Abstract
Enduring cognitive deficits exist in schizophrenic patients, long-term abusers of phencyclidine (PCP), as well as in animal PCP models of schizophrenia. It has been suggested that cognitive performance and memory processes are coupled with remodeling of pyramidal dendritic spine synapses in prefrontal cortex (PFC), and that reduced spine density and number of spine synapses in the medial PFC of PCP-treated rats may potentially underlie, at least partially, the cognitive dysfunction previously observed in this animal model. The present data show that the decrease in number of asymmetric (excitatory) spine synapses in layer II/III of PFC, previously noted at 1-week post PCP treatment also occurs, to a lesser degree, in layer V. The decrease in the number of spine synapses in layer II/III was sustained and persisted for at least 4 weeks, paralleling the observed cognitive deficits. Both acute and chronic treatment with the atypical antipsychotic drug, olanzapine, starting at 1 week after PCP treatment at doses that restore cognitive function, reversed the asymmetric spine synapse loss in PFC of PCP-treated rats. Olanzapine had no significant effect on spine synapse number in saline-treated controls. These studies demonstrate that the effect of PCP on asymmetric spine synapse number in PFC lasts at least 4 weeks in this model. This spine synapse loss in PFC is reversed by acute treatment with olanzapine, and this reversal is maintained by chronic oral treatment, paralleling the time course of the restoration of the dopamine deficit, and normalization of cognitive function produced by olanzapine.
Keywords: asymmetric spine synapse, olanzapine, phencyclidine, prefrontal cortex, pyramidal cell, schizophrenia
INTRODUCTION
Schizophrenia is a chronic mental disorder that affects about 1% of the population and is characterized by so-called ‘positive' and ‘negative' symptoms, as well as cognitive deficits (Freedman, 2003). Cognitive dysfunction, including prefrontal cortex (PFC)-dependent functions such as working memory, appears to be the most enduring and treatment-resistant feature of schizophrenia, which represent a severe clinical problem (Tamminga et al, 1998).
It has been suggested that cognitive performance and memory processes are coupled with remodeling of pyramidal dendritic spine synapses in both the PFC (Hof and Morrison, 2004; Nimchinsky et al, 2002) and hippocampus (Silva, 2003; Toni et al, 1999). By reducing the number of available neuronal circuits for memory storage, loss of spine synapses is believed to result in poor memory and cognitive performance. In fact, a recent study has demonstrated a strong correlation between the loss of asymmetric spine synapses in monkey PFC and cognitive impairment during aging (Peters et al, 2008). Several groups have reported dystrophic changes in frontal cortical pyramidal cell dendrites in schizophrenia, including decreases in dendritic length, branching, and spine density (Black et al, 2004; Broadbelt et al, 2002; Garey et al, 1998; Glantz and Lewis, 2000; Kalus et al, 2000; Kolluri et al, 2005), consistent with the hypothesis that these changes are involved in the cognitive dysfunction that typifies the disorder. It is not currently known though whether the pathological changes in the cortex in schizophrenia are fixed or can be altered by treatment with antipsychotic drugs.
