Abstract
The perforant pathway originates from cells in the entorhinal cortex and relays sensory information from the neocortex to the hippocampus, a region critical for memory function. Imaging studies have demonstrated structural alterations in the parahippocampal white matter in the region of the perforant pathway in people at risk for developing Alzheimer’s disease. It is not clear, however, if changes noted in this region are indicative of pathological aging or are a function of the normal aging process. We compared MRI-derived mesial temporal lobe volumes in 51 healthy older individuals and 40 young participants, with an emphasis on the parahippocampal white matter. Yearly clinical evaluations showed that 9 of the older cohort declined in cognitive function. Parahippocampal white matter, hippocampal and entorhinal cortex volumes were significantly reduced in healthy older people who remained stable over time compared to young participants. These findings suggest that volume differences in mesial temporal lobe gray and white matter structures may take place as a result of the normative aging process.
Keywords: imaging, aging, memory, hippocampus, entorhinal cortex
1. Introduction
The entorhinal cortex and hippocampus are part of the mesial temporal lobe memory system (Squire and Zola-Morgan 1991; Young et al., 1997). Neurons of the entorhinal cortex receive multimodal sensory information from primary sensory and association cortices (Amaral et al., 1987; Van Hoesen and Pandya 1975a; Van Hoesen et al., 1975) and relay this information to the hippocampus via the axons that make up the perforant pathway (Hyman et al., 1984; Van Hoesen and Pandya 1975b).
Quantitative structural magnetic resonance imaging (MRI) techniques provide a tool for examining alterations in brain anatomy in vivo during healthy and pathological aging. Such changes in anatomy can be used as a proxy measure of the underlying pathology in neurodegenerative diseases. For example, using such techniques, a number of studies have now reported that atrophy of the entorhinal cortex and hippocampus, structures known to be pathologically involved very early in Alzheimer’s disease (AD, Braak and Braak, 1991, 1995; Braak et al., 1998), can provide sensitive markers of risk for AD among older people with mild cognitive impairment (MCI) or subjective cognitive complaints (Cardenas et al., 2002; Chao et al., 2010; deToledo-Morrell et al., 2004; Devanand et al., 2007; Dickerson et al., 2001; Jack et al., 1999; Jessen et al., 2006; Killiany et al., 2000, 2002; Saykin et al., 2006; Stoub et al., 2005; Tapiola et al., 2008). These results are not surprising, since memory dysfunction is one of the earliest hallmarks of AD.
In addition to gray matter regions, there has recently been increased interest in assessing structural changes in white matter regions in those at risk for AD, as well as in healthy older adults (Allen et al., 2005; Bartzokis et al., 2001, 2003, 2004; Guttmann et al., 1998; Good et al., 2001; Jernigan et al., 2001; Raz et al., 2005; Resnick et al., 2003; Rogalski et al.,2009; Salat et al., 2009; Smith et al., 2007; Stoub et al., 2006). The studies that investigated the effects of aging on cerebral white matter found mostly diffuse decreases in white matter volume associated with aging (Allen et al., 2005; Guttmann et al., 1998; Jernigan et al., 2001; Raz et al., 2005; Resnick et al., 2003; Salat et al., 2009). However, age-related atrophy in the parahippocampal white matter has not received much attention.
Recent work from our laboratory has demonstrated decreased parahippocampal white matter volume in the region of the perforant pathway in people with amnestic mild cognitive impairment (aMCI), who are at risk for developing AD, compared to healthy older controls (Rogalski et al., 2009; Stoub et al., 2006). Such alterations in parahippocampal white matter could degrade information flow from the entorhinal cortex to the hippocampus and contribute to the memory deficit observed in aMCI and very mild AD. It is unclear, however, if volume changes in this region take place as a function of the aging process per se or are due to age-related pathological processes.
Investigations in animal models of aging have demonstrated that cell numbers remain the same in layer II of the entorhinal cortex, as well as in hippocampal CA3 and dentate gyrus regions (Rasmussen et al., 1996). However, there is a decrease in synaptophysin markers in CA3 (Smith et al., 2000) and a reduction in actual synapse numbers in the middle molecular layer of the hippocampal dentate gyrus (Geinisman et al., 1986, 1992). Additionally, electrophysiological experiments have shown a decrease in the presynaptic fiber potential in old, memory impaired rats (Barnes, 1979; Barnes and McNaughton, 1980), suggesting a pruning of axon collaterals from the perforant pathway to the dentate gyrus. If there is a cross-species correspondence in the types of brain changes that occur during aging, then the rodent data predict that humans should also show changes in the region of the perforant pathway as a function of age.
