HD=diagnosis of MetS based on historical exposition, with one or more treated components of the MetS normal at the time of survey.
At the HyperGEN clinic exam, BP was in the hypertensive range (i.e. ≥ 140 mmHg systolic or 90 mmHg diastolic) in 34% of the No-MetS group, 51% of the DD group (p<0.0001 vs No-MetS) and 7% of the HD group (p<0.0001 vs. other groups, Figure 1).
Figure 1.
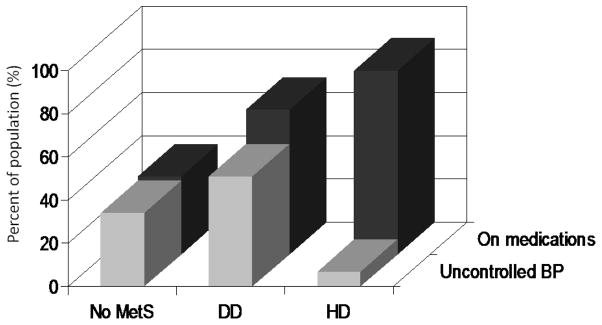
Frequency of prescriptions of anti-hypertensive medications (dark grey columns) and prevalence of uncontrolled blood pressure (BP) among treated participants without MetS (No-MetS), in those classified on the basis of direct measurements of components (DD) and in those classified on the basis of history of exposure (HD).
Distribution of classes of anti-hypertensive meds among hypertensive participants (diuretics, ACE-inhibitors, AT1 receptor blockers, Ca++ channel blockers, β-blockers, others), was similar in the three groups, except for β-blockers that were more often prescribed in hypertensive HD (p<0.0001).
In contrast to BP distribution and hypertension prevalence, central fat distribution exhibited similar higher prevalence in both MetS groups than in the No-MetS group. Obesity by BMI classification, was also more prevalent in DD (71%) and in HD (65%, p<0.05 vs. DD) than in No-MetS (33%, both p<0.0001).
Table 3 shows that BMI and waist circumferences were similarly greater in both the MetS groups than in the No-Mets, whereas blood pressure was higher only in the DD group, with the HD group exhibiting values very similar to the No-MetS group. Reported duration of hypertension and antihypertensive treatment was significantly longer in DD than in both HD and No-Mets (table 3, both p<0.0001). Plasma glucose, insulin, HOMAr, total cholesterol and triglycerides were also higher and HDL-cholesterol lower in both MetS groups, but DD exhibited more severe alterations than the HD group. LDL-cholesterol and heart rate were similarly higher in both MetS groups than in the No-Mets, without differences between them. Of note, whereas the natural log of circulating insulin was 25% higher in HD than in NoMetS, the difference between HD and DD was only 9%. Similar results were obtained with HOMAr.
Table 3.
General characteristics of the study population
| No-MetS (n=1775 ) | HD (n=328) | DD (n=512) | |
|---|---|---|---|
| Age (yrs) | 42.7±13.5 | 51.6±11.2* | 50.6±12.3* |
| BMI (kg/m2) | 28.6±6.4 | 33.1±6.7* | 33.9±6.9* |
| Waist (cm) | 95±16 | 108±14* | 110±14* |
| Diastolic BP (mmHg) | 71.7±11.2 | 70.7±8.9† | 75.8±11*.4 |
| Systolic BP (mmHg) | 121.0±19.4 | 122.4±14.4† | 135.6±20.8* |
| Duration of hypertension (yrs) | 13.7±10.1 | 13.0±9.8† | 16.0±11.2* |
| Duration of antihypertensive therapy (yrs) | 12.5±9.6 | 11.4±8.8† | 14.5±10.1* |
| Heart rate (bpm) | 66.0±10.6 | 68.7±11.8* | 69.9±11.4* |
| Creatinine (mg/dL) | 0.96±0.20 | 0.97±0.21 | 0.97±0.20 |
| Cholesterol (mg/dL) | 187.6±37.9 | 199.5±40.8*† | 207.0±38.5* |
| HDL-cholesterol (mg/dL) | 54.9±15.2 | 48.2±12.0*† | 41.7±10.9* |
| LDL-cholesterol (mg/dL) | 113.7±34.3 | 123.7±34.0* | 126.5±35.2* |
| Triglycerides (mg/dL) | 94.8±51.6 | 139.3±66.9*† | 203.3±102.9* |
| Fasting glucose (mg/dL) | 90.7±9.2 | 96.5±12.9*† | 108.0±11.8* |
| Fasting insulin (μUI/L) | 7.1±5.4 | 10.8±9.1*† | 13.0±11.8* |
| HOMAr index | 1.6±1.3 | 2.6±2.4*† | 3.6±4.0* |
p<0.05 vs. No MetS;
p<0.05 vs DD.