Long-term abusers of phencyclidine (PCP) develop an enduring cognitive dysfunction (Cosgrove and Newell, 1991), and animal models that use a subchronic PCP treatment paradigm simulate the schizophrenia-like lasting cognitive deficit (Jentsch et al, 1997a, 1997b). Our own studies demonstrate a significant reduction in the number of asymmetric synapses on dendritic spines of pyramidal neurons in layer II/III of PFC in the rodent PCP model at a time when the rats show cognitive deficits (Hajszan et al, 2006). Asymmetric and symmetric synapses are recognized to represent sites of excitatory (eg, glutamatergic) and inhibitory (eg, GABA) signal transmission, respectively, (Eccles, 1964). Layer II/III of the PFC was examined because preliminary data indicated a greater impact of PCP on synapses in this layer, and because layer II/III is critical for cognitive function, being the origin and termination of profuse corticocortical connections (Fuster, 1997). In addition, the mediodorsal thalamus, a region implicated in the pathology of schizophrenia (Cronenwett and Csernansky, 2010), projects heavily to layer III of PFC (Wang and Shyu, 2004), and virtually all of the inputs from mediodorsal thalamus to layer III of rat PFC make asymmetric synapses on dendritic spines (Rotaru et al, 2005). As the loss of dendritic spine synapses in the PFC may contribute in part to the PCP-induced cognitive dysfunction and decreased prefrontal cellular activity observed in the rat model (Jentsch and Roth, 1999), we have examined the time course of the loss of asymmetric spine synapses on pyramidal neurons in layer II/III, and the relationship of synapse loss to the cognitive deficits observed following PCP treatment. As there is a loss of dopamine (DA) tone in the PFC in our PCP model (Jentsch et al, 1998), we also examined the effects of the atypical antipsychotic drug, olanzapine, which preferentially elevates DA release and turnover in the PFC of rodents (Jentsch et al, 2000; Kuroki et al, 1999; Li et al, 1998). This agent, similar to clozapine, has been shown to have some benefit in treatment of cognitive deficits associated with schizophrenia (Keefe et al, 2007; Woodward et al, 2005). Olanzapine was chosen for this study as chronic administration of this atypical antipsychotic drug was shown to reverse the dystrophic changes in dendrites of PFC pyramidal neurons induced by DA denervation of the PFC (Wang and Deutch, 2008). In the current study, olanzapine was given chronically and acutely to determine whether the loss of PFC asymmetric spine synapses elicited by PCP treatment is influenced by treatment with this atypical antipsychotic drug.
MATERIALS AND METHODS
Animals
Adult male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in a group (three/cage), except for during oral drug treatment when single housing was necessary. Animals were maintained on a 12/12 h light/dark cycle and provided with unlimited access to water and rat chow. All animal protocols used in this study were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Yale University.
Effect of Repeated PCP on Asymmetric Synapse Number in Layer II/III and V of PFC
Two groups of rats received intraperitoneal (i.p.) injections of either 5 mg/kg PCP hydrochloride (n=6), Sigma-Aldrich, St Louis, MO, USA) dissolved in sterile saline or 1 ml/kg saline vehicle (n=6) twice daily for 7 days (Jentsch et al, 1997b). At 1 week later, rats were killed under pentobarbital anesthesia (to effect, ⩾60 mg/kg i.p.) by transcardial perfusion of heparinized saline followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.35) (Hajszan et al, 2006).
Time Course of the Effect of PCP on Asymmetric Synapse Number in Layer II/III of PFC
Two additional groups of rats received either repeated PCP (n=12) treatment or saline (n=8) for 7 days (as above). These rats were killed under pentobarbital anesthesia, as described above, at 2 weeks (PCP n=4, saline n=4), 3 weeks (PCP n=4), or 4 weeks (PCP n=4, saline n=4) after finishing PCP treatment. The dosage of PCP and the timing of this study were chosen to match our published data that demonstrated a selective decrease in prefrontal cortical DA turnover and cognitive deficits, which were sustained for 1 month after the last PCP injection (Jentsch, 1999; Jentsch et al, 1997b).
Effect of Chronic Olanzapine Following Repeated PCP on Spine Synapse Number
Two other groups of rats (eight per group) were administered either PCP or saline for 7 days, as described above, then four rats from each group were chronically treated with olanzapine or vehicle in their drinking water for 21 days, starting at 1 week after PCP withdrawal. The target dose of olanzapine was 7.5 mg/kg/day. Previous studies have shown that the chosen dose of olanzapine produces plasma levels in the rat that correspond to clinically effective concentrations in man (Aravagiri et al, 1999; Chay and Herman, 1998; Perry et al, 1997), and also occupies central D2 receptors at a level comparable to that seen clinically (Perez-Costas et al, 2008). Olanzapine was dissolved in 0.1 M acetic acid and diluted 1 : 100 in 0.75% sucrose-sweetened water. The weight of each rat and the volume it drank each day was measured daily. The initial concentration of olanzapine in drinking water was 0.105 mg/ml, but this was adjusted between 0.06 and 0.11 mg/ml to accommodate individual variation in daily consumption. In the first week, the mean intake of olanzapine was 8.9 mg/kg/day, the second week it was 8.2 mg/kg/day, and in the final week it was 8.1 mg/kg/day, equivalent to a mean of dose of 8.4 mg/kg/day over the 3-week period. Mean weight gain in the olanzapine-treated group over the 21-days treatment period was 137±4 g; in the control group the weight gain over this period was 137±6 g. Rats were killed under pentobarbital anesthesia by transcardial perfusion with fixative at 4 weeks after PCP withdrawal, as described above.
