Abstract
Objective
To evaluate the feasibility of utilizing [18F]-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT) to detect and quantify systemic inflammation in psoriasis patients.
Design
Case series with a nested case-control study.
Setting
Referral dermatology and preventive cardiology practices.
Participants
Six patients with psoriasis affecting >10% body surface area and 4 controls age and sex matched to 4 psoriasis patients for a nested case-control study.
Main Outcome Measures
FDG uptake in the liver, musculoskeletal structures, and aorta measured by mean Standardized Uptake Value (SUV), a measure of FDG tracer uptake by macrophages and other inflammatory cells.
Results
FDG-PET/CT identified numerous foci of inflammation in 6 patients with psoriasis within the skin, liver, joints, tendons, and aorta. Inflammation in the joints was observed in a patient with psoriatic arthritis as well as in 1 patient with no history of joint disease or joint symptoms. In a nested case-control study, FDG-PET/CT imaging demonstrated increased vascular inflammation in multiple segments of the aorta compared to controls. These findings persisted after adjustment for traditional cardiovascular risk factors in multivariate analysis (mean beta 0.33, p<0.001). Patients with psoriasis further demonstrated increased hepatic inflammation after adjusting for cardiovascular risk factors (beta 0.18, p<0.001), but the association was no longer significant when adjusted for alcohol intake (beta −0.25, p=0.07).
Conclusions
FDG-PET/CT is a sensitive tool for identifying inflammation and can be used to identify clinically observed inflammation in the skin and subclinical inflammation in the blood vessels, joints, and liver of patients with psoriasis.
Psoriasis is a chronic inflammatory disease affecting 2–3% of the adult population.1,2 It is associated with an inflammatory arthritis (psoriatic arthritis) in about 10% of patients with much higher frequencies in patients with more extensive skin disease.3 Psoriasis is also associated with increases in markers of inflammation in the skin and blood and increasingly is thought to be a systemic inflammatory disease and risk factor for incident diabetes, myocardial infarction, stroke, and premature cardiovascular (CV) death.4–18 The mechanism behind these associated comorbidities, however, remains unknown. It has been widely suggested that a possible common pathway linking psoriasis to metabolic and CV disease is chronic inflammation mediated by Th17 and Th1 cells.19–23 Yet despite evidence of systemic inflammation in psoriasis, few techniques have been successfully employed to identify and quantify locations of inflammation in vivo in these patients. Because traditional markers of systemic inflammation such as high sensitivity C-reactive protein (hsCRP) and erythrocyte sedimentation rate (ESR) only modestly correlate with psoriasis severity and do not provide regional information about disease involvement, novel assessments of inflammation in vivo are particularly important to understanding the impact of psoriasis on systemic inflammation and systemic comorbidities.24
The development of [18F]-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT), a validated technique used extensively in cancer and neuroimaging, enables highly precise, novel measurements of inflammatory activity including vascular, visceral, and whole body inflammation in vivo.25 Previous studies using animal models and immunohistochemistry in humans indicate that FDG-PET/CT is exquisitely sensitive for detecting macrophage activity, an important source of cellular inflammation in numerous tissues, including early stages of atherosclerosis in vessel walls.26–30 The high sensitivity of FDG-PET/CT to detect early, subclinical inflammation with minimal error from operator dependence, demonstrated in studies of vasculitis29,30, atherosclerosis31–34, and joint disease35–37, has expanded its use as a novel investigative tool to detect regional inflammatory disease. Importantly, observational studies indicate that aortic and carotid inflammation measured by FDG-PET/CT are strong predictors of future major vascular events.34,38,39 Furthermore, randomized controlled trials demonstrate that statin and therapeutic life style interventions improve vascular inflammation as measured by FDG-PET within 12 weeks and 16 months respectively.40,41
FDG-PET/CT therefore represents an innovative approach to studying systemic inflammation in a manner that is sensitive, quantifiable and anatomically localizable. Furthermore, inflammation detected by FDG-PET/CT has been shown to be responsive to modulation by anti-inflammatory medical treatments and predictive of future vascular events. Such a tool could therefore be valuable in further defining systemic inflammation in patients with psoriasis. We therefore conducted a proof-of-principle study to describe FDG tracer uptake consistent with inflammation in the skin, liver, blood vessels, and joints in a series of patients with moderate to severe psoriasis. The second part of the pilot study attempts to determine if FDG-PET/CT can discriminate systemic inflammation in a subset of our psoriasis patients compared to healthy controls using a nested case-control design.
METHODS
We conducted a proof-of-principle study (n=6) evaluating the role of FDG-PET/CT in psoriasis to detect sites of inflammation. This study enrolled patients ages 18 to 70 with psoriasis involving a body surface area (BSA) >10%. Because this study was designed to demonstrate the feasibility of applying FDG-PET/CT to this novel population, we allowed inclusion of treated psoriasis patients to ensure a broad range of patients and improve the generalizability of our experience. Therefore, psoriasis patients undergoing active light therapy, topical therapy, or being treated with stable doses (not changed within 12 weeks of study enrollment) of oral systemic medications (e.g. methotrexate) or biologics were eligible. We excluded individuals with diabetes mellitus, tobacco use, history of CV disease, uncontrolled hypertension (defined as systolic blood pressure >180 mmHg or diastolic blood pressure >95 mmHg), warfarin use, coagulopathy, pregnancy or lactation, regular use of alcoholic beverages (>2 drinks per day), non-skin malignancy within 5 years, HIV positive status, major surgery within 3 months of imaging, history of intravenous drug use within the last year, research drug study participation in the 6 weeks prior to imaging, or active infection within preceding 72 hours or other serious medical or psychiatric condition.