The present in vivo structural imaging study was undertaken to examine if healthy older individuals show volume changes, compared to younger adults, in the parahippocampal white matter region that includes the perforant pathway. In addition, we investigated the volumes of surrounding structures including the hippocampus and entorhinal cortex, regions important for episodic memory function, such as memory for events and things.
2. Subjects and Methods
2.1 Subjects
Participants included 40 young (mean age=27 years, range 22–36; 22 male and 18 female) and 51 healthy older individuals [mean age=77 years, range 65–89; 14 male and 37 female; mean Mini Mental State Examination (MMSE)=29, range 27–30]. The healthy older participants were recruited from the community for an ongoing longitudinal study (deToledo-Morrell et al., 2004), as well as from two longitudinal clinico-pathologic investigations of aging and AD in older individuals: the Religious Order Study (ROS; Mufson et al., 1999, Bennett et al., 2002) and the Rush Memory and Aging project (MAP; Bennett et al., 2005). Older subjects did not have any cognitive impairment at entry into the study based on neuropsychological tests carried out at the Rush Alzheimer’s Disease Center clinic; they were followed yearly with clinical evaluations (total mean follow-up period=7.1 ± 2.5 years, with a range of 3–12 years). Selection of healthy elderly participants required a normal neurological examination, normal cognition and a MMSE (Folstein et al., 1975) score of ≥ 27 (out of a maximum of 30 points). Young subjects were recruited from Rush University Medical Center students and employees, as well as their friends and family members. Subjects in both groups were excluded from entering the study if neurologic, psychiatric and systemic conditions, or a history of temporal lobe epilepsy that could affect mesial temporal lobe structures, were identified. Informed consent was obtained from all participants according to the rules of the Institutional Review Board of Rush University Medical Center.
2.2 MRI acquisition
All subjects received an MRI scan at entry into the study. Scans were acquired with a 1.5 Tesla General Electric Signa scanner, using the manufacturer’s 3D Fourier transform spoiled gradient recalled (SPGR) pulse sequence. Acquisition parameters consisted of: 124 contiguous images in the coronal plane, 1.6 mm thick, matrix=256×192, field of view=22 cm, TR/TE=34/7 msec, flip angle=35°, signals averaged=1.
2.3 Regions of Interest Volumes
The volumes of the parahippocampal white matter, entorhinal cortex and hippocampus were determined with the use of the Analyze software package (Mayo Clinic Foundation, Rochester, MN). To correct for individual differences in brain size, volumes were divided by total intracranial volume derived from sagittally formatted 5 mm slices (i.e., normalized). To compute intracranial volume, the inner table of the cranium was traced in consecutive sagittal sections spanning the entire brain. At the level of the foramen magnum, a straight line was drawn from the inner surface of the clivus to the occipital bone. Normalized volume for brain regions of interest was determined using the formula: absolute volume in mm3/intracranial volume in mm3 × 1000.
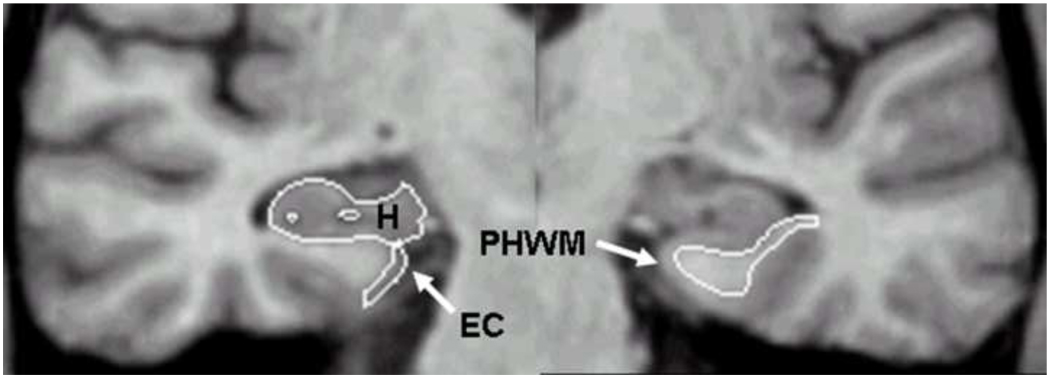
Figure 1 depicts the segmentation of the parahippocampal white matter, entorhinal cortex and hippocampus in a single coronal MRI section for a sample participant. All three volumes were measured from the same oblique coronal sections most commonly used for hippocampal volumetry. Briefly, volumes were computed separately for the right and left hemispheres from coronal slices reformatted to be perpendicular to the long axis of the hippocampus. The boundaries used for quantifying parahippocampal volume were published previously (Rogalski et al., 2009). Tracing of the parahippocampal white matter began with the slice in which the gyrus ambiens, amygdala and white matter of the parahippocampal gyrus were first visualized. The most caudal slice traced was one slice rostral to the first appearance of the lateral geniculate nucleus. The lateral border of the parahippocampal white matter was defined as the bend that signifies the junction between the parahippocampal white matter and the temporal stem. The medial border was defined as the point at which the white matter meets the gray matter of the entorhinal cortex.
