Abstract
Bipolar disorder and schizophrenia overlap in symptoms and may share some underlying neural substrates. The medial prefrontal cortex (MPFC) may have a crucial role in the psychophysiology of both these disorders. In this study, we examined the functional connectivity between MPFC and other brain regions in bipolar disorder and schizophrenia using resting-state functional magnetic resonance imaging (fMRI). Resting-state fMRI data were collected from 14 patients with bipolar disorder, 16 patients with schizophrenia, and 15 healthy control subjects. Functional connectivity maps from the MPFC were computed for each subject and compared across the three groups. The three groups showed distinctive patterns of functional connectivity between MPFC and anterior insula, and between MPFC and ventral lateral prefrontal cortex (VLPFC). The bipolar disorder group exhibited positive correlations between MPFC and insula, and between MPFC and VLPFC, whereas the control group exhibited anticorrelations between these regions. The schizophrenia group did not exhibit any resting-state correlation or anticorrelation between the MPFC and the VLPFC or insula. In contrast, neither patient group exhibited the significant anticorrelation between dorsal lateral prefrontal cortex (DLPFC) and MPFC that was exhibited by the control group. The decoupling of DLPFC with MPFC in bipolar disorder and schizophrenia is consistent with the impaired executive functioning seen in these disorders. Functional connectivity between MPFC and insula/VLPFC distinguished bipolar disorder from schizophrenia, and may reflect differences in the affective disturbances typical of each illness.
Keywords: fMRI, functional connectivity, anticorrelations, DLPFC, VLPFC, insula
INTRODUCTION
A significant body of genetic, imaging, and neuropsychology research has established that schizophrenia and bipolar disorder share substantial overlap in clinical features, as well as in contributing genetic factors (Lichtenstein et al, 2009). However, there is little specific evidence about shared and distinctive neural substrates of schizophrenia and bipolar disorder. There is evidence for a failure in the prefrontal cortex (PFC) modulation of anterior limbic structures such as the amygdala resulting in emotional dysregulation in bipolar disorder (Strakowski et al, 2005) and for inefficient cognitive information processing in the PFC in schizophrenia (Ragland et al, 2007).
The ventral and orbital part of the medial prefrontal cortex (MPFC), including Brodmann areas 32, 24, 25, and 10, has extensive and reciprocal connections to the limbic circuit and surrounding prefrontal cortical regions (Öngür and Price 2000), and has been suggested to contribute to the emotional regulation impairment in bipolar disorder (Phillips et al, 2008). Bipolar disorder patients exhibited elevated functional connectivity between MPFC and amygdala while viewing sad stimuli compared with healthy controls (Versace et al, 2010). Effective connectivity between MPFC and amygdala has been shown to differentiate bipolar disorder from major depression patients (Almeida et al, 2009). These results suggest that MPFC–limbic connectivity may underlie the affective disturbance in bipolar disorder.
MPFC is also critical in internal, self-referential processing (Gilbert et al, 2006), and has been suggested to underlie the impairments in reality monitoring in schizophrenia (Taylor et al, 2007; Vinogradov et al, 2008). MPFC is a major hub of the default mode network, which is typically more active during rest than during tasks that demand external attention, is thought to mediate internal mental activity (Buckner et al, 2008). Compared with healthy controls, schizophrenia patients exhibited hyperactivity in the MPFC during a working memory task and increased connectivity between MPFC and other regions of the default mode network (Whitfield-Gabrieli et al, 2009).
Based on this literature, we aimed to examine the MPFC functional connectivity differences in schizophrenia and bipolar disorder using resting-state functional magnetic resonance (fMRI). Coherent spontaneous blood oxygen level-dependent (BOLD) activity during rest among specific subsets of brain regions is thought to reflect the natural organization of brain networks (Biswal et al, 1995; see Buckner et al, 2008; Fox and Raichle, 2007, for a review). Abnormal resting-state functional connectivity patterns have been observed in both schizophrenia (Bluhm et al, 2007; Lynall et al, 2010; Whitfield-Gabrieli et al, 2009; Zhou et al, 2007) and bipolar disorder (Anand et al, 2009; Chepenik et al, 2010).
