Abstract
Background
Following severe trauma there is a profound elevation of catecholamines that is associated with a persistent anemic state. We have previously shown that beta blockade (BB) prevents erythroid growth suppression and decreases hematopoietic progenitor cell (HPC) mobilization following injury. Under normal conditions, granulocyte colony stimulating factor (G-CSF) triggers the activation of matrix metalloprotease-9 (MMP-9), leading to the egress of progenitor cells from the bone marrow (BM). When sustained, this depletion of BM cellularity may contribute to BM failure. This study seeks to determine if G-CSF plays a role in the BB protection of BM following trauma.
Methods
Male Sprague-Dawley rats were subjected to either unilateral lung contusion (LC)±BB, hemorrhagic shock (HS)±BB, or both LC/HS±BB. Propranolol (BB) was given immediately following resuscitation. Animals were sacrificed at 3 and 24 hours and HPC mobilization was assessed by evaluating BM cellularity and flow cytometric analysis of peripheral blood for HPCs. The concentration of G-CSF and MMP-9 was measured in plasma by ELISA.
Results
BM cellularity is decreased at 3hrs following LC, HS and LC/HS. HS and LC/HS resulted in significant HPC mobilization in the peripheral blood. The addition of BB restored BM cellularity and reduced HPC mobilization. Three hours following HS and LC/HS, plasma G-CSF levels more than double, however LC alone showed no change in G-CSF. BB significantly decreased G-CSF in both HS and LC/HS. Similarly, MMP-9 is elevated following LC/HS and BB prevents this elevation (390±100pg/ml vs. 275±80pg/ml**).
Conclusion
BB protection of the BM following shock and injury may be due to reduced HPC mobilization and maintenance of BM cellularity. Following shock, there is an increase in plasma G-CSF and MMP-9 which is abrogated by BB and suggests a possible mechanism how BB decreases HPC mobilization thus preserving BM cellularity. In contrast, BB protection of BM following LC is not mediated by G-CSF. Therefore, the mechanism of progenitor cell mobilization from the BM is dependent on the type of injury.
Keywords: catecholamine, propranolol, anemia, MMP-9, hematopoietic progenitor cells
INTRODUCTION
Under normal homeostatic conditions there is an ongoing slow efflux of stem and hematopoietic progenitor cells (HPCs) between the bone marrow (BM) and peripheral blood (1). The movement of these cells is complex and dependent upon neural regulation, disruption of adhesive interactions, and microenvironment alterations (1, 2). The nervous system orchestrates these interactions by its effects on the cells, the immune system, the BM and its vasculature and the supportive stromal microenvironment (3). One known mediator of BM mobilization is granulocyte colony stimulating factor (G-CSF), which has been shown alter the BM microenvironment through the release of proteolytic enzymes (2). The proteolytic enzymes involved include matrix metalloprotease-9 (MMP-9), neutrophil elastase, and cathepsin G. These enzymes cleave key linkages between HPCs and stromal cells (4). MMP-9 disrupts the linkage between the chemokine stromal derived factor (SDF-1) of the stromal cells and the cell surface receptor CXCR4 found on HPCs (5, 6). This SDF-1/CXCR4 interaction is essential for the maintenance of HPCs in their BM niche.
Previously, Livingston et al. (7) demonstrated that following severe trauma and hemorrhagic shock (HS) there is a persistent anemic state lasting at least two weeks. This erythropoietic dysfunction can last for the duration of the patient’s hospital stay regardless of the number of blood transfusions (8). Similarly, the adrenergic response to severe injury results in an elevation of catecholamine levels two to ten times normal (9-11). The cause of this persistent anemia is multifactorial but has been shown to be associated with a norepinephrine-induced prolonged suppression of BM erythroid cell growth (12). Norepinephrine not only suppresses BM erythroid growth in a dose dependent fashion it also increases HPC egress into the peripheral blood (13). The loss of HPC cells into the periphery decreases BM cellularity and contributes to the persistent anemia following shock and injury (14). However, the mediators involved in BM HPC mobilization following trauma and shock have not been described.