Acute Olanzapine
Two other groups of rats (eight per group) were administered either PCP or saline, for 7 days as described above, then at 1 week after ending PCP treatment, four from each group were acutely treated with olanzapine (1.5 mg/kg i.p.) or vehicle and killed 90 min later, as described above.
Electron Microscopic Stereology
After perfusion fixation, brains were removed and postfixed overnight in the same fixative without glutaraldehyde. Blocks were cut with the help of a coronal rat brain matrix (Zivic Laboratories, Pittsburgh, PA) to ensure that sectioning was made uniformly in the same coronal plane. The left or right prefrontal region was randomly selected from each brain for further processing. Subsequently, 100 μm coronal vibratome sections were cut and collected, beginning at the rostral pole of the forceps minor of corpus callosum and up to the rostral pole of the genu corpus callosum. The resultant vibratome sections per animal were divided into three portions using systematic, uniformly random sampling (Hajszan et al, 2006). As a result, sections #1, 4, 7, 10, and so on were sorted into the first portion, sections #2, 5, 8, 11, and so on went into the second portion, and sections #3, 6, 9, 12, and so on made up the third portion.
The number of spine synapses was calculated in layer II/III and layer V of the prelimbic cortex using the physical disector technique (Sterio, 1984) as published previously (Hajszan et al, 2006). Briefly, a randomly selected portion of prelimbic sections, containing 10 coronal vibratome sections collected at an interval of approximately 300 μm, were flat embedded in Durcupan (Electron Microscopy Sciences, Fort Washington, PA) between slides and coverslips. First, using these embedded sections, the volume of the two sampling areas (layer II/III and V of prelimbic cortex) was estimated separately using the Cavalieri Estimator module of the Stereo Investigator System (MicroBrightField, Villiston, VT) mounted on a Zeiss Axioplan 2 light microscope. Because the cytoarchitectonic borders of the prelimbic cortex are debated, we applied custom criteria to define our sampling area, as published previously (Hajszan et al, 2006). Thereafter, a trapezoidal-shape disector site for electron microscopic analysis was localized in both sampling areas using a systematic random approach, as previously described (see Figure 1 in Hajszan et al, 2006). At the disector sites, digitized electron micrographs at a final magnification of 11 000 × (Figure 1) were taken of the physical disector in a Tecnai-12 transmission electron microscope (FEI Company, Hillsboro, OR) furnished with a Hamamatsu digital camera system (Hamamatsu Photonics, Hamamatsu, Japan). This sampling technique provided 10 disectors for each of layer II/III and layer V, ie, 20 disectors per brain altogether.
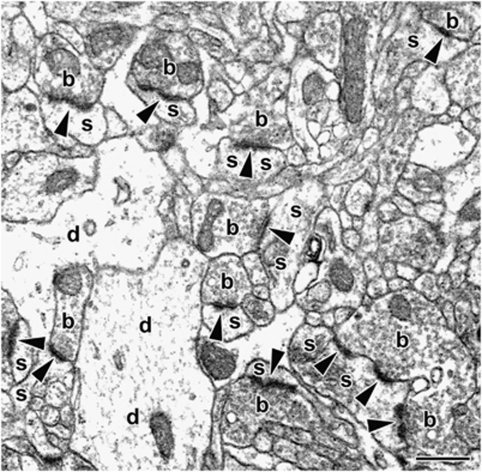
Figure 1.
Representative electron micrograph demonstrating asymmetric spine synapses between dendritic spines (s) and boutons (b) in layer II/III of rat prefrontal cortex. Arrowheads point to postsynaptic densities. d, dendritic shafts; scale bar= 500 nm.