We previously reported an independent component analysis (ICA) approach to detect default mode network abnormalities in schizophrenia and bipolar disorder (Öngür et al, 2010). The ICA approach identifies collections of brain regions (components) with similar time courses. Such an analysis is driven by patterns in the data, but it does not focus on individual brain regions nor does it provide information about which brain regions are anticorrelated with a given component. This last point is important because anticorrelations are a prominent feature of the brain's intrinsic functional organization and may reflect competitive relationships among different brain networks (Fox et al, 2005). Complementary to the ICA approach, seed-driven connectivity analysis allows for close examination of correlations and anticorrelations from a seed region of interest (ROI) to other regions in the brain, and has revealed anticorrelated relationships between brain regions in healthy subjects (Fox et al, 2005; Fransson, 2005; Uddin et al, 2009) and clinical populations (Hoptman et al, 2010; Whitfield-Gabrieli et al, 2009).
In this exploratory study, we used a data set largely overlapping with that in our previous report, but we asked a different and specific question, using a seed-driven approach. We examined the functional connectivity between a seed region in the MPFC with other brain regions (correlated or anticorrelated) in resting-state fMRI in three groups of participants: patients with bipolar disorder (N=14), patients with schizophrenia (N=16), and healthy controls (N=15). Previous studies that reported anticorrelations in resting-state connectivity have relied on global signal removal, a common noise correction technique. However, the nature of anticorrelations has been questioned since global signal removal artificially introduces negative correlations (Murphy et al, 2009). In our analysis, we used a different noise reduction approach, which did not rely on global signal removal (Behzadi et al, 2007). We hypothesized that bipolar disorder patients would show abnormal MPFC connectivity with anterior limbic regions and with cognitive regions such as the lateral PFC, whereas schizophrenia patients would show mostly abnormalities in connectivity between MPFC and cognitive regions such as the lateral PFC.
MATERIALS AND METHODS
Subjects
Following approval by the McLean Hospital Institutional Review Board, 39 bipolar I disorder patients, currently in a manic episode, 20 schizophrenia patients with acute exacerbation of psychosis and 15 healthy controls were recruited for this study. Usable data were obtained from 14 bipolar disorder patients, 16 schizophrenia patients, and 15 healthy controls (NC, BP, and SZ groups, respectively). All patients were hospitalized in a psychiatric in-patient unit. Participants with significant neurological or medical problems, current substance abuse, or history of substance dependence were excluded. Substance abuse was excluded if it occurred in the past month. Substance dependence was excluded if it occurred lifetime. These substance use disorder criteria apply to alcohol and substances as included in the Structured Clinical Interview for DSM-IV (SCID), but not to nicotine. Nicotine use was allowed. Every bipolar disorder patient was taking lithium (N=6) or depakote (N=8), and an antipsychotic at scan time (none on clozapine, one on first-generation (typical) antipsychotic, and remainder on second-generation (atypical) antipsychotics). All schizophrenia patients were taking an antipsychotic (three on clozapine and remainder on other second-generation antipsychotics); four of these patients were also on lithium and two on depakote.
The bipolar disorder cohort had a particularly high rate of attrition, and this was largely due to manic patients' inability to complete the scan. In total, 22 bipolar disorder subjects did not complete the study (an additional three had unusable data due to excessive motion during the scan, or substance use disorder revealed subsequent to the scan). The 22 bipolar disorder patients who did not complete the study had markedly more severe psychotic symptoms (Positive and Negative Syndrome Scale (PANSS)=82.0) as well as moderately more severe manic and depressed symptoms (Young Mania Rating Scale (YMRS)=26.9; Montgomery–Asberg Depression Rating Scale (MADRS)=18.4) than those who did (see Table 1). This pattern suggests that our findings may not be generalizable to all patients in a manic episode, especially those with significant psychotic symptoms. Schizoaffective patients were included in the SZ cohort in this study. In all, seven patients in the SZ group were diagnosed as having schizoaffective disorder in the SCID. All of these patients had chronic psychotic symptoms, were hospitalized with an acute psychotic exacerbation, and were not in a mood episode at the time of the study. Thus, their presentation was similar to that of patients with schizophrenia.
Table 1. Subject Demographic and Clinical Information.
| Normal control subjects (N=15) | Bipolar disorder (N=14) | Schizophrenia (N=16) | Statistical evaluation | |
|---|---|---|---|---|
| Age (years) | 37.3±2.4 | 32.7±3.0 | 41.6±2.6 | F(2,42)=3.21, p=0.05 |
| Gender | 6 F, 9 M | 5 F, 9 M | 7 F, 9 M | χ2: 2.01, p=0.90 |
| Age of onset | — | 22.4±2.5 | 21.1±1.5 | t(25)=0.42, p=0.68 |
| MADRS | — | 11.1±0.9 | 17.6±1.9 | t(27)=2.97, p=0.006 |
| YMRS | — | 23.5±1.9 | 15.1±2.3 | t(27)=2.74, p=0.01 |
| PANSS | — | 59.3±3.5 | 84.7±4.7 | t(27)=4.33, p<0.001 |
| CPZ equivalents | — | 287.2±38.5 | 504.7±106.3 | t(25)=1.86, p=0.074 |
Abbreviations: CPZ, chlorpromazine; F, female; M, male; MADRS, Montgomery–Asberg Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale.