The regulation of the catecholamine response with BB has been shown to be beneficial in humans after burns, trauma, non-cardiac surgery and traumatic brain injury as measured by improved outcomes (15-17). Previous work, in the context of tissue injury-induced BM dysfunction and shock-induced BM dysfunction has shown that non-selective BB with propranolol reduced suppression of BM HPC growth and reduced HPC mobilization (13). In addition, specifically the beta-2 and beta-3 adrenergic receptors appear to mediate the effects of reduced BM suppression and HPC mobilization (13). The exact mechanism involved in the BB protection of BM and its impact on HPC mobilization has not yet been elucidated.
Therefore, the aim of this study was to further characterize HPC mobilization after injury and shock in an animal model and analyze G-CSF and MMP-9 levels. In addition, these mediators will also be examined to determine if they play a role in the BB protection of BM following trauma.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 300-400g were housed under barrier-sustained conditions and kept at 25°C with 12 hour light/dark cycles. Animal were provided ad lib access to water and food (Teklad22/5 Rodent Diet W-8640; Harlan Teklad, Madison, WI). The animal facility environment and animals were maintained in accordance with the regulations detailed in the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care and Use Committee approved all animal protocols.
Reagents
Propranolol hydrochloride (BB) was purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS), Iscove’s Modified Dulbecco’s Medium (IMDM), and trypan blue were obtained from Invitrogen (Carlsbad, CA). Sodium pentobarbital was purchased from Lundbeck Inc. (Deerfield, ILL) and heparin was obtained from Hospira Inc (Lakefront, ILL).
Experimental Design
In order to evaluate the effect of BB on G-CSF and HPC mobilization, rats (N=4-8 animals/group) were randomly allocated to one of the following seven study groups: unmanipulated control (UC), lung contusion (LC) ± BB, hemorrhagic shock (HS) ± BB, or LC followed by HS (LC/HS) ± BB (Table 1). Those animals that received BB were given a non-selective BB, propranolol, at 10 mg/kg IP after lung injury alone or approximately ten minutes following resuscitation with shed blood. BM cellularity and HPC mobilization was assessed at three hours following injury and/or shock and animals were sacrificed at three and twenty-four hours to obtain G-CSF and MMP-9 data. Peripheral blood was acquired through cardiac puncture and BM was harvested from bilateral femurs.
Table 1.
Experimental groups defined. UC=unmanipulated control; LC=lung contusion; BB=beta blockade; HS=hemorrhagic shock; LC/HS=combined lung contusion followed by hemorrhagic shock; N=number of animals/group at the time of sacrifice
| Group | Lung Contusion |
Hemorrhagic Shock |
Intraperitoneal Injection |
N for 3h Sacrifice |
N for 24h Sacrifice |
|---|---|---|---|---|---|
| UC | - | - | - | 8 | 8 |
| LC | Yes | - | - | 6 | 5 |
| LC + BB | Yes | - | Propranolol | 6 | 5 |
| HS | - | Yes | - | 6 | 4 |
| HS + BB | - | Yes | Propranolol | 7 | 4 |
| LC/HS | Yes | Yes | - | 8 | 5 |
| LC/HS + BB | Yes | Yes | Propranolol | 8 | 6 |
Tissue Injury Model
Experimental animals were weighed and anesthetized with IP injections of sodium pentobarbital (50mg/kg). Unilateral LC was induced by using a blast wave of a percussive nail gun (Craftsman 968514 Stapler, Sears Brands Chicago, IL) applied to a 12mm metal plate applied to the right axilla of the rat. This model has been shown to produce a clinically significant LC as demonstrated by radiography and histology (18). After 10 minutes of recovery time animals were randomly allocated to one of the above experimental groups.