DD=diagnosis of MetS based on direct measurements of the components.
HD=diagnosis of MetS based on historical exposition, with one or more treated components of the MetS normal at the time of survey.
Echocardiographic markers of preclinical CV disease
After controlling for age, sex and race, LV dimension and mass, relative wall thickness and LA dimension were similarly greater in both HD and DD than in No-Mets. There was no difference in these values between the two MetS groups (Table 4). The prevalence of LV hypertrophy was also similarly higher in the two MetS group than in the No-MetS.
Table 4.
Markers of preclinical cardiovascular disease in different diagnosis of MetS. Comparison is adjusted for age, sex, and race.
| No-MetS (n=1775 ) | HD (n=328) | DD (n=512) | |
|---|---|---|---|
| LV mass index (g/m2.7) | 36.8±10.6 | 41.3±9.8* | 41.9±10.5* |
| Prevalence of LVH (%) | 13% | 22%* | 24%* |
| Relative wall thickness | 0.32±0.05 | 0.34±0.05* | 0.34±0.06* |
| LV dimension index (cm/m) | 3.01±0.25 | 3.06±0.26* | 3.07±0.27* |
| LA dimension index (cm/m) | 1.96±0.29 | 2.08±0.29* | 2.10±0.27* |
| Ejection fraction (%) | 64.3±11.2 | 65.2±12.7 | 65.3±11.3 |
| Midwall shortening (%) | 18.0±2.0 | 17.6±2.0* | 17.6±2.0* |
| LA systolic force (kdynes) | 9.2±4.4 | 12.4±4.6* | 13.1±5.9* |
p<0.05 vs. No MetS;
p<0.05 vs. DD.
DD=diagnosis of MetS based on direct measurements of the components.
HD=diagnosis of MetS based on historical exposition, with one or more treated components of the MetS normal at the time of survey.
Ejection fraction was similar in the three groups. Midwall shortening was similarly slightly lower and LA systolic force more than 30% greater in both MetS groups than in no-MetS, without appreciable difference between HD and DD.
These results did not change when duration of antihypertensive therapy was added as a covariate to the ANCOVA model.
Standardized residuals of the ANCOVA of LV mass index were linearly correlated with the natural log of both fasting insulin and HOMAr (both r=0.11, p<0.0001).
DISCUSSION
This cross-sectional study suggests that MetS may not be a dynamic condition, subjected to improvement of CV condition if individual components (high blood pressure and dyslipidemia) are controlled by treatment without intervention on the other components (central obesity / impaired fasting glucose-insulin resistance). Whether or not MetS was diagnosed on the basis of measured abnormalities or also using clinical history in individuals on treatment for hypertension or dyslipidemia, the association of MetS with the echocardiographic markers of CV risk used in this study did not change substantially.
The only significant difference found between DD and HD was in the level of BP, which was substantially lower when diagnosis was made on the basis of clinical history, reflecting the widespread use of antihypertensive treatment and the relatively better control of this risk factor compared to the other components of MetS. These findings indicates that inclusion by history of treatment rather then, or in addition to, abnormal lab values is justified in trials recruiting patients with MetS.
Based on the differences found between the two different diagnostic criteria for MetS, our data indicate that even partial recovery of the syndrome, with control of some of the risk factors, may not be effective in reducing target organ damage and, as a possible consequence, CV risk. This finding is even more impressive, considering that, among the components of MetS, BP was relatively controlled in the HD group. Despite this relatively good control, HD participants exhibited higher LV mass and LA dimension, more pronounced concentric LV geometry and significant abnormality of LV and LA function, similar to the alterations found in DD group in which high BP was substantially more prevalent. This deviation from normality of markers of preclinical CV disease in HD could not be, therefore, attributable to uncontrolled hypertension.