We then conducted a sub-study to evaluate the ability of this modality to discriminate inflammation with high sensitivity by comparing psoriasis patients from our case series to healthy controls matched based on age, sex and a restricted BMI <30. Of the 6 psoriasis patients in our case series, two were excluded from this analysis: one had an existing diagnosis of psoriatic arthritis (a known cause of systemic inflammation), and a second had a BMI of 35.7 (obesity is associated with systemic inflammation). The sub-study focused on quantification of subclinical vascular inflammation and hepatic inflammation in both groups to test the hypothesis that psoriasis is associated with increased inflammation in vivo compared to controls. To compare vascular inflammation assessed by FDG-PET/CT, we matched psoriasis patients by age and gender to prospectively recruited controls (n=3) and a single historical control from another FDG-PET/CT study at our institution. The inclusion and exclusion criteria for controls were similar to those for our psoriasis subjects except controls were excluded if they had known chronic inflammatory diseases. All 4 psoriasis patients included in this analysis would have otherwise qualified as healthy controls (if they did not have psoriasis) based on the criteria utilized.
All subjects underwent a whole body FDG-PET/CT scan using the standard protocol described herein. Subjects fasted for 8 hours prior to the FDG-PET/CT scan. Fasting serum glucose levels were checked by fingerstick to assure glucose<150mg/dL prior to FDG administration. Whole body PET/CT image acquisition [Gemini TF, Philips Medical Systems] commenced ~60 minutes after intravenous administration of 140 µCi/kg FDG. Axial, sagittal, and coronal PET reconstructions were interpreted with and without attenuation correction using non-contrast CT images for attenuation correction and anatomical correlation of FDG uptake. After qualitative review of PET and CT images, 2D circular regions of interests (ROI) were manually placed on PET images around the external aortic contour, around the hepatic margin, and around articular spaces on all transverse slices passing through these structures using low dose CT images for anatomic guidance. Mean standardized uptake values (SUV) and areas of each ROI were measured for each slice using dedicated PET/CT image analysis software which auto-calculates the SUV per slice within the specified ROI [Extended Brilliance Workstation, Philips Healthcare, Bothell, WA]. Total numbers of slices differ slightly from subject to subject depending on body habitus and anatomical variation, but these differences do not alter interpretation of regional data.33 Details of this methodology have been published previously by our group and have been validated for the quantification of atherosclerosis, hepatic, and joint inflammation.33,37,42–45
In our case-series (n=6), our primary outcomes were defined as any finding on FDG-PET/CT consistent with inflammation, measured by SUV, the unit for FDG uptake, in the vasculature, viscera, and musculoskeletal system. Vascular SUV were compared descriptively to expected norms based on age and sex from published literature.31 In the nested case-control sub-study, our primary outcome was aortic mean SUV, defined as the average of all mean SUV recorded from sequential 2D ROI in the aorta. Aortic mean SUV was calculated for 5 segments of the aorta: ascending aorta, aortic arch, descending thoracic aorta, suprarenal abdominal aorta, and infrarenal abdominal aorta. These segments were defined to consider separately various regions of aortic disease that are associated with different clinical phenotypes (e.g. aortic arch disease with stroke, abdominal aortic disease with abdominal aortic aneurysm). As a secondary analysis, we also examined hepatic SUV in psoriasis and controls. Unadjusted analyses were performed using two-sided t-tests and Wilcoxon-Mann-Whitney tests for continuous data and Fisher’s exact tests for dichotomous data. Linear regression was performed to adjust for known CV risk factors (age, gender, hypertension, LDL cholesterol, and body mass index (BMI)) with mean SUV as the outcome. Hypertension in our models was adjusted as a dichotomous variable; all other variables were continuous. To study the effect of psoriasis on hepatic inflammation, we adjusted for CV risk factors including BMI, and further adjusted analysis for alcohol use in the multivariate regression model. Finally, to accommodate within subject correlation of SUV, we performed random effects regression models. Given that we did not observe any difference in estimates for psoriasis, we present the beta-coefficient from the linear regression models. All analyses were performed in STATA 11 [StataCorp, College Station, Texas]. The study was approved by the Institutional Review Board at the University of Pennsylvania and complies with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
RESULTS
Psoriasis demonstrates increased FDG uptake in the body detectable by PET/CT
The characteristics and measurements of FDG uptake in the vasculature, liver, and musculoskeletal structures of all 6 patients with psoriasis in the case series are shown in Table 1. Psoriasis patients had the disease for a median of 14 years (interquartile range 3–24) and had a median BSA of 14% (IQR 11–19%), median Physician’s Global Assessment of 2.5 (IQR 2.0–2.7) and median Psoriasis Area Severity Index 9.9 (IQR 7.2–12.1) (Table 1). Current psoriasis therapies (within three months) included topical steroids (N=4), combination treatment with methotrexate and abatacept (N=1), and narrow band phototherapy (N=1). Thus 5 patients were not on systemic treatment or phototherapy at the time of the study. One patient (i.e. the individual treated with methotrexate/abatacept) had a diagnosis of psoriatic arthritis established by rheumatology that was clinically symptomatic, and another patient had a history of anterior cruciate ligament repair 12 months prior to the study and was asymptomatic. The remaining patients had no history of joint disease or joint symptoms. CV risk factors are noted in Table 1, with three patients having diagnosed hyperlipidemia on statins and one having hypertension on a single agent.
Table 1.