Before synapse counting, the pictures were coded for blind analysis. Asymmetric spine synapses were counted according to the rules of the disector technique (Sterio, 1984), aided by a counting grid with an area of 79 μm2, and usually there were at least 100 per sampling area. Synapsing spines were identified by the presence of synaptic densities, as well as by the absence of mitochondria, microtubules, and synaptic vesicles. Boutons were recognized by the presence of synaptic vesicles and mitochondria. Asymmetric spine synapses were identified between spines and boutons by the characteristically electron-dense postsynaptic density (Figure 1, arrowheads). The average volumetric density of asymmetric spine synapses (synapse/μm3) within each sampling area was then determined by dividing the sum of counted synapses by the disector volume (118.8 μm3). Finally, the average volumetric density was multiplied by the volume of the sampling area, determined earlier, to arrive at the total number of asymmetric spine synapses. For more details of this technique, please see our previous paper (Hajszan et al, 2006). As interneurons in the rat PFC are essentially aspiny and receive the vast majority of their synaptic inputs on dendritic shafts (Gabbott et al, 1997), the counted asymmetric spine synapses in the present study are assumed to be located on pyramidal cells.
RESULTS
Asymmetric Spine Synapse Loss in Layer II/III and Layer V of PFC of PCP-Treated Rats
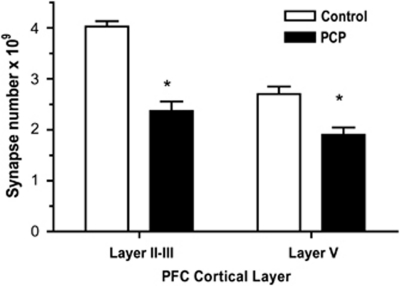
Initially, we extended our earlier finding that demonstrated a significant PCP-induced loss of asymmetric spine synapses in layer II/III of PFC to layer V. At 7 days after PCP treatment, we observed a 30% decrease in the number of asymmetric spine synapses in layer V of PFC (Figure 2) compared with the 41% reduction we observed in layer II/III (Hajszan et al, 2006). The number of asymmetric dendritic spine synapses was significantly higher in layer II/III than in layer V in the saline-treated control group (4.0 and 2.7 billion, respectively, t(6)=3.5, p<0.05). Our remaining studies focused on layer II/III, as the total number of spine synapses and the spine synapse loss in PCP-treated rats was less robust in layer V than layer II/II, and because of the importance of layer II/III to cognition (see Introduction).
Figure 2.
Loss in asymmetric spine synapses in layer II/III and layer V of PFC in PCP-treated rats at 1 week after PCP administration. Unpaired two-tailed t-test showed a significant effect of PCP treatment compared with saline-treated controls in layer II/III (t(10)=7.7, p<0.0001) and in layer V (t(10)=3.9, p<0.005) indicated by asterisks. There were six rats in each group.
Time Course of PCP-induced PFC Asymmetric Spine Synapse Loss
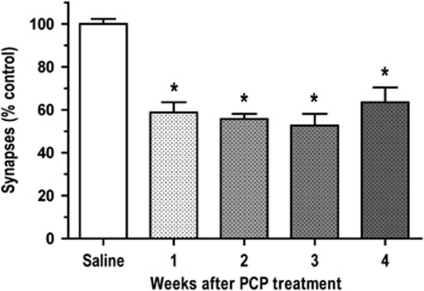
In rats withdrawn from repeated PCP treatment, there was a persistent loss of spine synapses in PFC. A 1 month time-course study of asymmetric spine synapse number in layer II/III of PFC after PCP treatment revealed that the decrease in number of spine synapses was sustained throughout the whole period. The decrease in number of asymmetric spine synapses observed at 2, 3, and 4 weeks after PCP treatment was not significantly different from the 41% decrease in number of spine synapses in layer II/III of the PFC occurring at 1 week after PCP administration (Figures 2 and 3).
Figure 3.
Sustained loss of asymmetric synapses in layer II/III of PFC following PCP treatment. One-way ANOVA showed a significant effect of treatment group on synapse number (F(4, 23)=30.0, p<0.0001), indicated by asterisks. Scheffe's test showed that all time points (1, 2, 3, or 4 weeks) examined after PCP were not significantly different from each other, but all time points were significant decreased compared with the saline group (p<0.0001). There were 4–8 rats in each group.