Alternative candidates to explain the persistently abnormal values of these markers despite relative BP control, are central obesity and/or insulin resistance. We have previously shown in the Strong Heart Study that the prevalence of LV hypertrophy in participants with normal BP but MetS by ATP III criteria is similar to prevalence in participants with hypertension but without MetS (25). In the HyperGEN study (5), the probability of clear-cut LV hypertrophy in obese normotensive participants with abnormal lipid profile and diabetes was similar to the probability of LV hypertrophy in hypertensive subjects without metabolic abnormalities, but this could be due to the impact of diabetes on LV geometry (26). This is not the case in the present analysis because diabetic participants were excluded. Prevalence of the abnormal cardiovascular phenotype in MetS participants despite partial correction of the syndrome could not be attributed to abnormal BP, diabetes or less anti-hypertensive or lipid-lowering therapy, which were, in contrast, given more frequently. BMI and central fat distribution were the only components of MetS that had near-identical value in both MetS groups. Insulin resistance and hyperinsulinemia could also play a role in the association between abdominal adiposity and markers of preclinical cardiovascular disease, as previously reported (27), and suggested by the correlation between standardized residuals from ANCOVA and both circulating insulin and HOMA index. It is also relevant that, whereas circulating insulin and insulin resistance were substantially higher in HD than in NoMetS, the differences between HD and DD were negligible, indicating that insulin resistance was a characteristics also in HD.
Because this is a cross-sectional observational study, it is possible that participants in the HD group had been exposed to the clusters of risk factors for longer time, or initially had more severe abnormality, a potential stimulus to starting treatment more frequently than in those in the DD group. If this bias was encountered, it is also possible that their target organ damage was more severe than in the group less aggressively treated. In this case, a cross-sectional analysis would not be able to detect improvement in markers of preclinical CV disease that were even more abnormal prior to therapy. Other potential confounders could be elements of life style that were not considered in this analysis, such as diet (salt, vegetable and fat intake) and physical activity. However, persistence of abnormal values of these markers despite good control of BP is consistent with recent longitudinal data from the LIFE study (28), demonstrating that, for comparable effects on BP, therapy-induced regression of both ECG and echocardiographic LV hypertrophy is blunted in participants with MetS.
There is still debate on whether MetS increases CV risk beyond what might be predicted by single components. The evidence is increasing that MetS is a condition that requires medical attention (1,2,29–35) and should yield prompt and appropriate therapy to reduce risk burden (32,35). Similar to arterial hypertension, MetS is also associated with target organ damage (2,31,33,36–38) in hypertensive and non-hypertensive subjects (25,39). And, similar to arterial hypertension, a substantial part of risk attributable to MetS in the absence of diabetes can be explained by coexisting LV hypertrophy (2). If the complete control of the syndrome, including therefore control of central obesity, is the key issue for reducing target organ damage in the context of MetS, as it appears in this study, more effort should be devoted in controlling and preventing all components, namely central obesity and insulin resistance. Consistent with indications from the Diabetes Prevention Program (40–42), our study suggests that greater efforts should be devoted in the attempt to control these risk factors in addition to blood pressure and lipid profile.
As stated above, this study is a cross-sectional survey and cannot assess temporal evolution. However, our study also suggests that appropriate longitudinal investigations are needed to evaluate the impact of control of components of MetS on reduction or regression of preclinical CV disease.
APPENDIX.
List of HyperGEN Participating Institutions and Principal Staff
| Network Center/Univ. of Utah Field Center: | Steven C. Hunt, Roger R. Williams (deceased), Hilary Coon, Paul N. Hopkins, Janet Hood, Lily Wu, Jan Skuppin |
| Univ. of Alabama at Birmingham Field Center: | Albert Oberman, Cora E. Lewis, Michael T. Weaver, Phillip Johnson, Susan Walker, Christie Oden |
| Boston University/Framingham Field Center: | R. Curtis Ellison, Richard H. Myers, Yuqing Zhang, Luc Djoussé, Jemma B. Wilk, Greta Lee Splansky |
| University of Minnesota Field Center: | Donna Arnett, Aaron R. Folsom, Mike Miller, Jim Pankow, Gregory Feitl, Barb Lux |
| University of North Carolina Field Center: | Gerardo Heiss, Barry I. Freedman, Kari North, Kathryn Rose, Amy Haire |
| Data Coordinating Center, Washington Univ.: | D.C. Rao, Michael A. Province, Ingrid B. Borecki, Avril Adelman, Derek Morgan, Karen Schwander, David Lehner, Aldi Kraja, Stephen Mandel |
| Central Biochemistry Lab, Univ. of Minnesota: | John H. Eckfeldt, Catherine Leiendecker-Foster, Ronald C. McGlennen, Greg Rynders, Michael Y. Tsai, Jean Bucksa |
| Molecular Genetics Laboratory, Univ. of Utah: | Mark Leppert, Steven C. Hunt, Jean-Marc Lalouel, Robert Weiss |
| National Heart, Lung, & Blood Institute: | Susan E. Old, Millicent Higgins (retired), Cashell Jaquish, Martha Lundberg, Mariana Gerschenson. |
Acknowledgments
Supported in part by grants HL 55673, HL54471, HL54472, HL54473, HL54495, HL54496, HL54509, HL54515 from the National Heart, Lung and Blood Institute, and grant M10RR0047-34 (GCRC) from the National Institutes of Health, Bethesda, MD.