Six Patients with Psoriasis Demonstrate Whole Body Inflammation Detected by FDG-PET CT
| ID | Cardiovascular History |
Psoriasis History |
Current psoriasis treatment (within 3 months) |
Past psoriasis treatments (greater than 3 months prior) |
Psoriatic Arthritis |
FDG-PET/CT Vascular, SUVmean (SD) |
FDG PET/CT Liver, SUVmean (SD) |
FDG-PET/CT Musculoskeletal | |
|---|---|---|---|---|---|---|---|---|---|
| Subject 1 41-year-old Malea |
No CV history HDL: 36 LDL: 106 BMI: 29.9 hsCRP: 2.9 Medications: Fish oil |
Duration: 20 years BSA: 13 PGA: 2 PASI: 7.2 |
|
|
No | AsA | 1.84 (0.08) |
2.28 (0.36) |
|
| Arch | 1.70 (0.06) | ||||||||
| TA | 1.59 (0.16) | ||||||||
| SRAA | 1.75 (0.15) | ||||||||
| IRAA | 1.61 (0.13) | ||||||||
| Subject 2 40-year-old Male |
Hyperlipidemia HDL: 33 LDL: 161 BMI: 36.7 hsCRP: 2.5 Medications: Statin |
Duration: 24 years BSA: 20 PGA: 2.66 PASI: 12.1 |
|
|
Yes |
AsA | 1.67 (0.05) | 1.76 (0.19) |
|
| Arch | 1.53 (0.09) | ||||||||
| TA | 1.43 (0.14) | ||||||||
| SRAA | 1.50 (0.07) | ||||||||
| IRAA | 1.37 (0.13) | ||||||||
| Subject 3 56-year-old Malea |
Hyperlipidemia HDL: 52 LDL: 90 BMI: 29.1 hsCRP: 0.1 Medications: Statin, aspirin, fish oil |
Duration: 8 years BSA: 19 PGA: 3.3 PASI: 17 |
|
|
No | AsA | 1.51 (0.06) | 1.64 (0.23) |
|
| Arch | 1.40 (0.06) | ||||||||
| TA | 1.72 (0.10) | ||||||||
| SRAA | 1.40 (0.07) | ||||||||
| IRAA | 1.29 (0.15) | ||||||||
| Subject 4 53-year-old Male |
Hyperlipidemia, hypertension HDL: 36 LDL: 69 BMI: 35.7 hsCRP: 2.0 Medications: Statin, diuretic, aspirin, fish oil |
Duration: 25 years BSA: 15 PGA: 2.8 PASI: 10.1 |
|
|
No | AsA | 1.72 (0.04) | 1.56 (0.50) |
|
| Arch | 1.62 (0.10) | ||||||||
| TA | 1.45 (0.16) | ||||||||
| SRAA | 1.47 (0.05) | ||||||||
| IRAA | 1.50 (0.14) | ||||||||
| Subject 5 58-year-old Femalea |
No CV history HDL: 87 LDL:104 BMI: 18.3 hsCRP: n/a Medications: None |
Duration: 2 years BSA: 11 PGA: 2 PASI: 9.6 |
|
|
No | AsA | 1.52 (0.04) | 1.80 (0.14) |
|
| Arch | 1.33 (0.08) | ||||||||
| TA | 1.41 (0.06) | ||||||||
| SRAA | 1.34 (0.05) | ||||||||
| IRAA | 1.47 (0.13) | ||||||||
| Subject 6 44-year-old Malea |
No CV history HDL: 38 LDL: 165 BMI: 24.3 hsCRP: 8.4 Medications: None |
Duration: 3 years BSA: 10.2 PGA: 2.3 PASI: 6.5 |
|
|
No | AsA | 1.40 (0.00) | 1.84 (0.26) |
|
| Arch | 1.42 (0.42) | ||||||||
| TA | 1.46 (0.08) | ||||||||
| SRAA | 1.36 (0.11) | ||||||||
| IRAA | 1.19 (0.18) | ||||||||
Subject is included in the nested case-control study.
AsA – Ascending aorta; Arch – Aortic arch; TA – Descending thoracic aorta; SRAA – Suprarenal abdominal aorta; IRAA – Infrarenal abdominal aorta; CV – cardiovascular; NB-UVB – narrow band ultraviolet B therapy; BMI – body mass index; SUV – Standardized Uptake Value.
Whole body FDG-PET/CT imaging revealed areas of systemic inflammation including vascular, articular, periarticular, visceral, and skin territories in patients with psoriasis. Focal areas of inflammation in the skin observed clinically as areas of plaque psoriasis corresponded to areas of skin inflammation observed on FDG-PET/CT (Figure 1). Additionally, among the 5 patients without diagnosis or symptoms of psoriatic arthritis, vascular inflammation in all 5 aortic segments was increased compared to age-adjusted normal values (between 1.0–1.2 SUV corrected for the scanner utilized).32,33 FDG-PET/CT of the liver also demonstrated increased metabolic activity, likely indicative of increased hepatic inflammation. In addition, both clinical and sub-clinical joint inflammation were detected in the sample (Table 1). Of note, one patient without psoriatic arthritis, joint symptoms, or previous joint surgery demonstrated focal areas of FDG uptake on FDG-PET/CT in various tendons, entheses, and joint spaces, particularly in the knees (with maximum SUV of 3.0 on the right and 2.2 on the left), consistent with inflammatory arthritis.37 Similar findings of tendon, entheses, and joint inflammation were demonstrated on FDG-PET/CT for the patient with clinical psoriatic arthritis (Table 1).
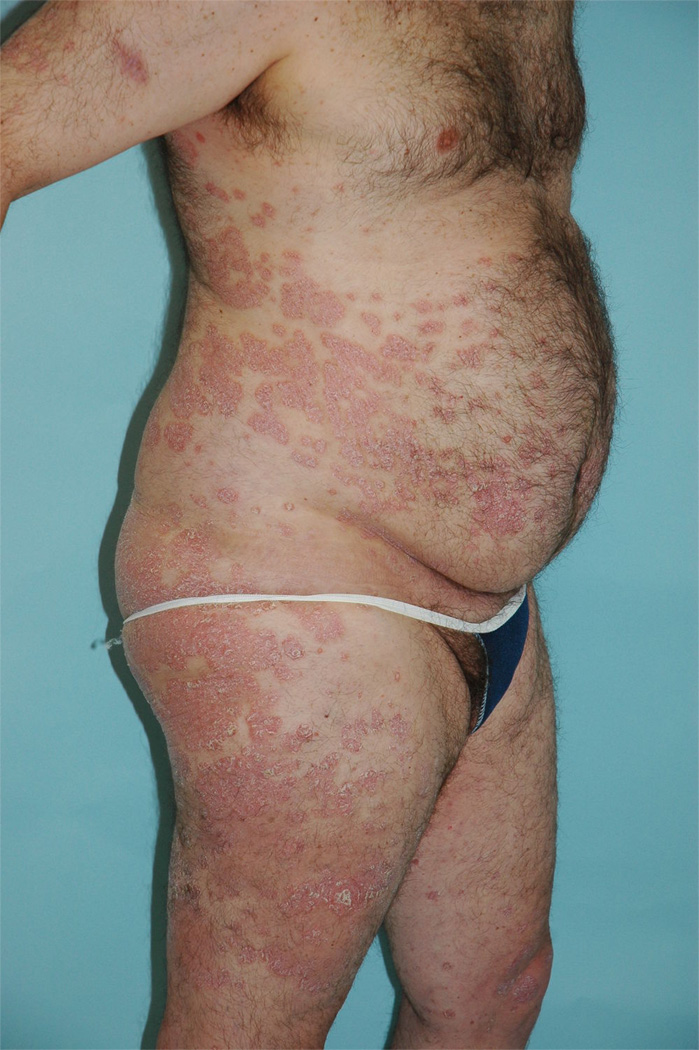
Figure 1. FDG-PET/CT imaging of skin correlates with observed skin inflammation.