Figure 1.
A single coronal slice illustrating the segmentation of the parahippocampal white matter (PHWM), entorhinal cortex (EC) and hippocampal formation (H).
Entorhinal cortex volume was quantified with the use of a protocol developed and validated in our laboratory, technical details of which are presented in Goncharova et al. (2001). For the entorhinal cortex, tracing began with the first section in which the gyrus ambiens, amygdala and the white matter of the parahippocampal gyrus first appeared visible. The superomedial border in rostral sections was the sulcus semiannularis and in caudal sections the subiculum. The shoulder of the collateral sulcus was used as the lateral border. The latter is a somewhat conservative criterion that allowed consistency in tracings and avoided the use of different lateral borders depending on individual differences in the depth of the collateral sulcus (see, for example, Insausti et al., 1998). The lateral border was constructed by drawing a straight line from the most inferior point of the white matter to the most inferior tip of the gray matter. The last section measured was three 1.6 mm sections rostral to the image in which the lateral geniculate nucleus first appeared visible.
The protocol and validation procedures used for quantifying hippocampal volume were published previously (deToledo-Morrell et al., 1997; Wilson et al., 1996). Tracings of the hippocampus started with the first section where it could be clearly differentiated from the amygdala by the alveus and included the fimbria, dentate gyrus, the hippocampus proper and the subiculum. Tracings continued on all consecutive 1.6 mm thick images until the slice before the full appearance of the fornix.
2.4 Memory testing
Episodic memory function was tested using the verbal version of the Buschke ‘controlled learning’ task (Buschke and Grober, 1986; Grober and Buschke, 1987). This task has been shown to distinguish between “apparent” and “genuine” memory deficits in elderly individuals (Buschke and Grober, 1986; Grober and Buschke, 1987), since it controls for attention or the use of inefficient strategies in acquiring information. Participants were asked to learn a list of 16 items presented four at a time as previously described (deToledo-Morrell et al., 2000a). Items were shown as line drawings with one picture in each quadrant of a card. When a category cue was given verbally, the subject had to search, point to and name the object from that category. After this was done for four items, immediate cued recall of the four items was tested by presenting each category cue to the subject. If the subject failed to recall an item in response to its cue, the item was shown again, and the entire process was repeated until immediate cued recall was correct for the four items. Then, the next set of four items was presented until all 16 items were correctly retrieved during immediate cued recall. The search and naming procedure ensured that all participants used the same strategy in processing information and that the items have been correctly encoded.
After the subjects learned the items, three trials of free recall were administered, with each trial being preceded by 20 seconds of interference. On each trial, subjects were allowed a maximum of 2 minutes to name as many of the learned items as possible. Next, a category cue was provided for each item missed on that trial. If the subject still failed to recall the item with the cue, he/she was reminded of the missed item which he/she then repeated. An additional trial of free recall was administered after approximately 60 min to test for delayed recall.
2.5 Statistical Analyses
To evaluate group and hemisphere differences for all measured structures separate 2-way repeated measures analyses of covariance (ANCOVA) were used with gender as the covariate. Group differences in the performance of the verbal memory task were assessed with 1-way ANCOVAs with gender as the covariate.