Mean±SD, where appropriate.
The structural MRI scans obtained in this study were reviewed by a radiologist, and participants with structural brain abnormalities were excluded. All participants had negative urine toxicology tests. We used an Informed Consent Survey that asks 10 simple questions about the study, such as ‘What illness is being studied?' and ‘If you don't want to, do you have to be in this study?' Every participant answered these questions correctly, and answers were documented. Patients were assessed using the SCID, YMRS, MADRS, and PANSS on the day of the study. Healthy controls were assessed using the SCID. Chlorpromazine (CPZ) equivalents were calculated for participants taking antipsychotic medications (Woods, 2003). Demographic and clinical variables and statistical comparisons are in Table 1. This data set is largely overlapping but not identical with that from another report that used an ICA to examine default mode network abnormalities in bipolar disorder and schizophrenia (Öngür et al, 2010). All 14 patients with BP in this analysis were included in the previous study and constitute a subset of the cohort in that study. Of the 16 SZ patients in this study, 14 were included in the previous study; additional data that became available from two participants were also included in this study.
Procedure
Data were acquired on a 3T Siemens scanner using a standard head coil. T1-weighted whole-brain anatomy images (MPRAGE sequence, 256 × 256 voxels, 1 × 1.3 mm2 in-plane resolution, 1.3-mm slice thickness) were acquired. In addition, all participants underwent a resting fMRI scan of 10 min with the instructions ‘keep your eyes open and think of nothing in particular'. Resting scan images were obtained in 42 3.5-mm-thick transverse slices, covering the entire brain (interleaved EPI sequence, T2*-weighted images; 3.5-mm in-plane resolution, repetition time=2.5 s, echo time=24 ms, flip angle=82, 64 × 64 voxels). To minimize effects of scanner signal stabilization, the first two images were omitted from all analysis.
Data Analysis
fMRI data preprocessing was carried out in SPM5 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Images were slice-time corrected, realigned, normalized, spatially smoothed with a 6-mm kernel, and temporally band-pass filtered (0.009<f<0.08 Hz). Functional connectivity analysis was performed using a seed-driven approach with in-house, custom software (Whitfield-Gabrieli and Nieto Castanon, under review). We investigated the connectivity patterns from MPFC. To make it feasible to compare our results with existing resting-state connectivity findings, we created the MPFC seed based on coordinates from previous studies (Fox et al, 2005; Whitfield-Gabrieli et al, 2009). The MPFC seed was a 10-mm sphere centered in the anterior cingulate gyrus around MNI coordinates (−1, 49, −2) and included BA 32 and 10. Unlike previous resting-state fMRI analyses in which the mean global brain signal was commonly removed from the time course (Fox et al, 2005; Fransson, 2006), our analysis used a component-based noise correction method (aCompCor) to estimate physiological and other spurious sources of noise (Behzadi et al, 2007). Significant principle components of the signals from white matter and CSF regions were removed together with movement-related covariates. Our analysis approach enhances the sensitivity and specificity of positive correlations and produces comparable anticorrelations compared with the standard approach with global signal removal (Chai et al, under review).
In the first-level analysis, a correlation map was produced for each subject by extracting the residual BOLD time course from the MPFC seed and computing Pearson's correlation coefficients between that time course and the time course of all other voxels. Correlation coefficients were converted to normally distributed z-scores using Fisher's transform to allow for second-level General Linear Model analyses. In the second-level random effects analysis, connectivity maps from the MPFC seed from all participants were entered into a three-level analysis of variance (ANOVA), to identify regions with different MPFC connectivity among the three groups of participants. Regions from the ANOVA that survived a height threshold of p<0.001 at the voxel level and an extent threshold of FWE-corrected p<0.05 at the cluster level were used as ROIs in post hoc ROI analysis. The connectivity value (Fisher's z-score) between the MPFC seed and each ROI was extracted from the MPFC connectivity map from each participant, to further examine the nature of the connectivity between the MPFC and each of these ROIs in each group (correlated or anticorrelated). Fisher's z-values extracted from the ROIs were converted back to correlation coefficients (r-values) for reporting purposes. Because the differences in age among the three groups approached significance, we conducted an additional analysis in which the age of each participant was included as a covariate to account for effects related to age differences among the groups.