(a) Photograph of patient with psoriasis showing extensive multifocal plaques. (b) Corresponding PET image from FDG-PET/CT study in same patient demonstrates skin inflammation in similar distribution.
A nested case-control study shows differences in vascular and hepatic inflammation detected by FDG-PET/CT
We next matched controls to four patients from our psoriasis sample by age and gender. Subjects in the sub-study were restricted to BMI <30. The characteristics of this nested case-control study are shown in Table 2. Patients with psoriasis (n=4, 75% male, age 50 (43–57)) had similar, low-risk CV profiles compared to controls (n=4, 75% male, age 49 (43–52)) (Table 2). Both psoriasis patients and controls also had median BMI in the overweight category (26.9 (21.5–29.5) vs. 29.7 (28.5–29.9), respectively). We show an image from the PET component of a FDG-PET/CT study in a patient with psoriasis and a control patient as an example of how the difference in inflammation detected between these two subjects is visualized using FDG-PET/CT (Figure 2).
Table 2.
Demographic & Laboratory Results for Psoriasis Patients and Controls
| Psoriasis Median (IQR) n=4 |
Controls Median (IQR) n=4 |
p-value | Normal Values |
|
|---|---|---|---|---|
| Age | 50 (43–57) | 49 (43–52) | 0.66 | - |
| Male, count (%) | 3 (75%) | 3 (75%) | 1.00 | - |
| BSA, % | 12 (10.6–16) | - | - | - |
| Physician’s Global Assessment (PGA) | 2.2 (2.0–2.8) | - | - | - |
| Psoriasis Area Severity Index (PASI) | 8.4 (6.9–13.3) | - | - | - |
| Diagnosed psoriatic arthritis, count (%) | 0 (0%) | - | ||
| Tobacco use, count (%) | 0 (0%) | 0 (0%) | 1.00 | - |
| BMI | 26.9 (21.5–29.5) | 29.7 (28.5–29.9) | 0.22 | 18.5–24.9 |
| Fasting blood glucose | 94 (89–107) | 86 (72–96) | 0.23 | 70–99 mg/dL |
| Systolic blood pressure | 133 (123–142) | 124 (118–134) | 0.48 | 90–140 mmHg |
| Diastolic blood pressure | 81 (72–84) | 73 (69–80) | 0.56 | 60–90 mmHg |
| Diagnosed hypertension, count (%) | 1 (25%) | 1 (25%) | 1.00 | - |
| Antihypertensive therapy, count (%) | 0 (0%) | 1 (25%) | 1.00 | - |
| Total Cholesterol | 214 (182–225) | 188 (186–206)a | 0.66 | ≤200 mg/dL |
| Triglycerides | 120 (84–194) | 262 (165–290)a | 0.15 | ≤150 mg/dL |
| HDL | 45 (37–70) | 50 (18–66)a | 0.66 | ≥40 mg/dL |
| LDL | 105 (97–136) | 118 (62–123)a | 0.58 | ≤100 mg/dL |
| Statin therapy, count (%) | 1 (25%) | 0 (0%) | 1.00 | - |
| hsCRP | 2.9 (0.1–8.4)a | 1.5 (0.4–2.0) | 0.37 | ≤3.0 mg/L |
| ALT | 38 (18–68) | 31 (22–51) | 0.73 | 17–63 U/L |
| AST | 28 (19–43) | 41 (23–62) | 0.50 | 15–41 U/L |
| Alkaline phosphatase | 71 (56–92) | 71 (57–95) | 0.91 | 32–91 U/L |
| Total bilirubin | 0.8 (0.8–1.0) | 0.8 (0.6–1.0) | 0.52 | 0.3–1.2 mg/dL |
| Alcohol consumption, count (%) | ||||
| Non-drinker | 2 (50%) | 3 (75%) | 1.00 | - |
| Social drinker (<7 drinks/week) | 2 (50%) | 1 (25%) | - |
n=3
IQR – Interquartile Range; n/a – not available
Diabetes mellitus, a traditional cardiovascular risk factor, is excluded from this table as it was an exclusion criterion in this study.