Hemorrhagic Shock Model
If not previously anesthetized for LC, animals were given sodium pentobarbital (50mg/kg IP). Using aseptic technique, the right internal jugular vein and femoral artery were cannulated with polyethylene tubing (PE-50 and PE-10, respectively) containing heparinized saline (10 units/ml). The femoral artery tubing was then connected to a continuous blood pressure monitoring device (BP-2 Digital Blood Pressure Monitor; Columbus Instruments; Columbus, Ohio) for measurement of mean arterial pressure (MAP) and heart rate (HR). Animals were bled to a MAP of 30-35mmHg for 45 minutes. Temperature was maintained at approximately 37°C with the use of an electric heating pad under the surgical platform. Shed blood was also maintained at approximately 37°C and was re-infused at a rate of 1ml/min following the shock period.
Bone Marrow Cellularity
BM cells were obtained by flushing both femurs from individual rats with a total of 1mL IMDM supplemented with 10% FBS. A suspension was prepared by passing cells through a 40μm sterile nylon strainer to remove particulate matter. Total viable cell counts were then determined by 0.4% Trypan blue staining using a hemocytometer.
Measurement of Plasma G-CSF and MMP-9
Following sacrifice, peripheral blood samples were centrifuged at 10,000rpm for ten minutes at 10°C to obtain plasma, which was collected and stored at −80°C. Plasma samples were analyzed for G-CSF and MMP-9 using commercial colorimetric sandwich ELISA kits (CUSABIO Biotech Company LTD, Wuhan, China and R&D Systems Inc., Minneapolis, MN). Assays were performed according to the provided manufacturer’s instructions. All standards and samples were assayed in duplicate.
Flow Cytometry
The frequency of CD117+ and CD 71+ cells was quantified in unfractionated peripheral blood samples using an established, single-platform enumeration method. Briefly, 100 μl of peripheral blood (one million cells) was labeled with 10 μL of BD Pharmingen™ mouse anti-rat CD71 antibody conjugated with fluorescein isothiocyanate and 10 μL of BD Pharmingen™ rat anti-mouse CD117 (c-Kit) antibody conjugated to phycoerythrin (BD Biosciences, Franklin Lakes, NJ) for 30 minutes. Following ammonium chloride erythrocyte lysis, cells were then centrifuged at 300G x five minutes and supernatant was discarded. Cells were washed 3 times and fixed with BD Cytofix™ solution (BD). Cells were analyzed using BD FACSCalibur flow cytometer (BD) equipped with CellQuest software (BD). Samples from each group were stained and run in duplicate and an event count of 30,000 was obtained for each run. Following acquisition of data further analysis was performed using Flow Jo v.7.2.4 (Tree Star, Ashland, OR).
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparison post test with GraphPad Prism (Version 4.0, San Diego, CA). Results were considered significant if *P <0.05.
RESULTS
Circulating HPCs Increase early after Injury and Shock
An assessment of BM cellularity demonstrates that three hours following LC alone and HS alone there is a significant loss of BM cells as compared to control animals (124±23* and 107±24* vs. 198±15) (Table 2). The addition of HS to LC further increases the loss of BM cells as compared to control animals (86±21* vs. 198±15).
Table 2.
BM cellularity restored with use of beta blockade. N=6-8/group
| Group | Cells × 106ml/femur | |
|---|---|---|
| UC | 198 ± 15 | |
| No BB | + BB | |
| LC | 124 ± 23 * | 215 ± 31 ** |
| HS | 107 ± 24 * | 195 ± 56 ** |
| LC/HS | 86 ± 21 * | 238 ± 7 ** |
P<0.05 vs. UC
P<0.05 vs. non-BB counterpart
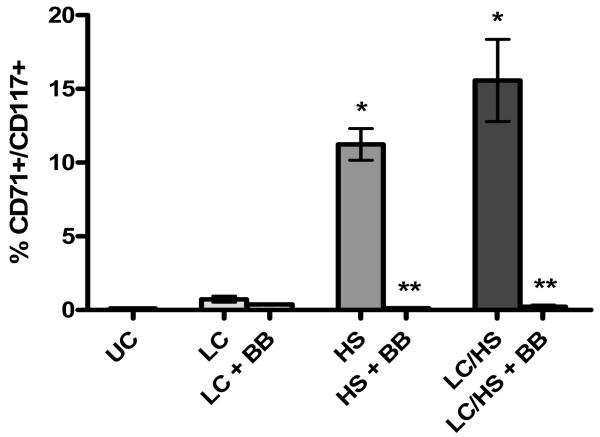
Using flow cytometry, HPCs mobilized into peripheral blood were defined as those cells expressing both CD71 and CD117. In UC animals only 0.1% of cells are circulating HPCs and three hours following LC alone there is increased HPC mobilization into peripheral blood (0.9%). The presence of shock (HS alone or LC/HS) significantly increased HPC mobilization at three hours as compared to UC animals (11%* and 16%* vs. 0.1%) (Figure 1). This demonstrates that circulating HPCs increase early after injury which results in decreased BM cellularity.