Owing to patient heterogeneity in our sample and the modest sample size in each group, we performed a data simulation to test the reliability of our results. Bootstrapping (resampling with replacement) was performed on the extracted connectivity values between the MPFC and each ROI (Supplementary Information).
In addition to the whole-brain connectivity analysis described above, we also conducted a subsidiary analysis to specifically examine MPFC–amygdala resting-state functional connectivity in the bipolar disorder and healthy control groups, given previous evidence for the importance of MPFC and amygdala in emotional regulation. The left and right amygdala ROIs were defined using WFUpickatlas (Maldjian et al, 2003). Connectivity values (Fisher's z-score) between the MPFC seed and the amygdala ROIs were extracted from the MPFC connectivity map from each bipolar disorder and healthy control subject. Between-group t-tests were conducted to determine if the two groups differed in MPFC–amygdala connectivity.
RESULTS
There were significant differences among the groups for connectivity between the MPFC seed and bilateral dorsal lateral prefrontal cortex (DLPFC), right ventral lateral prefrontal cortex (VLPFC), and left insula (ANOVA F (2,42) test, cluster-level FWE-corrected p<0.05; Table 2). We examined the nature of the differences in these four ROIs defined by the ANOVA via post hoc ROI level t-tests.
Table 2. Regions that Showed Significant Group Differences in Connectivity with MPFC.
| BA | x | y | z | z-Score | Cluster size | p-Value | |
|---|---|---|---|---|---|---|---|
| L DLPFC | 46 | −52 | 42 | 10 | 5.13 | 49 | 0.034 |
| 46 | −52 | 40 | 18 | 3.86 | |||
| R DLPFC | 46 | 50 | 36 | 18 | 3.90 | 55 | 0.019 |
| R VLPFC | 47 | 40 | 20 | −4 | 4.29 | 104 | 0.0004 |
| 47 | 40 | 26 | −12 | 3.78 | |||
| L insula | −38 | 12 | 4 | 4.32 | 51 | 0.028 | |
| −34 | 8 | −2 | 4.30 |
Coordinates (x, y, z) are based on MNI brain (Montreal Neurologic Institute). BA, Brodmann area; z-score, z-score equivalent of the ANOVA F statistic at the peak voxel within each cluster; p-value, FWE-corrected cluster-level p-value.
MPFC–DLPFC Connectivity
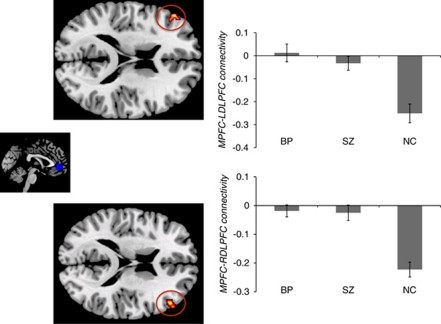
The control group exhibited significant anticorrelations between MPFC and DLPFC (left DLPFC, mean r=−0.25, t(14)=5.75, p<0.001; right DLPFC, mean r=−0.22, t(14)=7.89, p<0.001). This differed from both patient groups, who showed no significant correlations between MPFC and DLPFC (p's>0.3) (Figure 1). Both patient groups differed significantly from the control group in right DLPFC (SZ vs controls, t(29)=4.14, p<0.001; BP vs controls, t(27)=4.46, p<0.001), and left DLPFC (SZ vs controls, t(29)=4.95, p<0.001; BP vs controls, t(27)=5.64, p<0.001). MPFC–DLFPC connectivity did not differ between the bipolar disorder and schizophrenia groups (p's>0.3).
Figure 1.
Functional connectivity between medial prefrontal cortex (MPFC) and dorsal lateral prefrontal cortex (DLPFC). Bars represent the average connectivity between the MPFC seed and the DLPFC region of interests (ROIs) among the subjects within each group. Error bars represent standard errors. Connectivity value for each subject was calculated as the correlation between the time course from the DLPFC ROIs and the time course from the MPFC seed. Left panel: the MPFC seed. Middle panel: top, left DLPFC ROI (BA46); bottom: right DLPFC ROI (BA46). Right panel: Functional connectivity between MPFC and left DLPFC (top), and right DLPFC (bottom). BP, bipolar disorder; NC, healthy controls; SZ, schizophrenia.