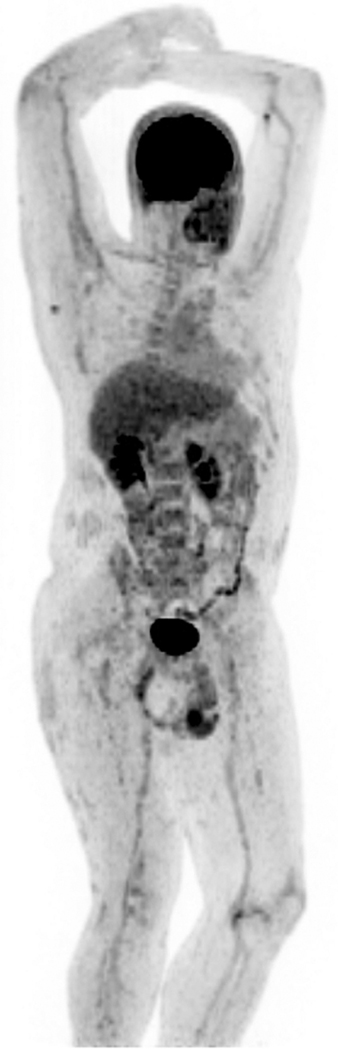
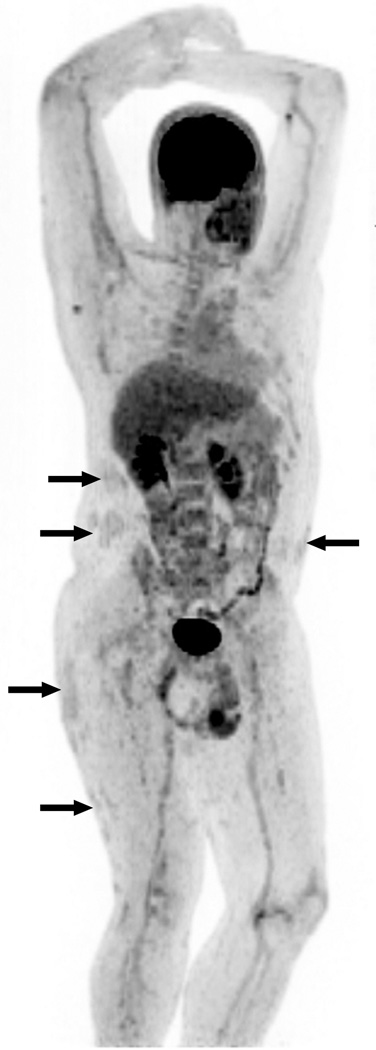
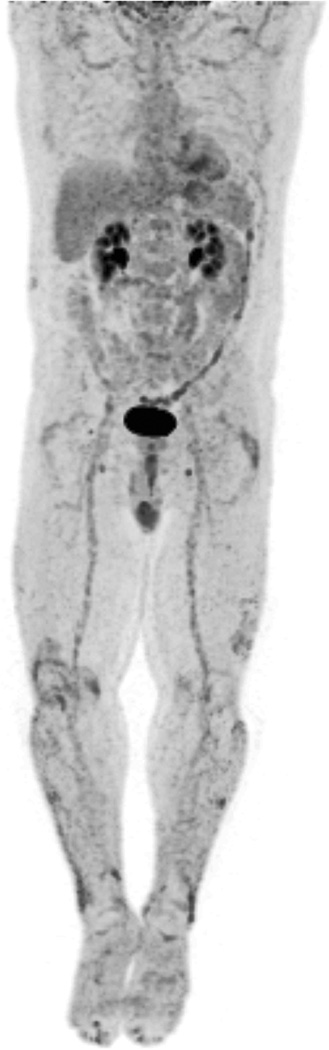
Figure 2. FDG-PET/CT imaging Shows Multifocal Inflammation in Patient with Psoriasis Compared to Control.
PET image from FDG-PET/CT study in patient with psoriasis is shown at left. FDG-PET image from control patient is shown at right for comparison (no abnormal FDG uptake). (A) FDG uptake in right knee joint (SUV 3.0) and distal right quadriceps tendon, left trochanteric bursa, and left ankle in asymptomatic psoriasis patient. (B) Moderate diffusely increased FDG uptake throughout liver (SUV 1.64) consistent with increased hepatic inflammation. (C) Diffuse FDG uptake in aortic wall (SUV 1.29–1.72) and in femoral arterial tree, consistent with vascular inflammation. (D) Focal areas of FDG uptake in skin consistent with inflammation in thick plaques in lower extremities.
From these subjects, we analyzed a total of 386 slices of the aorta in patients with psoriasis and compared them to 317 slices of the aorta in controls. We also performed quantitative analysis of the liver using 161 slices for psoriasis and compared them to 154 slices for controls. FDG-PET/CT detected greater inflammation in the aorta in patients with psoriasis compared to controls (p<0.001). After stratifying analysis to specific segments of the aorta, we report that the SUV within each aortic segment were notably higher in psoriasis patients compared to controls (Table 3). In multivariable regression, psoriasis remained highly associated with SUV in each aortic segment after adjusting for traditional CV factors and BMI compared to controls, and we show the beta coefficient for psoriasis in the multivariate model (Table 4). Finally, compared to controls, patients with psoriasis demonstrated increased hepatic inflammation in multivariate analysis adjusting for known confounders and risk factors for liver disease including age, gender, plasma triglycerides and BMI (beta 0.18, p<0.001). However, when the model was further adjusted for alcohol use (beta 0.76, p<0.001), the relationship between hepatic inflammation and psoriasis was no longer significant (beta −0.25, p=0.07).
Table 3.
Psoriasis is associated with Increased Vascular Inflammation in the Aorta
| Region | Vascular Inflammation, mean SUV (SD)a | ||
|---|---|---|---|
| Psoriasis | Controls | P-valueb | |
| Ascending Aorta | 1.56 (0.16) | 1.49 (0.08) | 0.015 |
| Aortic Arch | 1.45 (0.15) | 1.38 (0.14) | 0.087 |
| Descending Thoracic Aorta | 1.54 (0.16) | 1.43 (0.14) | <0.001 |
| Suprarenal Abdominal Aorta | 1.46 (0.20) | 1.37 (0.09) | 0.002 |
| Infrarenal Abdominal Aorta | 1.40 (0.22) | 1.29 (0.15) | <0.001 |
Vascular inflammation for each region was calculated by the weighted average of mean SUV per slice in the specified aortic segment. Mean SUV indicates the average degree of FDG uptake per slice and measures the degree of inflammatory activity.
P-values are reported for a two-sided t-test.
Table 4.