This hypertension network is funded by cooperative agreements (U10) with NHLBI: HL54471, HL54472, HL-54473, HL54495, HL54496, HL54497, HL54509, HL54515.
Footnotes
Statement on potential conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Simone G, Olsen MH, Wachtell K, Hille DA, Dahlof B, Ibsen H, Kjeldsen SE, Lyle PA, Devereux RB. Clusters of metabolic risk factors predict cardiovascular events in hypertension with target-organ damage: the LIFE study. J Hum Hypertens. 2007;21:625–632. doi: 10.1038/sj.jhh.1002203. [DOI] [PubMed] [Google Scholar]
- 2.de Simone G, Devereux RB, Chinali M, Roman MJ, Lee ET, Resnick HE, Howard BV. Metabolic syndrome and left ventricular hypertrophy in the prediction of cardiovascular events: The Strong Heart Study. Nutr Metab Cardiovasc Dis. 2009;19:98–104. doi: 10.1016/j.numecd.2008.04.001. [Erratum in: Nutr Metab Cardiovasc Dis. 2009;19:520] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devereux RB, Alderman MH. Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation. 1993;88:1444–1455. doi: 10.1161/01.cir.88.4.1444. [DOI] [PubMed] [Google Scholar]
- 4.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mockrin SC, Hunt SC. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 5.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and -cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Roman MJ, de Simone G, O’Grady MJ, Paranicas M, Yeh JL, Fabsitz RR, Howard BV. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart Study. Circulation. 1997;96:1416–1423. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Morgan D, Paranicas M, Fishman D, Arnett DK. Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension. 2001;38:417–423. doi: 10.1161/01.hyp.38.3.417. [DOI] [PubMed] [Google Scholar]
- 11.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 12.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux RB, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two- dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB. Normalization for body size and population-attributable risk of left ventricular hypertrophy The Strong Heart Study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 16.Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, Poisa P, Rizzoni D, Castellano M, Agabiti-Rosei E. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–738. doi: 10.1161/01.HYP.0000121223.44837.de. [DOI] [PubMed] [Google Scholar]
- 17.Laukkanen JA, Kurl S, Eranen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 18.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Dubin J, Wallerson DC, Cody RJ, Devereux RB. Comparative accuracy of Doppler echocardiographic methods for clinical stroke volume determination. Am Heart J. 1990;120:116–123. doi: 10.1016/0002-8703(90)90168-w. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Devereux RB, Ganau A, Hahn RT, Saba PS, Mureddu GF, Roman MJ, Howard BV. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–807. doi: 10.1016/s0002-9149(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 21.de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, Laragh JH. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension [published erratum appears in J Am Coll Cardiol 1994 Sep;24 (3):844] J Am Coll Cardiol. 1994;23:1444–1451. doi: 10.1016/0735-1097(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 22.de Simone G, Devereux RB, Koren MJ, Mensah GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265. doi: 10.1161/01.cir.93.2.259. [DOI] [PubMed] [Google Scholar]