Connectivity Between MPFC and VLPFC, and Between MPFC and Insula
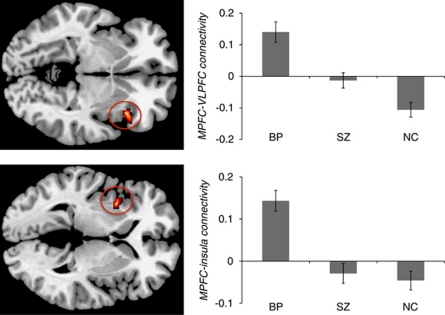
The bipolar disorder group showed significant positive correlations between MPFC and left insula (mean r=0.15, t(13)=5.81, p<0.001), and between MPFC and right VLPFC (mean r=0.14, t(13)=4.35, p=0.001) (Figure 2). In contrast, the control group exhibited significant anticorrelations between MPFC and right VLPFC (mean r=−0.11, t(14)=4.49, p=0.001), and between MPFC and left insula (mean r=−0.05, t(14)=2.20, p=0.045). The schizophrenia group did not show any significant correlation between the MPFC and VLPFC (p>0.6) or insula (p>0.2). The bipolar disorder group differed significantly from both the schizophrenia and the control groups in the MPFC–insula (BP vs controls, t(27)=5.77, p<0.001; BP vs SZ, t(28)=5.04, p<0.001) and MPFC–VLPFC (BP vs controls, t(27)=6.19, p<0.001; BP vs SZ, t(28)=3.85, p=0.001) connectivity. The schizophrenia group differed from the control group in MPFC–VLPFC connectivity (t(29)=2.71, p=0.011).
Figure 2.
Functional connectivity between medial prefrontal cortex (MPFC) and ventral lateral prefrontal cortex (VLPFC), and between MPFC and insula. Top panel: right VLPFC region of interest (ROI) (BA 47); bottom panel: left insula ROI. BP, bipolar disorder; NC, healthy controls; SZ, schizophrenia. Error bars represent standard errors.
Within either patient group, we did not find correlations between MPFC connectivity and clinical symptom scores. Age of onset correlated with the MPFC–right DLPFC connectivity within the bipolar disorder group (r=0.6, p=0.02). This correlation was not significant after correcting for multiple comparisons. Connectivity values and CPZ equivalents were not significantly correlated in either bipolar disorder (p's>0.12) or schizophrenia (p's>0.21) patients. It was not feasible to examine the relationship between connectivity and different classes of medication in our data because medication was too varied.
When we included subjects' age as a covariate in the ANCOVA, we found the same brain regions that showed connectivity differences among the three groups. Post hoc ROI analysis of these regions showed the same pattern of results as before, after age was accounted for. We have included the details of these additional analyses in Supplementary Information (Supplementary Table S1). Bootstrap resampling yielded the same pattern of results (Supplementary Table S2). Connectivity results from the healthy control group are presented in Supplementary Table S3 and Supplementary Figure S1.
MPFC–amygdala connectivity was not different between the bipolar disorder group and the healthy control group (left, p=0.6; right, p=0.2). MPFC was positively correlated with bilateral amygdala in the control group (left, t(14)=2.86, p=0.01; right, t(14)=4.06, p=0.001). In the bipolar disorder group, MPFC was positively correlated with the left amygdala (t(13)=3.15, p=0.008).
DISCUSSION
In this study, we used a seed-based approach to probe the resting functional connectivity of the MPFC, a region of the prefrontal cortex implicated in both bipolar disorder and schizophrenia. Connectivity between the MPFC and other prefrontal regions revealed differences among the groups. First, neither patient group exhibited the significant anticorrelation between MPFC and DLPFC seen in the control group. The observed absence of anticorrelated activity between MPFC and DLPFC in both bipolar disorder and schizophrenia is consistent with the similarities in cognitive dysfunction known to exist in these two patient groups. Second, only the bipolar disorder group exhibited positive MPFC–insula and MPFC–VLPFC correlations; the schizophrenia group did not exhibit any resting correlation between these regions and the control group exhibited anticorrelations between MPFC and insula, and between MPFC and VLPFC. The distinctive patterns in MPFC–insula and MPFC–VLPFC functional connectivity differentiated the bipolar disorder group from both the schizophrenia group and the control group.