Association of Psoriasis and Vascular and Hepatic Inflammation After Adjusting for Confounding Variables
| Beta-coefficient for Psoriasis on mean SUV (p-value)a | ||
|---|---|---|
| Anatomic Region | Model adjusted for age, gender, and BMI | Model adjusted for age, gender, BMI, hypertension, and LDL cholesterol |
| Ascending Aorta | 0.17 (<0.001) | 0.37 (<0.001) |
| Aortic Arch | 0.16 (0.002) | 0.28 (<0.001) |
| Descending Thoracic Aorta | 0.21 (<0.001) | 0.27 (<0.001) |
| Suprarenal Abdominal Aorta | 0.25 (<0.001) | 0.40 (<0.001) |
| Infrarenal Abdominal Aorta | 0.20 (<0.001) | 0.32(<0.001) |
| Model adjusted for age, gender, BMI, triglycerides | Model adjusted for age, gender, BMI, triglycerides, and alcohol | |
| Liver | 0.28 (<0.001) | −0.25 (0.07) |
Because no patients in this study had diabetes mellitus or tobacco use, diabetes mellitus status and tobacco use were not included in the fully adjusted model.
COMMENT
To our knowledge, this is the first description of in vivo whole body inflammation in psoriasis detected by FDG-PET/CT simultaneously in three tissues: viscera, vasculature, and musculoskeletal structures. We demonstrate that FDG-PET/CT, a technique validated to assess vascular inflammation, can be employed in patients with psoriasis to visualize and quantify anatomic regions of inflammation.33,42,43,46–48 In our case series, FDG-PET/CT detected numerous areas of inflammation in patients with psoriasis, including increased inflammation in the blood vessels, in the liver, in articular and periarticular structures, and in the skin. Interestingly, all of these findings occurred in psoriasis patients who otherwise felt well (only one patient who had an established diagnosis of psoriatic arthritis had joint symptoms), and had no clinically significant abnormalities in their laboratory data except for one patient (subject 6) who had an elevated hs-CRP of 8.4 mg/L. These foci of inflammatory activity were observed with levels of inflammation far exceeding the range of normal for joint, liver, and vascular inflammation despite a lack of evidence of clinical disease.45
In our nested case-control study, FDG-PET/CT imaging revealed vascular inflammation more severe than age and gender-matched controls. The corresponding magnitude of SUV difference observed between psoriasis patients and controls (0.20) in multiple segments of the aorta is equivalent to the magnitude of vascular inflammation observed over two additional decades of aging.33 Furthermore, FDG-PET/CT imaging of psoriasis patients demonstrated high risk findings49,50, including diffusely increased vascular inflammation in each segment of the aorta that remained significant after adjusting for traditional CV risk factors and BMI. We also observed that psoriasis was associated with increased hepatic inflammation in multivariate analysis adjusting for confounders and risk factors for liver disease including plasma triglycerides and BMI. This relationship however was no longer significant when adjusted for alcohol intake, suggesting that either patients underreported their alcohol use, because inclusion in the study permitted only light drinking (less than two drinks per day), or that patients with psoriasis may be more susceptible to liver dysfunction in the face of alcohol use. Despite this observation, this degree of hepatic inflammation is similar to that seen in patients with hepatic steatosis or chronic active hepatitis.43 This finding warrants careful follow up and further study, and may present a compelling opportunity to explore the concept of the “psoriatic liver.” Finally, our observations of musculoskeletal inflammation are consistent with previous studies using conventional imaging techniques to demonstrate findings consistent with psoriatic arthritis such as articular inflammation and enthesitis in patients with psoriasis.51–53 In particular, the diffuse distribution of subclinical articular and periarticular involvement observed on imaging suggests that FDG-PET/CT may be a more feasible approach for detecting subclinical psoriatic arthritis than traditional imaging modalities such as ultrasound or MRI.
The findings of excess vascular, hepatic, musculoskeletal, and cutaneous inflammation on FDG-PET/CT are novel and suggest the potential of FDG-PET/CT technology to investigate systemic inflammation. The underlying inflammation linking chronic inflammatory disease states such as atherosclerosis, metabolic syndrome, diabetes mellitus, and psoriatic arthritis with psoriasis is captured in vivo by FDG-PET/CT, thereby providing a measureable phenotype (i.e. biomarker) in a dynamic disease such as psoriasis. In this study, we demonstrate this novel application of FDG-PET/CT, which has exquisite sensitivity for detecting picomolar to nanomolar concentrations of glucose uptake with inflammatory cells. We further report a systematic approach to image analysis which can be used in future studies to regionally and globally quantify inflammation in the aorta, liver and joints.
We note, however, that the findings we report are based on a pilot study which has important limitations, and thus additional studies are necessary to confirm and extend our findings. First, the small sample size and referral-based source of our patients may affect the generalizability of our results. However, our study is comparable in size and design to other landmark studies using FDG-PET/CT in inflammatory disease states.25,58,59 We also note that use of FDG-PET/CT is limited by the need for sophisticated hardware and software that may not be widely available, the need for subjects to be willing to fast for eight hours, and radiation exposure similar to that of a standard contrast-enhanced CT scan. As a result, patients may need to be highly motivated to enter studies using FDG-PET/CT, which may lead to particular challenges in recruiting healthy controls. Additionally, in this pilot study subjects were not required to undergo washout of topical or ultraviolet light treatments, and systemic treatments were permissible as long as the dose had been stable for 3 months. Thus, additional studies in psoriasis patients who are treatment naïve or have washouts of psoriasis therapies will be necessary to further interpret our findings. Furthermore, the nested-case control study is subject to error introduced by selection bias and confounding. For example, it is possible that one group could have been more health conscious than the other (i.e. selection bias), although the laboratory and anthropometric data do not support this possibility as a potential source of error. We carefully adjusted for confounding variables on which we had detailed data, however, incomplete measurement of confounders such as diet and exercise could affect our measurement of association. Additionally, while our study attempted to adjust for LDL cholesterol and hypertension, it is possible that incomplete adjustment for these confounders still exist and we did not have LDL data for one of the controls. Finally, three patients with psoriasis in the case series, and one patient with psoriasis in the nested case-control study were on active statin therapy. While statins may interfere with vascular inflammation, we would expect that they attenuate vascular inflammation, as demonstrated in a previously published clinical trial using FDG-PET/CT,40 and therefore would bias our results towards the null. In addition, we note that none of the study participants had existing CV disease and most CV risk prediction tools, such as the Framingham Risk Score, use current blood pressure and LDL cholesterol measurements to assess CV risk without regard to medication usage because biometric data is more significantly correlated with CV outcomes.50
In this study, we demonstrate that FDG-PET/CT represents a powerful molecular imaging modality to assess whole body inflammation in association with psoriasis. Further studies are needed to 1) confirm and extend our results of increased vascular and hepatic inflammation in a larger series of patients, carefully selecting cases and controls and measuring confounding variables to determine if increased inflammation measured by FGD-PET is due to psoriasis, its treatments, or other associated factors; 2) understand how to relate these findings to clinical prognosis; and 3) assess the effect of psoriasis treatment on the inflammation observed in various tissues.