3. Results
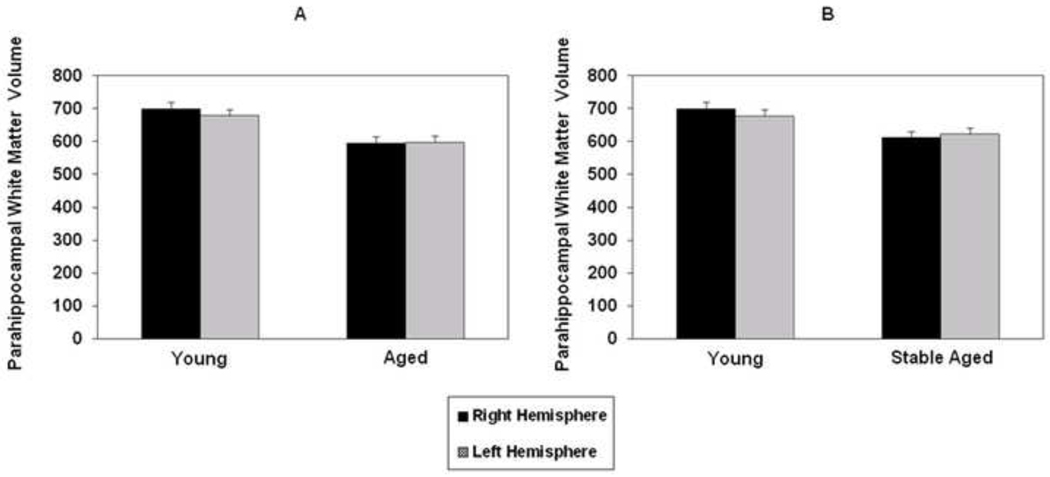
Mean right and left parahippocampal white matter volumes for the healthy old and young participants are presented in Figure 2A. A repeated measures ANCOVA found significant group [F(2,88)=10.94, p=0.001], but not hemisphere effects, with no significant interaction between them.
Figure 2.
Mean normalized right and left parahippocampal white matter volume in all aged subjects (A) and only those who remained stable during the follow-up period (B) compared to young participants. Vertical bars represent the standard error of the mean.
Yearly clinical evaluations available for the healthy old cohort demonstrated that of the original 51 participants enrolled, 9 declined in cognitive function during the 7 year follow-up period, with 6 of the 9 receiving a diagnosis of AD (McKhann et al., 1984; mean age of all declining participants was 79 years, range 70–83; 5 male and 4 female; mean MMSE=28, range 27–30). The remaining 3 participants received a diagnosis of amnestic mild cognitive impairment (MCI). Those diagnosed with amnesitc MCI were found to have a deficit in memory only, but did not meet criteria for dementia (Petersen et al., 1999). When young subjects were compared to only the 42 old participants who remained stable over time, the difference in parahippocampal white matter volume between the two groups was smaller, but still significant [F(1,79)=5.99, p=0.017; see Figure 2B]. These findings suggest that volume reduction in this region is present not only as a function of pathological aging, but also, although to a lesser extent, as a result of the normal aging process.
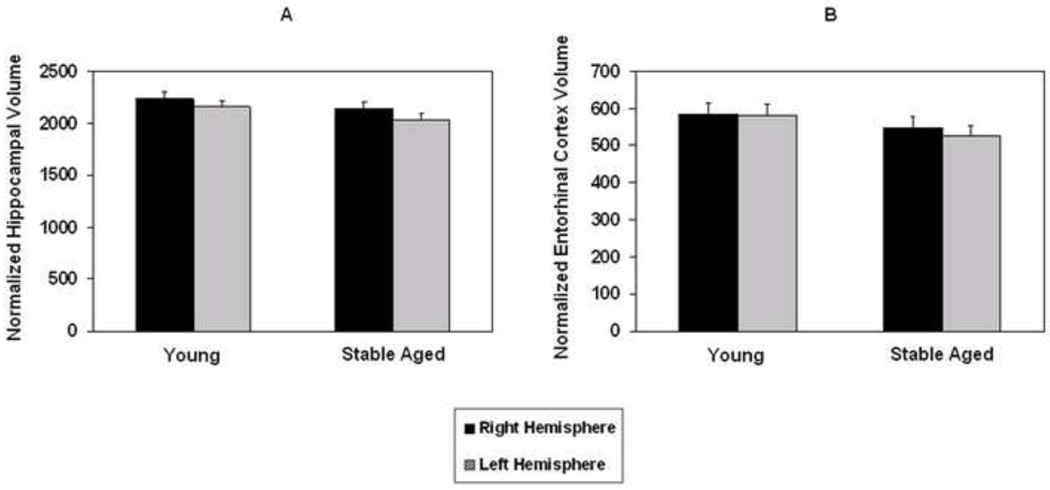
Since the entorhinal cortex and hippocampus are essential for episodic memory function and are connected via the perforant pathway, we determined if there were age related volumetric changes in these two structures. Volumes for the entorhinal cortex and hippocampus for old participants who remained stable compared to the young are shown in Figure 3. A repeated measures ANCOVA comparing these two groups showed significant group [F(1,79)=7.26, p=0.009] and hemisphere [F(1,79)=6.51, p=0.013] differences in hippocampal volume, with no interaction between them. The hemisphere effect was due to the right hippocampus volume being larger than the left for both groups. The analysis for entorhinal cortex volume revealed only a significant group difference [F(1,79)=6.41, p=0.013].