The decoupling of the DLPFC from the MPFC in bipolar disorder and schizophrenia is consistent with the results of task-related activation fMRI studies in these disorders. Reduced activations in DLPFC have been widely reported during executive function tasks in schizophrenia (Weinberger et al, 1986; see, Minzenberg et al, 2009, for meta-analysis) and in bipolar disorder (Lagopoulos et al, 2007; Monks et al, 2004; Perlstein et al, 2001; Townsend et al, 2010). MPFC is part of the default mode network, which consists of a set of brain regions that are more active during rest than during cognitive tasks. Dorsal executive control regions including the DLPFC are anticorrelated with the MPFC in healthy subjects during rest (Fox et al, 2005; Fransson, 2005; Kelly et al, 2009; Uddin et al, 2009). During tasks that demand external attention and executive control, such as working memory tasks, DLPFC is strongly activated, whereas activation in the MPFC is suppressed (Greicius et al, 2003; McKiernan et al, 2003). Our observations in schizophrenia are consistent with a working memory study that also showed reduced anticorrelation between MFPC and DLPFC in patients with schizophrenia and their first-degree relatives (Whitfield-Gabrieli et al, 2009). Our findings suggest that the cognitive deficits in the patient groups are in part due to a decoupling of MPFC and DLPFC at baseline, which is also manifested during task execution as a failure to activate the DLPFC and failure to suppress MPFC.
By contrast with the DLPFC, the MPFC–insula and MPFC–VLPFC connectivity patterns distinguished the bipolar disorder group from both schizophrenia and healthy control groups. Compared with the schizophrenia group and healthy controls, the bipolar disorder group showed hyperconnectivity between MPFC and insula/VLPFC regions. The MPFC, VLPFC, and the insula are important nodes of the emotional processing network (Elliott et al, 2004; Mayberg et al, 1999; Phillips et al, 2003a, 2003b). Elevated activations in VLPFC have been observed in bipolar patients during an emotional Go-NoGo task (Elliott et al, 2004) and while viewing emotional faces (Lawrence et al, 2004), suggesting that dysfunction of the VLPFC may underlie the deficit in mood regulation in bipolar patients. The MPFC and the VLPFC are anatomically connected as part of the medial prefrontal network, which has a major role in eliciting visceral and somatic responses to emotionally salient stimuli (Öngür and Price 2000). In contrast to the elevated MPFC–VLPFC connectivity in bipolar disorder group, the control group showed significant anticorrelations between these regions. The VLPFC and anterior insula, as well as the DLPFC have been previously identified as part of the ‘task-positive network', which is typically active during task performance than during rest, and has been found to correlate negatively with the default mode network (Fox et al, 2005; Fransson, 2006; Kelly et al, 2008). Seeley et al (2007) showed that the resting-state network for emotional saliency processing, which includes VLPFC and insula, is dissociable from the executive control network, which links DLPFC and parietal regions. The salience network may be important for switching between external attention and internal processes (Sridharan et al, 2008). The abnormally high connectivity between the MPFC and insula/VLPFC regions in bipolar patients could contribute to not only the executive control deficits, but also their overly heightened attention to internal emotionally salient processing, which might result in substantial dysregulation of emotion and disturbed responses to affectively salient stimuli (rewarding or aversive) in bipolar disorder (Goodwin and Jamison, 2007). Our findings could be evidence for an inappropriate coupling between emotional regulation regions (eg, MPFC/ACC) and emotional processing regions (eg, insula), such that emotional stimuli capture processing resources more easily in bipolar disorder patients.
The bipolar disorder and healthy control groups in our study did not differ in resting-state connectivity between MPFC and amygdala. Versace et al (2010) showed that bipolar disorder patients exhibited higher functional connectivity between MPFC and amygdala while viewing sad stimuli, but reduced MPFC–amygdala connectivity while viewing happy stimuli compared with healthy controls. In healthy participants, amygdala–MPFC connectivity at rest has been linked to individual differences in self-reported anxiety level (Kim et al, 2010). These evidence suggests that the interaction between amygdala and MPFC might be state-dependent. Future functional connectivity studies should take in to account differences in anxiety levels during rest.