Acknowledgement
Funding/Support: This study was supported in part by a grant from the National Psoriasis Foundation (NNM), by K23HL097151-01 (NNM), by a grant from the Doris Duke Charitable Foundation (YY), 1P30 ES013508-05 from the National Institute of Environmental Health Sciences, NIH (DAT), NHLBI RO1HL089744 (JMG), and P50 HL-083799-SCCOR (Penn).
Role of Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr(s) Mehta, Gelfand, Alavi, Torigian and Yu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mehta, Gelfand
Acquisition of data: Mehta, Torigian, Alavi, Saboury, Foroughi, Yu, Krishnamoorthy, Antigua, Raper, Baer, Van Voorhees, Gelfand
Analysis and interpretation of data: Mehta, Yu, Gelfand, Torigian, Saboury, Foroughi, Krishnamoorthy, Antigua
Drafting of the manuscript: Mehta, Yu, Torigian
Critical revision of the manuscript for important intellectual content: Gelfand, Alavi
Statistical analysis: Mehta, Yu, Saboury, Foroughi
Obtained funding: Mehta, Torigian
Administrative, technical, or material support: Baer, Raper, Antigua, Krishnamoorthy
Study supervision: Baer, Mehta, Gelfand, Torigian, Alavi
All Financial Interests (including pharmaceutical and device products):
Employment: none
Consultancies: Gelfand – Amgen, Pfizer, Novartis, Centocor, Celgene
Van Voorhees – Amgen, Abbott, Genentech, Incyte, Warner Chilcott, Connetics, Bristol Myers Squibb, IDEC, Centocor, VGX, Xtrc, Leo
Honoraria: Van Voorhees – Amgen, Abbott, Genentech, Incyte, Warner Chilcott, Connetics, Bristol Myers Squibb, IDEC, Centocor, VGX, Xtrc, Leo
Speakers bureau: none
Stock ownership or options: Van Vorhees – Merck; Torigian – Pfizer
Expert testimony: none
Grants: Gelfand – Amgen, Abbott, Novartis, Pfizer; Torigian – Pfizer
Van Voorhees – Amgen, Astellas, Bristol Myers Squibb, Roche
Patents filed, received, pending, or in preparation: none
Royalties: none
Donation of medical equipment: none
REFERENCES
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005 Dec;141(12):1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009 Feb;60(2):218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005 Oct;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009 Apr;145(4):379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauchli YB, Jick SS, Meier CR. Psoriasis and the risk of incident diabetes mellitus: a population-based study. Br J Dermatol. 2008 Sep 6; doi: 10.1111/j.1365-2133.2008.08814.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010 Jun;24(6):661–666. doi: 10.1111/j.1468-3083.2009.03481.x. [DOI] [PubMed] [Google Scholar]
- 7.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol. 2009 May;160(5):1048–1056. doi: 10.1111/j.1365-2133.2008.09020.x. [DOI] [PubMed] [Google Scholar]
- 8.Balci DD, Balci A, Karazincir S, et al. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol. 2009 Jan;23(1):1–6. doi: 10.1111/j.1468-3083.2008.02936.x. [DOI] [PubMed] [Google Scholar]
- 9.Davidovici BB, Sattar N, Jorg PC, et al. Psoriasis and Systemic Inflammatory Diseases: Potential Mechanistic Links between Skin Disease and Co-Morbid Conditions. J Invest Dermatol. May 6; doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006 Oct 11;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 11.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2009 Dec 27; doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009 Jun;145(6):700–703. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 13.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009 Oct;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006 Nov;55(5):829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007 Feb;156(2):271–276. doi: 10.1111/j.1365-2133.2006.07562.x. [DOI] [PubMed] [Google Scholar]
- 16.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008 Jul;20(4):416–422. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisondi P, Girolomoni G. Psoriasis and atherothrombotic diseases: disease-specific and non-disease-specific risk factors. Semin Thromb Hemost. 2009 Apr;35(3):313–324. doi: 10.1055/s-0029-1222610. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216(2):152–155. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 19.Libby P. Inflammation in atherosclerosis. Nature. 2002 Dec 19–26;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 20.Nishibu A, Han GW, Iwatsuki K, et al. Overexpression of monocyte-derived cytokines in active psoriasis: a relation to coexistent arthropathy. J Dermatol Sci. 1999 Sep;21(1):63–70. doi: 10.1016/s0923-1811(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 21.Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol. 2009 Jul;161(1):1–7. doi: 10.1111/j.1365-2133.2009.09281.x. [DOI] [PubMed] [Google Scholar]
- 22.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009 Jan;129(1):79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010 May;130(5):1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM. Psoriasis, inflammation, and vascular risk: a problem more than skin deep? European Heart Journal. 2010 April 1;31(8):902–904. doi: 10.1093/eurheartj/ehq042. 2010. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Zaidi H, Houseni M, et al. Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: implications for normal variation, aging and diseased states. Semin Nucl Med. 2007 May;37(3):223–239. doi: 10.1053/j.semnuclmed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med. 2001 Apr;26(4):314–319. doi: 10.1097/00003072-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Rudd JHF, Warburton EA, Fryer TD, et al. Imaging Atherosclerotic Plaque Inflammation With [18F]-Fluorodeoxyglucose Positron Emission Tomography. Circulation. 2002 June 11;105(23):2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. 2002. [DOI] [PubMed] [Google Scholar]