Figure 3.
Mean normalized right and left hippocampal (A) and entorhinal cortex (B) volumes in old participants who remained stable during the follow-up period compared to young subjects. Vertical bars represent the standard error of the mean.
Memory testing was carried out on 35 of the 40 young participants in the study and on all the old participants. Separate ANCOVAs comparing the stable old and the 35 young subjects revealed a significant difference between the two groups in verbal free recall following the third trial [F(1,74)=7.80, p=0.001] and in verbal delayed recall [F(1,74)=10.32, p<0.0001].
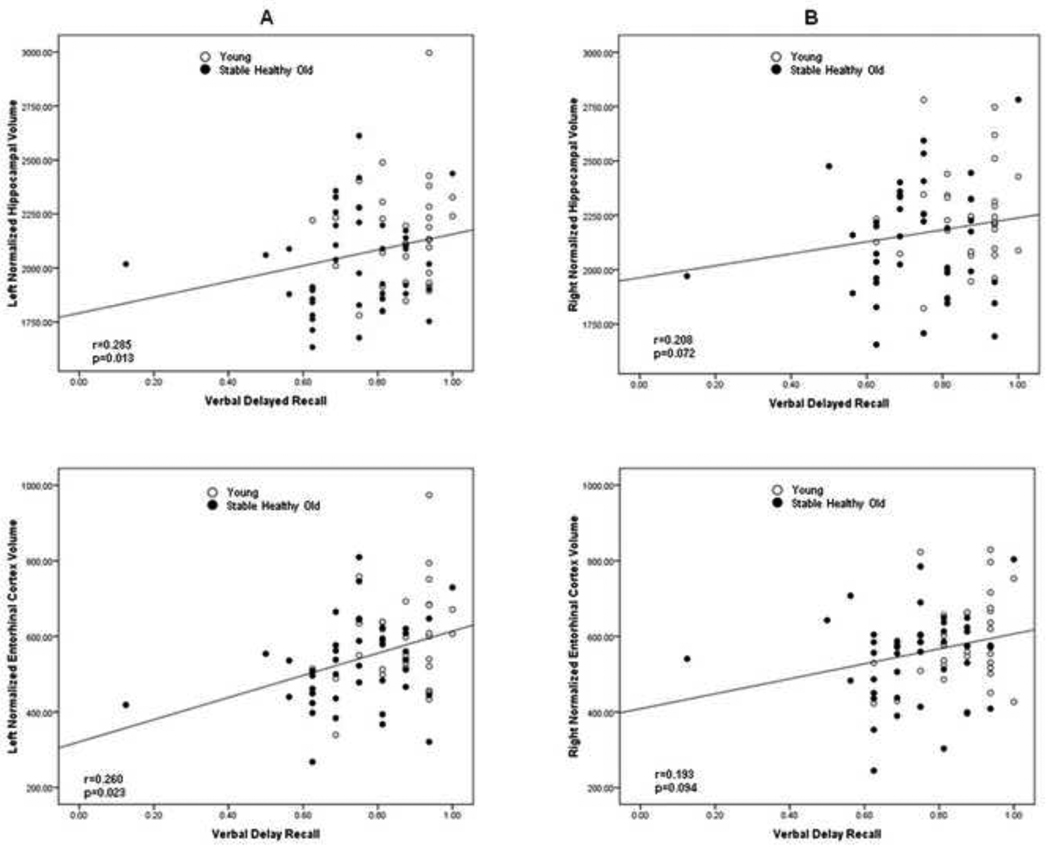
We examined the relation between memory performance and right and left hemisphere volumes for the hippocampus, entorhinal cortex and the parahippocampal white matter, in both the young and stable old participants. Each of the three measures was entered singly into a partial correlation analysis with verbal free recall for the third trial and delayed recall as separate dependant variables and gender entered first. The analyses showed a significant relationship between delayed verbal recall and left hippocampal (r=0.285; p=0.013), as well as left entorhinal cortex (r=0.262; p=0.023) volumes (Figure 4). There were no significant relationships between delayed recall and either right hippocampal or right entorhinal cortex volumes. Neither left nor right parahippocampal white matter volumes were related to delayed verbal memory performance. Finally, there was no significant relationship between any of the left or right hemisphere volumes and verbal free recall following the third trial.