- 23.Chinali M, de Simone G, Liu JE, Bella JN, Oberman A, Hopkins PN, Kitzman DW, Rao DC, Arnett DK, Devereux RB. Left atrial systolic force and cardiac markers of preclinical disease in hypertensive patients: the Hypertension Genetic Epidemiology Network (HyperGEN) Study. Am J Hypertens. 2005;18:899–905. doi: 10.1016/j.amjhyper.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Chinali M, de Simone G, Roman MJ, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Devereux RB. Left atrial systolic force and cardiovascular outcome the strong heart study. Am J Hypertens. 2005;18:1570–1576. doi: 10.1016/j.amjhyper.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93:40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 27.Verdecchia P, Reboldi G, Schillaci G, Borgioni C, Ciucci A, Telera MP, Santeusanio F, Porcellati C, Brunetti P. Circulating insulin and insulin growth factor-1 are independent determinants of left ventricular mass and geometry in essential hypertension. Circulation. 1999;100:1802–1807. doi: 10.1161/01.cir.100.17.1802. [DOI] [PubMed] [Google Scholar]
- 28.de Simone G, Okin PM, Gerdts E, Olsen MH, Wachtell K, Hille DA, Dahlöf B, Kjeldsen SE, Devereux RB. Clustered metabolic abnormalities blunt regression of hypertensive left ventricular hypertrophy: the LIFE study. Nutr Metab Cardiovac Dis. 2009;19:634–640. doi: 10.1016/j.numecd.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 29.de Simone G, Devereux RB, Chinali M, Best LG, Lee ET, Galloway JM, Resnick HE. Prognostic Impact of Metabolic Syndrome by Different Definitions in a Population with High Prevalence of Obesity and Diabetes: The Strong Heart Study. Diabetes Care. 2007;30:1851–1856. doi: 10.2337/dc06-2152. [DOI] [PubMed] [Google Scholar]
- 30.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de Rekeneire N, Rubin S, Ding J, Kritchevsky SB. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 31.Chinali M, de Simone G, Roman MJ, Best LG, Lee ET, Russell M, Howard BV, Devereux RB. Cardiac markers of pre-clinical disease in adolescents with the metabolic syndrome: the strong heart study. J Am Coll Cardiol. 2008;52:932–938. doi: 10.1016/j.jacc.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 33.Mancia G, Bombelli M, Corrao G, Facchetti R, Madotto F, Giannattasio C, Trevano FQ, Grassi G, Zanchetti A, Sega R. Metabolic syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study: daily life blood pressure, cardiac damage, and prognosis. Hypertension. 2007;49:40–47. doi: 10.1161/01.HYP.0000251933.22091.24. [DOI] [PubMed] [Google Scholar]
- 34.Ingelsson E, Sullivan LM, Fox CS, Murabito JM, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O’Donnell CJ, Wang TJ, D’Agostino RB, Wolf PA, Vasan RS. Burden and prognostic importance of subclinical cardiovascular disease in overweight and obese individuals. Circulation. 2007;116:375–384. doi: 10.1161/CIRCULATIONAHA.107.688788. [DOI] [PubMed] [Google Scholar]
- 35.Gotto AM, Jr, Blackburn GL, Dailey GE, III, Garber AJ, Grundy SM, Sobel BE, Weir MR. The metabolic syndrome: a call to action. Coron Artery Dis. 2006;17:77–80. doi: 10.1097/00019501-200602000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Huang P, Kraja AT, Tang W, Hunt SC, North KE, Lewis CE, Devereux RB, de Simone G, Arnett DK, Rice T, Rao DC. Factor relationships of metabolic syndrome and echocardiographic phenotypes in the HyperGEN study. J Hypertens. 2008;26:1360–1366. doi: 10.1097/HJH.0b013e3282ffdc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, Daikuhara H, Nakamura H, Taoka T, Kohno M. Left ventricular diastolic dysfunction as assessed by echocardiography in metabolic syndrome. Hypertens Res. 2006;29:897–903. doi: 10.1291/hypres.29.897. [DOI] [PubMed] [Google Scholar]
- 38.Mule G, Cottone S, Mongiovi R, Cusimano P, Mezzatesta G, Seddio G, Volpe V, Nardi E, Andronico G, Piazza G, Cerasola G. Influence of the metabolic syndrome on aortic stiffness in never treated hypertensive patients. Nutr Metab Cardiovasc Dis. 2006;16:54–59. doi: 10.1016/j.numecd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Cuspidi C, Meani S, Valerio C, Sala C, Fusi V, Zanchetti A, Mancia G. Age and target organ damage in essential hypertension: role of the metabolic syndrome. Am J Hypertens. 2007;20:296–303. doi: 10.1016/j.amjhyper.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;19(142):611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, Temprosa M. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]