- 28.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006 Nov 7;48(9):1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 29.Wassélius J, Larsson S, Jacobsson H. FDG-Accumulating Atherosclerotic Plaques Identified with <sup>18</sup>F-FDG-PET/CT in 141 Patients. Molecular Imaging and Biology. 2009;11(6):455–459. doi: 10.1007/s11307-009-0223-2. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa M, Ishino S, Mukai T, et al. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004 Jul;45(7):1245–1250. [PubMed] [Google Scholar]
- 31.Worthley SG, Zhang ZY, Machac J, et al. In vivo non-invasive serial monitoring of FDG-PET progression and regression in a rabbit model of atherosclerosis. Int J Cardiovasc Imaging. 2009 Mar;25(3):251–257. doi: 10.1007/s10554-008-9377-2. [DOI] [PubMed] [Google Scholar]
- 32.Bural GG, Torigian DA, Botvinick E, et al. A pilot study of changes in (18)F-FDG uptake, calcification and global metabolic activity of the aorta with aging. Hell J Nucl Med. 2009 May-Aug;12(2):123–128. [PubMed] [Google Scholar]
- 33.Bural GG, Torigian DA, Chamroonrat W, et al. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008 Mar;35(3):562–569. doi: 10.1007/s00259-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 34.Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation. 2008 Apr 22;117(16):2061–2070. doi: 10.1161/CIRCULATIONAHA.106.652313. [DOI] [PubMed] [Google Scholar]
- 35.Tateishi U, Imagawa T, Kanezawa N, et al. PET assessment of disease activity in children with juvenile idiopathic arthritis. Pediatr Radiol. 2010 Nov;40(11):1781–1788. doi: 10.1007/s00247-010-1716-5. [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi Y, Arii K, Kumon Y, et al. Positron emission tomography/computed tomography: a clinical tool for evaluation of enthesitis in patients with spondyloarthritides. Rheumatology (Oxford) 2010 Feb;49(2):348–354. doi: 10.1093/rheumatology/kep379. [DOI] [PubMed] [Google Scholar]
- 37.Kubota K, Ito K, Morooka M, et al. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann Nucl Med. 2009 Nov;23(9):783–791. doi: 10.1007/s12149-009-0305-x. [DOI] [PubMed] [Google Scholar]
- 38.Paulmier B, Duet M, Khayat R, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008 Mar-Apr;15(2):209–217. doi: 10.1016/j.nuclcard.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009 Oct;50(10):1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 40.Tahara N, Kai H, Ishibashi M, et al. Simvastatin Attenuates Plaque Inflammation: Evaluation by Fluorodeoxyglucose Positron Emission Tomography. Journal of the American College of Cardiology. 2006;48(9):1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 41.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008 Aug;49(8):1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 42.Bural GG, Torigian DA, Chamroonrat W, et al. Quantitative assessment of the atherosclerotic burden of the aorta by combined FDG-PET and CT image analysis: a new concept. Nucl Med Biol. 2006 Nov;33(8):1037–1043. doi: 10.1016/j.nucmedbio.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Bural G, Torigian D, Burke A, et al. Quantitative Assessment of the Hepatic Metabolic Volume Product in Patients with Diffuse Hepatic Steatosis and Normal Controls Through Use of FDG-PET and MR Imaging: A Novel Concept. Molecular Imaging and Biology. 2010;12(3):233–239. doi: 10.1007/s11307-009-0258-4. [DOI] [PubMed] [Google Scholar]
- 44.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional Imaging of Inflammatory Diseases Using Nuclear Medicine Techniques. Seminars in Nuclear Medicine. 2009;39(2):124–145. doi: 10.1053/j.semnuclmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Beckers C, Ribbens C, Andre B, et al. Assessment of Disease Activity in Rheumatoid Arthritis with 18F-FDG PET. J Nucl Med. 2004 June 1;45(6):956–964. 2004. [PubMed] [Google Scholar]
- 46.Rudd JHF, Narula J, Strauss HW, et al. Imaging Atherosclerotic Plaque Inflammation by Fluorodeoxyglucose With Positron Emission Tomography: Ready for Prime Time? Journal of the American College of Cardiology. 2010;55(23):2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 47.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009 Mar;39(2):124–145. doi: 10.1053/j.semnuclmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Wehrli NE, Bural G, Houseni M, Alkhawaldeh K, Alavi A, Torigian DA. Determination of age-related changes in structure and function of skin, adipose tissue, and skeletal muscle with computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med. 2007 May;37(3):195–205. doi: 10.1053/j.semnuclmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 50.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez M, Filippucci E, De Angelis R, et al. Subclinical Entheseal Involvement in Patients with Psoriasis: An Ultrasound Study. Semin Arthritis Rheum. 2010 Aug 5; doi: 10.1016/j.semarthrit.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Coates LC, McGonagle DM, Hodgson R, et al. Imaging in psoriasis and psoriatic arthritis: GRAPPA 2008. J Rheumatol. 2010 Feb;37(2):448–452. doi: 10.3899/jrheum.090955. [DOI] [PubMed] [Google Scholar]
- 53.Offidani A, Cellini A, Valeri G, Giovagnoni A. Subclinical joint involvement in psoriasis: magnetic resonance imaging and X-ray findings. Acta Derm Venereol. 1998 Nov;78(6):463–465. doi: 10.1080/000155598442809. [DOI] [PubMed] [Google Scholar]