Figure 4.
Scatterplots showing the relationship between left (A) and right (B) normalized hippocampal and entorhinal cortex volume and delayed verbal recall.
4. Discussion
The purpose of this study was to determine if older healthy individuals show age-related volume changes in the mesial temporal lobe regions with age, with a specific emphasis on the parahippocampal white matter in the region that includes the perforant pathway. Based on studies in rodent models of aging (Barnes, 1979; Barnes and McNaughton, 1980; Geinisman et al., 1986, 1992) implicating the loss of axon collaterals from the perforant pathway to the dentate gyrus, we hypothesized that similar changes would exist in humans as they age.
The most important finding reported here was that alterations in parahippocampal white matter in the region that includes the perforant pathway occur in healthy older individuals. The strength of this report is that the inclusion of yearly clinical follow-up made it possible to observe cognitive decline in the old participants over time. This allowed the examination of changes in the regions of interest in stable old participants to determine “genuine” age effects. The finding that parahippocampal white matter volumes were reduced in the cognitively stable old cohort suggests that although atrophy in this region occurs as a consequence of early AD-related disease pathology (Kalus et al., 2006; Stoub et al., 2006; Rogalski et al., 2009; Salat et al., 2010; Wang et al., 2010), it is also present, to a lesser extent, during the normal aging process. In addition, Bartzokis (2004) has argued that late-myelinating regions of the brain are more susceptible to myelin breakdown. He cites the parahippocampal region as having a very long cycle of myelination, going into the fifth decade of life which may aid in explaining these findings.
Structural changes in white matter have been reported in the healthy aging population (Allen et al., 2005; Bartzokis et al., 2001, 2003, 2004; Guttmann et al., 1998; Jernigan et al., 2001; Raz et al., 2005; Resnick et al., 2003; Salat et al., 2009; Smith et al., 2007). One of the above studies (Salat et al., 2009), showed a relation between age and white matter volume reduction in multiple regions throughout the brain including the parahippocampal gyrus. However, these authors did not provide a direct comparison of older and young participants, nor did they provide longitudinal follow-up data to track what proportion of their old participants declined in cognitive status over time.
In addition to structural volumetric studies, the integrity of white matter in healthy aging has been investigated using diffusion tensor imaging (DTI) techniques (Head et al., 2004; O’Sullivan et al., 2001; Sullivan et al., 2001; Sullivan et al., 2010; Yassa et al., 2010; Ziegler et al., 2008). Only one of these studies, however, focused on the area of the parahippocampal white matter (Yassa et al., 2010). Using an ultrahigh-resolution protocol and a metric of diffusion not commonly used, these investigators found signal degradation in the perforant pathway in older adults compared to young participants. They did not, however, assess the volume of the white matter in this region.
The results reported here show a significant difference in entorhinal cortex volume between the stable old and young groups. This finding is consistent with previous imaging reports (Du et al., 2006; Fjell et al., 2009; Goncharova et al., 2001; Raz et al., 2005, 2010) that show reduced volumes in healthy aging. However, there have been other studies reporting preservation of entorhinal cortex volume (Insausti et al., 1998; Juottonen et al., 1998). The age-related change in the entorhinal cortex suggests that volume differences in the parahippocampal white matter region may be due to axonal loss in the perforant pathway, as well as other processes such as collateral pruning or demyelination.
The significant reduction in hippocampal volume in the stable old cohort is consistent with the majority of reports in the literature (Head et al., 2008; Jack et al., 2002; Jernigan et al., 2001; Raz et al., 2004, 2005; Rodrigue et al., 2004). However, there have also been human studies (Sheline et al., 1999; Sullivan et al., 2005) and studies in nonhuman primates (Shamy et al., 2006) reporting no changes in hippocampal volume with age.