Figure 1.
Increased HPC mobilization into peripheral blood following injury and shock which is prevented with use of BB. N=6-8 animals/group; * P <0.05 vs. UC; ** P <0.05 vs. non-BB counterpart
BB Decreases HPC Mobilization
All experimental groups that received BB (LC+BB, HS+BB, and LC/HS +BB) had maintenance of BM cellularity similar to that of control levels (215±31, 195±56, and 238±7 vs. 198±15 (Table 2). Therefore, there was a statistically significant increase in BM cellularity with the use of BB as compared to their non-BB counterpart (Table 2). In addition, the use of BB reduced the degree of HPC mobilization in peripheral blood to that of control levels (LC+BB 0.4%, HS+BB 0.1%, LC/HS+BB 0.2% vs. UC 0.1%) (Figure 1). There was a statistically significant decrease in HPC mobilization in HS+BB and LC/HS+BB as compared to HS alone and LC/HS.
Plasma G-CSF and MMP-9 Increase after Injury and Shock
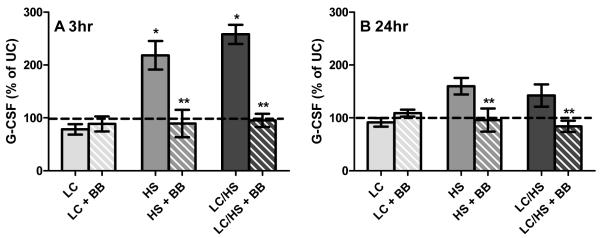
Parallel to the loss of BM cellularity and increased HPC mobilization into the peripheral blood, plasma G-CSF levels significantly more than double three hours following shock (HS and LC/HS) (Figure 2A). The peak level of G-CSF was higher in the plasma of rats that had both shock and injury (LC/HS) as compared to HS alone (Figure 2A). Twenty four hours following HS and LC/HS, G-CSF remains elevated but not significantly (Figure 2B). There was not a significant increase in G-CSF levels 3 or 24 hours following LC alone (Figure 2A and 2B).
Figure 2A and 2B.
Plasma G-CSF levels increase at 3 hour (2A) (N=6-8 animals/group) and remain elevated at 24 hours (2B) (N=4-8 animals/group) following HS and LC/HS and the use of BB prevents this elevation. * P <0.05 vs. UC; ** P <0.05 vs. non-BB counterpart
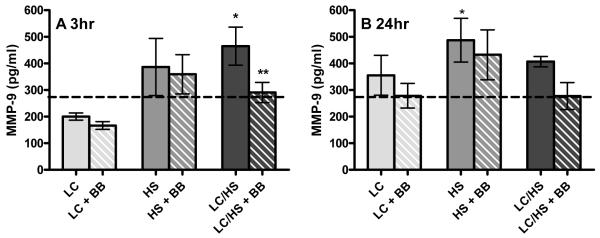
Examining MMP-9 levels, we find a similar trend to that of G-CSF. Three hours following HS and LC/HS, plasma MMP-9 levels significantly increased as compared to control (387±115 and 465±160* vs. 274±112 pg/mL) (Figure 3A). Twenty four hours following HS and LC/HS, MMP-9 remain elevated but it did not reach statistical significance (Figure 3B). There was not a significant increase in MMP-9 levels 3 or 24 hours following LC alone (Figure 3A and 3B).