Schizophrenia patients in our study showed MPFC–VLPFC connectivity intermediate to those observed in the control group and the bipolar disorder group. Although abnormal VLPFC activation in schizophrenia has been reported, the exact nature of this abnormality is unclear. Some studies point to abnormal activation in the VLPFC (eg, blunted activation in the right VLPFC during a Go-NoGo task in patients with schizophrenia (Kaladjian et al, 2007) and a positive correlation between right VLPFC abnormalities and measures of impulsivity (Kaladjian et al, 2011), but others have found the VLPFC in schizophrenia to be intact (Barch et al, 2001; Manoach et al, 2005). Often, VLPFC abnormalities are detected in the presence of abnormal activity in the DLPFC, a region more consistently implicated in cognitive dysfunction in schizophrenia. It has been suggested that VLPFC abnormalities, especially VLPFC hyperactivity, may reflect extension and compensatory recruitment of cortical activity due to inefficient DLPFC functioning (Minzenberg et al, 2009; Tan et al, 2005). Our MPFC–VLPFC connectivity results suggest that the mechanisms underlying VLPFC recruitment may be different between bipolar disorder and schizophrenia. The bipolar disorder group deviated more from the control group in MPFC–VLPFC connectivity than did the schizophrenia group; this may reflect the greater deficits in emotional processing in bipolar disorder. Notably, extremes of mood are typical of bipolar disorder, whereas flat affect is typical of schizophrenia.
Our previous report used ICA to identify the default mode network in bipolar disorder and schizophrenia, and found reduced MPFC connectivity in both bipolar disorder and schizophrenia patients compared with the healthy controls, thereby confirming the MPFC abnormality in these patients (Öngür et al, 2010). This study focused on MPFC using a seed-driven approach, which allowed us to examine any possible competitive relationships (anticorrelations) from MPFC to other brain regions. The ICA and seed-based analyses provided converging but distinct information. Although both analyses confirmed the abnormal connectivity of the MPFC in bipolar disorder and schizophrenia patients, this study showed that the anticorrelation patterns between MPFC and VLPFC/insula distinguished bipolar disorder from schizophrenia patients.
Even though all patients in the schizophrenia group had comparable SCID diagnoses, these diagnoses do not guarantee that all participants have similar clinical presentation or neurobiological abnormalities. In fact, there is significant heterogeneity within our diagnostic groupings. Although results of the Monte Carlo simulation suggest that our findings were robust against outliers in our modest-sized samples, future valid neurobiological assessments are needed to help categorize patients more accurately.
All of the bipolar disorder and schizophrenia participants in this study were taking medications. The participants with bipolar disorder were each taking an antipsychotic medication as well as a mood-stabilizing medication, whereas those with schizophrenia were on one or two antipsychotic medications and a subset were also taking mood-stabilizing medications. The CPZ equivalents for the two groups indicated that the bipolar disorder participants were taking lower doses of antipsychotics as compared with schizophrenia participants, although the difference did not reach statistical significance. There is previous evidence that suggests medication may influence brain connectivity. Antipsychotic medication may cause grey matter volume changes in certain brain structures in schizophrenia (Tomelleri et al, 2009). Dopamine receptor antagonist has been shown to reduce global and local network efficiency in healthy adult brains (Achard and Bullmore, 2007). After short-term antipsychotic treatment, first-episode schizophrenia patients showed decreased functional connectivity across brain regions compared with pretreatment (Lui et al, 2010). However, the patient groups in our study showed increased connectivity compared with healthy controls. Connectivity values did not correlate with CPZ equivalent scores in either patient group in our study. Still, we cannot rule out the influence of antipsychotic medication on our results, as it is unclear as to what the long-term effects of antipsychotic treatment are on brain connectivity. Additional studies are needed to document these effects.
We recruited bipolar disorder participants who were in a manic episode (mean YMRS score 23.5) and schizophrenia participants who were hospitalized with acute exacerbation of psychosis (mean PANSS score 84.7). Within each patient group, we did not find significant relationships between symptom severity and MPFC functional connectivity. We are aware of one study that compared brain activation in a face-viewing paradigm in a bipolar disorder patient cohort during manic and euthymic states (Chen et al, 2010), but similar work has not been carried out on cortical connectivity. In addition, cross-study comparisons of mania vs euthymia or acute vs stable schizophrenia are not fruitful owing to differences in clinical populations, study design, and data analysis. Future studies are needed to elucidate the relationship between functional connectivity and symptom severity.
It is known that cognition suffers in bipolar disorder and schizophrenia patients during episodes of illness (Lewandowskia et al, 2010), so it would not be surprising if brain networks underlying cognitive function appeared more abnormal. Likewise, the major disruption in affective regulation seen in bipolar mania may generate or at least amplify the MPFC–VLPFC/insula hyperconnectivity we observed. A broader question is: to what extent does the default mode network, the task-positive network, and the connections between them change dynamically during different mental states? As cognitive task demands impact connectivity in these networks in health and in mental illness (Whitfield-Gabrieli et al, 2009), it is reasonable to assume that clinical states also have an impact. Our data cannot address this issue, and future resting-state fMRI studies of patient groups in different clinical states will be needed.