Human lesion and imaging investigations have shown that the left hippocampus is involved in verbal information processing, while the right processes non-verbal information (Abrahams et al., 1997; deToledo-Morrell et al., 2000a, 2000b; Jones-Gotman, 1986; Maguire et al., 1997; Rosen et al., 2003; Smith and Milner 1981). Consistent with these previous investigations, the present study showed significant relationships between left hippocampal and left entorhinal cortex volumes and delayed verbal recall. In addition, these findings support previous reports demonstrating a relationship between hippocampal volume and delayed memory performance in the healthy aging population (Golomb et al., 1993, 1994, 1996).
In the present study, the relation between parahippocampal white matter volume and episodic memory function was not significant. Because atrophy or degradation of information flow from the entorhinal cortex to the hippocampus would be expected to be subtle in healthy elderly people, this finding is, perhaps, not surprising. It is remarkable, however, that the change in white matter in this region resembles that found in normally-aging rodents, and suggests that this alteration may be fundamental to brain aging across mammalian species. The reduction in white matter volume reported here may reflect not only pruning of afferent and efferent fibers in the region of the parahippocampal gyrus that includes the perforant pathway, but also partial demyelination in remaining fibers, as has been observed in frontal and visual cortex of a non-human primate model of aging (Nielsen and Peters, 2001; Peters and Sethares, 2002; Makris et al., 2007).
Table 1.
Memory recall scores of participants
| Stable Healthy Old |
Declining Healthy Old |
Young | |
|---|---|---|---|
| N | 42 | 9 | 35 |
|
Buschke Verbal Free (Mean Percent Correct ± SD) |
76 ± 10 | 68 ± 10 | 86 ± 10 |
|
Buschke Verbal Delay (Mean Percent Correct ± SD) |
73 ± 15 | 76 ± 10 | 86 ± 10 |
Acknowledgments
This work is supported by grants from the National Institute on Aging, National Institutes of Health P01 AG09466, P30 AG10161 and R01 AG17917 and from the Evelyn F. McKnight Brain Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors report no actual or potential conflicts of interest or financial gains. The data contained in the manuscript being submitted has not been previously published, has not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging. All procedures were approved by the institutional review board of the participating institutions. All authors have reviewed the manuscript and approve of its contents.
References
- Abrahams S, Pickering A, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;23:11–24. doi: 10.1016/s0028-3932(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol. Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J. Comp. Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: A neuropsychological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J. Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH. Age-related changes in frontal and temporal volume in men: a magnetic resonance imaging study. Arch. Gen. Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer’s disease: a magnetic resonance imaging study. Arch. Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogenous age-related breakdown of white matter structural integrity: implications for cortical ”disconnection” in aging and Alzheimer’s disease. Neurobiol. Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer related changes. Acta. Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–288. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer’s disease related cortical lesions. J. Neural Transm. Suppl. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- Buschke H, Gorber E. Genuine memory deficits in age-associated memory impairment. Dev. Neuropsychol. 1986;2:287–307. [Google Scholar]
- Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, Chui HC, Schuff N, Weiner MW. Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol. Aging. 2002;23:1–8. doi: 10.1016/s0197-4580(02)00130-6. [DOI] [PubMed] [Google Scholar]
- Chao LL, Mueller SG, Buckley ST, Peek K, Raptentsetseng S, Elman J, Yaffe K, Miller BL, Kramer JH, Madison C, Mungas D, Schuff N, Weiner MW. Evidence of neurodegeneration in brains of older adults who do not yet fulfill MCI criteria. Neurobiol. Aging. 2010;31:368–377. doi: 10.1016/j.neurobiolaging.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deToledo-Morrell L, Sullivan MP, Morrell F, Wilson RS, Bennett DA, Spencer S. Alzheimer's disease: in vivo detection of differential vulnerability of brain regions. Neurobiol. Aging. 1997;18:463–468. doi: 10.1016/s0197-4580(97)00114-0. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer’s disease. Hippocampus. 2000a;10:136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer’s disease: In vivo detection of entorhinal cortex atrophy. Ann. N.Y. Acad. Sci. 2000b;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol. Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampla and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer’s disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol. Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J. Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. A practical method for grading the mental status of patients for the clinician. J. Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Golomb J, de Leon MJ, Kluger A, Tarshish GC, Ferris SH. Hippocampal atrophy in normal human aging: an association with recent memory impairment. Arch. Neurol. 1993;50:967–976. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Convit A, Mittelman M, Cohen J, Rusinek SH, De Santi S, George AE. Hippocampal formation size in normal human aging: a correlate of delayed secondary memory performance. Learning & Memory. 1994;1:45–54. [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Mittelman M, Cohen J, George AE. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- Goncharova II, Dickerson BC, Stoub TR, deToledo-Morrell L. MRi of human entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiol. Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnstrude IS, Ashbumer J, Henson RN, Friston KJ, Frakowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H. Genuine memory deficits in dementia. Dev. Neuropsychol. 1987;3:13–36. [Google Scholar]