Figure 3A and 3B.
Plasma MMP-9 levels significantly increase at 3 hour (3A) (N=6-8 animals/group) and 24 hours (3B) (N=4-8 animals/group) after LC/HS and the use of BB prevents this elevation. * P <0.05 vs. UC; ** P <0.05 vs. non-BB counterpart
The Impact of BB on Plasma G-CSF and MMP-9 levels
The decrease in HPC mobilization with BB correlates to G-CSF levels. At three hours following injury, the addition of BB significantly decreased G-CSF levels in both HS and LC/HS groups as compared to their non-BB counterparts and reduced the G-CSF level to that of control animals (Figure 2A). The level of G-CSF remains similar to that of control animals at twenty-four hours in all experimental groups that received BB (LC+BB, HS+BB, and LC/HS +BB) (Figure 2B).
MMP-9 levels were lower at three and twenty-four hours in the LC/HS+BB group as compared to LC/HS (3h: 291±76* vs. 465±160 and 24h: 278±102 vs. 407±43 pg/mL). In the HS+BB group there was a trend toward lower MMP-9 levels at three and twenty-four hours but it did not reach statistical significance (Figure 3A and 3B). There was no significant difference in MMP-9 levels in LC+BB group compared to controls at three or twenty-four hours (Figure 3A and 3B).
DISCUSSION
The trafficking of HPCs from BM to peripheral blood and eventually to injured or inflamed tissue, is believed to be beneficial and aid in host defense and integral to wound healing (18, 19). This phenomenon of stress induced HPC mobilization is best illustrated by Katayama et al. (20) who showed a critical link between the sympathetic nervous system, particularly norepinephrine, and G-CSF induced mobilization. Previously we have shown that norepinephrine causes a dose-dependent reduction of BM HPC growth along with increased HPC mobilization from the BM (12-14). Therefore, severe trauma along with a prolonged hypercatecholamine state may be deleterious resulting in inhibition of BM HPC growth and continued loss of HPCs into peripheral blood that leads to persistent anemia and abnormal tissue healing. Similarly, in major burn injuries, there is a systemic release of catecholamines which induces a stress state and has been shown to delay burn wound healing (21). In the current study we demonstrate that BM cellularity is decreased early following LC, HS and LC/HS and this decrease in BM cellularity is associated with increased HPC mobilization into peripheral blood. Eleven percent of circulating mononuclear cells are HPCs following HS alone and this increases to 16% following both tissue injury and shock (LC/HS). Kollet et al. (22) demonstrated that osteoclast activation following stress and hemorrhage also mobilized progenitor cells into peripheral blood. When comparing patients suffering from acute myocardial infarction to those with stable angina, there is also a differential release of progenitor cells into peripheral blood, 8.6 cells/μl vs. 3.4 cells/μl (23). Current data supports the finding that the severity of the injury plays a key role in the egress and mobilization of progenitor cells from the BM.
Following both HS and LC/HS, we demonstrate that plasma G-CSF levels more than double and this correlates with the large increase in the percentage of HPCs that can be found circulating in peripheral blood. This finding is supported by prior studies that demonstrate that the use of exogenous G-CSF leads to the mobilization of cells from the BM (24, 25, 26). The induction of HPC mobilization in both non-injured rats and humans via G-CSF injection is time and dose dependent resulting in peripheral blood levels of HPC peaking up to 100-fold above baseline at 3-6 days after G-CSF administration (25, 26). In addition, several cytokines have been suggested to increase the number of circulating progenitor cells, including IL-1, IL-6, IL-8 and IL-12 (27). Our study demonstrates a similar and somewhat larger magnitude of increase in circulating HPCs as early as 3 hours following HS and LC/HS. Similar to our HPC mobilization findings, plasma G-CSF levels have the greatest elevation following combined LC/HS injury. Plasma G-CSF levels have been shown to be elevated during physiological stress in pediatric burn patients and following endurance and short-duration exercise (28, 29). In addition, there is a direct correlation between endogenous G-CSF levels and the mobilization of progenitor cells in patients with acute myocardial infarction (23). In ICU patients, twenty-four hour G-CSF levels appear to be predictive of worsening organ dysfunction and early mortality in severe sepsis (30). G-CSF may be a marker of ongoing HPC mobilization in these patients.