Although our analysis approach allowed us to examine local connectivity patterns from a specific seed region in the brain, graph-based analysis has shown great promise in studying large-scaled networks in clinical and healthy populations (Bullmore and Sporns, 2009). Network metrics such as path length and clustering can provide valuable information on the brain organizational differences among different populations. For example, brain organization in schizophrenia patients has been shown to be less economical and lacked the small-world properties shown in healthy participants (Liu et al, 2008), and has reduced hierarchies (Bassett et al, 2008). Future studies should directly compare the large-scaled complex network properties in bipolar disorder and schizophrenia. Based on findings that small-world network metrics in the brain have high heritability (Smit et al, 2008), Bullmore and Sporns, (2009) proposed that network metrics could be used as a marker to predict genetic risks for a neuropsychiatric disorder. It is possible that the scale of local connectivity differences of the MPFC observed in this study could also be useful to assess the developmental stages at which individuals might become predisposed to schizophrenia or bipolar disorder.
In summary, MPFC functional connectivity showed both commonalities and differences between the schizophrenia and bipolar disorder groups in this exploratory analysis comparing the two disorders. Both schizophrenia and bipolar disorder participants had deficient anticorrelated activity between the MPFC and DLPFC, and between MPFC and insula/VLPFC, suggesting a decoupling between regions of the default mode network and the task-positive network. The inappropriate high level of connectivity between MPFC and the VLPFC was specific to participants with bipolar disorder, and may reflect the greater prevalence of affective disturbances and extreme mood states in this patient group compared with schizophrenia.
Acknowledgments
This research was supported by 5K23MH079982-03 (Dr Öngür) from the National Institute of Mental Health and by the Poitras Center for Affective Disorders Research at the McGovern Institute for Brain Research at MIT. We thank Satra Ghosh for advice on data analysis, and the three anonymous reviewers for their helpful comments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. Abnormal amygdala–prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Nieto Castanon A, Öngür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global regression (under review) [DOI] [PMC free article] [PubMed]
- Chen CH, Suckling J, Ooi C, Jacob R, Lupson V, Bullmore ET, et al. A longitudinal fMRI study of the manic and euthymic states of bipolar disorder. Bipolar Disord. 2010;12:344–347. doi: 10.1111/j.1399-5618.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- Chepenik LG, Raffo M, Hampson M, Lacadie C, Wang F, Jones MM, et al. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res. 2010;182:207–210. doi: 10.1016/j.pscychresns.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR.2007Manic-Depressive Illness2nd edn.Oxford University Press: New York [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AM, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull. 2010;36:1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–193. doi: 10.1016/j.schres.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsitivity and neural correlates of response inhibition in schizophrenia. Psychol Med. 2011;41:291–299. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ.2010Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest Cereb Cortex(in press). [DOI] [PMC free article] [PubMed]
- Lagopoulos J, Ivanovski B, Malhi GS. An event-related functional MRI study of working memory in euthymic bipolar disorder. J Psychiatry Neurosci. 2007;32:174–184. [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lewandowskia KE, Cohena BM, Öngur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychological Medicine. 2010;41:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131 (Part 4:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by ‘resting state' functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manoach DS, White N, Lindgren KA, Heckers S, Coleman MJ, Dubal S, et al. Intact hemispheric specialization for spatial and shape working memory in schizophrenia. Schizophr Res. 2005;78:1–12. doi: 10.1016/j.schres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic–cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SC, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced. Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC.2008A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder Mol Psychiatry 13829833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJ, Stam CJ, Posthuma D, Boomsma DI, de Geus EJ. Heritability of ‘small-world' networks in the brain: a graph theoretical analysis of resting-state EEG functional connectivity. Hum Brain Mapp. 2008;29:1368–1378. doi: 10.1002/hbm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61:1171–1178. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Tomelleri L, Jogia J, Perlini C, Bellani M, Ferro A, Rambaldelli G, et al. Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia. Eur Neuropsychopharmacol. 2009;19:835–840. doi: 10.1016/j.euroneuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 2010;182:22–29. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, et al. Abnormal left and right amygdala–orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Schulman BJ, Simpson GV. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb Cortex. 2008;18:2532–2539. doi: 10.1093/cercor/bhn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence [see comments] Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto Castanon A.A functional connectivity toolbox for correlated and anticorrelated networks(under review). [DOI] [PubMed]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.