- Guttmann CRG, Jolesz FA, Kikinins R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes in normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: Cell specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Insausti R, Insausti AM, Sobreviela MT, Salinas A, Martinez Penuela JM. Human medial temporal lobe in aging: Anatomical basis of memory preservation. Microscopy Research and Technique. 1998;43:8–15. doi: 10.1002/(SICI)1097-0029(19981001)43:1<8::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jessen F, Feven L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L. Volume reduction of the entorhinal cortex in subjective memeory impairment. Neurobiol. Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M. Right hippocampal excision impairs learning and recall of a list of abstract designs. Neuropsychologia. 1986;24:659–670. doi: 10.1016/0028-3932(86)90005-9. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkanen K, Soininen H. Volumes of the entorhinal cortex and perirhinal cortices in Alzheimer’s disease. Neurobiol. Aging. 1998;19:15–22. doi: 10.1016/s0197-4580(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Ballunat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, Nyffeler T, Buri C, Federspiel A, Kunz D, Schroth G, Kiefer C. Examining the gateway to the limbic system with diffusion tensor imaging: The perforant pathway in dementia. Neuroimage. 2006;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann. Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale AM, Benner T, Wald LL, Wu O, Tuch DS, Caviness VS, Moore TL, Killiany RJ, Moss MB, Rosene DL. Frontal connections and cognitive changes in normal aging rhesus monkeys: A DTI study. Neurobiol. Aging. 2007;28:1556–1567. doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers. J. Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadian EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Chin EY, Cochran EJ, Beckett LA, Bennett DA, Kordower H. Entorhinal cortex beta amyloid load in individuals with mild cognitive impairment. Exp. Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Peters A. The effects of aging on the frequency of nerve fibers in rhesus monkey striate cortex. Neurobiol. Aging. 2000;21:621–628. doi: 10.1016/s0197-4580(00)00169-x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical disconnection as a mechanism for age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus collosum of the monkey. J. Comp. Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE>, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and ourcome. Arch. Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobio. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue K, Williamson A, Acker J. Aging, sexual dimorphism, and hemisheric asymmetry of the cerebral cortex: replicability of regional diffrences in volume. Neurobiol. Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging heathly adults: General trends, individual differences and modifiers. Cereb. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J. Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J. Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Murphy CM, deToledo-Morrell L, Shah RC, Moseley ME, Bammer R, Stebbins GT. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment a diffusion tensor imaging study. Behav. Neurol. 2009;21:51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A, Prull MW, Gabrieli JDE, Stoub T, O’Hara R, Friedman L, Yesavage JA, deToledo-Morrell L. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav. Neurosci. 2003;117:1150–1160. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Van Der Kouwe AJW, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol. Aging. 2010;31:244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predit rocognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol. Aging. 2006;27:1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophsin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: A voxel-based morhphometric study in healthy elderly. Neurobiol. Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, deToledo-Morrell L. MRI predictors of risk of incident Alzheimer’s disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proc. Natl, Acad. Sci. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol. Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol. Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T, Pennanen C, Tapiola M, Tervo S, Kivipelto M, Hanninen T, Pihlajamaki M, Laakso MP, Hallikainen M, Hamalainen A, Vanhanen M, Helkala EL, Vanninen R, Nissinen A, Rossi R, Frisoni GB, Soininen H. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol. Aging. 2008;29:31–38. doi: 10.1016/j.neurobiolaging.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res. 1975a;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975b;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Wang C, Stebbins GT, Medina D, Shah RC, Bammer R, Moseley ME, deToledo-Morrell L. Atrophy and dysfunction of parahippocampal white matter in mild Alzheimer’s disease. Neurobiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.020. [Epub ahead of press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Sullivan MP, deToledo-Morrell L, Stebbins GT, Bennett DA, Morrell F. Association of memory and cognition in Alzheimer’s disease with volumetric estimates of temporal lobe structures. Neuropsychology. 1996;10:459–463. [Google Scholar]
- Yassa M, Tugan Muftuler L, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc. Natl, Acad. Sci. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J. Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]