In this study elevation of plasma MMP-9 levels following LC/HS is consistent with studies that evaluate the BM milieu following G-CSF administration (31). MMP-9, has been shown to accumulate in the plasma following mobilization with G-CSF and serves to regulate the BM microenvironment (3, 4). Our plasma levels of MMP-9 following HS alone were higher later at twenty-four hours and LC/HS levels were higher earlier at three hours not as elevated at twenty-four hours. This finding may due to the higher G-CSF levels early after LC/HS. This finding is supported by Levesque et al. (25) who demonstrated that MMP-9 levels gradually increased and the highest levels were found at the time of maximal progenitor mobilization.
In our animal model, the use of BB following shock and injury led to preservation of BM cellularity similar to that of control animals. The addition of BB also reduced HPC mobilization as shown with flow cytometry of peripheral blood following HS alone and LC/HS. Thus reduced HPC mobilization and maintenance of BM cellularity with the use of BB may be crucial to the protection of BM. Our findings are supported by Katayama et al. (20) who demonstrated that the use of a beta-2 agonist augmented G-CSF induced mobilization and propranolol use diminished the mobilization of HPCs in normal mice. BB protects the BM from erythroid growth suppression and diminish HPC mobilization without delaying healing of injured tissue (13, 14). High dose propranolol (25 mg/kg) was shown to block the deleterious effects of circulating catecholamines and improve cutaneous wound healing in burned chronically stressed mice (21).
The study also demonstrates that while HS and LC/HS significantly increase plasma G-CSF, the use of BB prevented this increase. Similarly, MMP-9 is elevated following LC/HS and BB reduced this elevation. Therefore, following shock the increase in G-CSF and MMP-9 that is abrogated by BB may explain the decreased HPC mobilization. BB appears to protect BM through via a neurally regulated chemokine pathway. Given that G-CSF and its downstream target MMP-9 are modulated by BB, it is likely that BB is exerting its effect high in the mobilization pathway. The significant correlation between propranolol administration, endogenous plasma G-CSF levels, and HPC mobilization into peripheral blood could be a potential therapeutic option for BM protection following severe traumatic injury and shock.
Interestingly, while BB did preserve BM cellularity following tissue injury alone (LC), the protection of BM does not appear to be mediated by G-CSF. This finding may be due to the LC used in this model resulting in insufficient physiologic stress to generate a detectable G-CSF response or perhaps a the time points selected were not appropriate to detect an elevation of G-CSF . It is also possible that since LC alone represents a normal tissue healing process, it may be stromal cell mediated rather than HPC mediated and therefore it is not associated with G-CSF elevation. There is evidence of a differential regulation of progenitor cell mobilization as demonstrated by Kolonin et al. (32). VEGF has been shown to be another mediator involved in endothelial progenitor cell mobilization and has been shown to be increased during neo-angiogenesis and normal wound healing (32, 33). In our study examining specifically the mobilization of HPCs rather than BM stromal cells, we found that the degree of HPC mobilization was associated with the presence of shock and proportional to the severity of injury. The degree of HPC mobilization paralleled G-CSF and MMP-9 values in the HS and LC/HS groups.
In summary, following HS and LC/HS, the decreased HPC mobilization seen with the use of BB is likely due to reduced levels of plasma G-CSF and subsequently MMP-9. Our results indicate that the BB protection of BM following shock and injury is due to reduced HPC mobilization and thus maintenance of BM cellularity. In addition, the egress of progenitor cells following tissue injury alone is not mediated by G-CSF. Therefore, the mechanism of progenitor cell egress from the BM may be dependent on the type and severity of injury.
ACKNOWLEDGEMENTS
This research was supported in part by the NIH K08GM078304-01 and the Clowes ACS/AAST Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lapid K, Vagima V, Kollet O, Lapidot T. In: Egress and mobilization of hematopoietic stem and progenitor cells. StemBook, editor. Harvard Stem Cell Institute; Cambridge: 2008. [Google Scholar]
- 2.Magnon C, Frenette PS. In: Hematopoietic stem cell trafficking. StemBook, editor. Harvard Stem Cell Institute; Cambridge: 2008. [Google Scholar]
- 3.Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 4.McQuibban GA, Butler GS, Gong JH, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 5.Nervi B, Link D, DiPersio J. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 6.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 7.Livingston DH, Anjaria D, Wu J, et al. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corwin HL, Surgenor SD, Gettinger A. Transfusion practice in the critically ill. Crit Care Med. 2003;31:S668–S671. doi: 10.1097/01.CCM.0000099348.99451.84. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca RB, Mohr AM, Wang L, et al. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–890. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 10.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 11.Breslow MJ, Ligier B. Hyperadrenergic states. Crit Care Med. 1991;19:1566–79. doi: 10.1097/00003246-199112000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Penn A, Mohr AM, Shah SG, et al. Dose response relationship between norepinephrine and erythropoiesis: Evidence for a critical threshold. J Surg Res. 2010;163:e85–90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beiermeister KA, Keck BM, Sifri ZC, et al. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 adrenergic receptors following injury. J Trauma. 2010;69:338–343. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhassan IO, Hannoush EJ, Alzate W, et al. Beta blockade prevents hematopoietic progenitor cell suppression following hemorrhagic shock. Surg Inf. 2010;11:214. doi: 10.1089/sur.2010.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Eng J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 16.Arbabi S, Ahrns KS, Wahl WL, et al. Beta-blockade use is associated with improved outcomes in adult burn patients. J Trauma. 2004;56:265–271. doi: 10.1097/01.TA.0000109859.91202.C8. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Teixeira P, David JS, et al. Beta-Blockers in isolated blunt head Injury. J Am Coll Surg. 2008;206:432–438. doi: 10.1016/j.jamcollsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Badami CD, Livingston DH, Sifri ZC, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 19.Shah S, Ulm J, Sifri ZC, et al. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67:315–322. doi: 10.1097/TA.0b013e3181a5c9c7. [DOI] [PubMed] [Google Scholar]
- 20.Katayama Y, Battista M, Kao W, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Romana-Souza B, Porto LC, Monte-Alto-Costa A. Cutaneous wound healing of chronically stressed mice is improved through catecholamine blockade. Exp Derm. 2010;19:821–829. doi: 10.1111/j.1600-0625.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 22.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 23.Leone AM, Rutella S, Bonanno G, et al. Endogenous G-CSF and CD34* cell mobilization after acute myocardial infarction. Int J Card. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Duhrsen U, Villeval JL, Boyd J, et al. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074–2081. [PubMed] [Google Scholar]
- 25.Levesque JP, Hendy J, Takamatsu Y, et al. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 26.Sato N, Sawada K, Takahashi TA, et al. A time course study for optimal harvest of peripheral blood progenitor cells by granulocyte colony-stimulating factor in healthy volunteers. Exp Hematol. 1994;22:973–978. [PubMed] [Google Scholar]
- 27.Cottler-Fox MH, Lapidot T, Petit I, et al. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 28.Mikhal’chik EV, Piterskaya JA, Budkevich LY, et al. Comparative study of cytokine content in the plasma and wound exudate from children with severe burns. Bull Exp Biol Med. 2009;148:771–775. doi: 10.1007/s10517-010-0813-7. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Nakaji S, Yamada M, et al. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. [PubMed] [Google Scholar]
- 30.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Critical Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HK, De La Luz Sierra M, Kimmel Williams C, et al. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolonin MJ, Simmons PJ. Combinatorial stem cell mobilization. Nat Biotechnol. 2009;27:252–253. doi: 10.1038/nbt0309-252. [DOI] [PubMed] [Google Scholar]
- 33.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]