Olanzapine Reverses PCP-induced Loss of PFC Asymmetric Spine Synapses
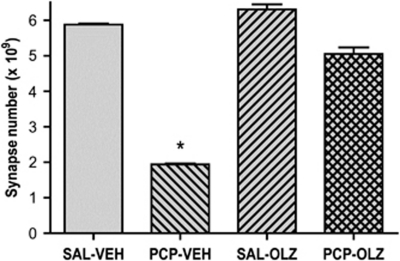
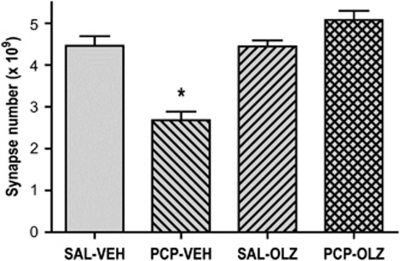
Administration of olanzapine or vehicle was initiated at 1 week after PCP treatment and continued for 3 weeks (Figure 4). A significant decrease in number of asymmetric spine synapses was observed at 1 week following PCP treatment. Oral administration of olanzapine (8 mg/kg/day) produced a striking restoration of spine synapse number. The number of asymmetric synapses in saline-treated rats given olanzapine was not significantly different from the number in saline-treated rats given vehicle.
Figure 4.
Chronic oral olanzapine reverses PCP-induced loss of asymmetric spine synapses in layer II/III of PFC. One-way ANOVA revealed a significant effect of treatment group (F(3, 12)=284, p<0.001). Scheffe's post-hoc test showed that PCP-treated rats given chronic oral vehicle (PCP-VEH) had a significant decrease in number asymmetric synapses in PFC compared with saline-treated rats given chronic oral vehicle administration (SAL-VEH) indicated by an asterisk; this effect was reversed in PCP-treated rats given chronic oral administration of olanzapine (PCP-OLZ). The number of asymmetric synapses in saline-treated rats given chronic oral olanzapine (SAL-OLZ) was not significantly different from the number in saline-treated rats given chronic vehicle (SAL-VEH). There were four rats in each group.
Other rats were killed at 90 min after an acute dose of olanzapine, given at 1 week after PCP or saline treatment. Analysis of the PFC revealed that olanzapine treatment led to a rapid reversal of the asymmetric spine synapse loss induced by PCP (Figure 5). There was no significant difference in number of asymmetric spine synapses in saline-treated rats given olanzapine compared with saline-treated rats given vehicle.
Figure 5.
Acute treatment with olanzapine (1.5 mg/kg i.p.) reverses PCP-induced loss of asymmetric spine synapses in layer II/III of PFC. One-way ANOVA revealed a significant effect of treatment group (F(3, 14)=24.8, p<0.0001). Scheffe's post-hoc test showed that PCP-treated rats given acute vehicle (PCP-VEH) had a significant decrease in number asymmetric synapses in PFC compared with saline-treated rats given acute vehicle administration (SAL-VEH) indicated by an asterisk; this effect was reversed in PCP-treated rats given acute olanzapine (PCP-OLZ). The number of asymmetric synapses in saline-treated rats given acute olanzapine (SAL-OLZ) was not significantly different from the number in saline-treated rats given acute vehicle (SAL-VEH). There were four rats in each group, except SAL-OLZ, which contained three rats.
DISCUSSION
The present data clearly show that in the rat PCP model there is a marked, lasting, and consistent loss of asymmetric synapses on dendritic spines of pyramidal neurons in layer II/III of PFC, which can be reversed by subsequent olanzapine treatment. These findings extend our earlier study (Hajszan et al, 2006) by showing that the loss of asymmetric spine synapses in the PFC of PCP-treated animals is sustained for at least a month, which parallels the time course of cognitive deficits and decreased DA turnover in PFC observed in this model (Abdul-Monim et al, 2003; Jentsch et al, 2006; McLean et al, 2010). Furthermore, chronic treatment with olanzapine, an atypical antipsychotic drug, which increases DA turnover and release in PFC, (Dazzi et al, 2004) and ameliorates the PCP-induced cognitive deficits of PCP-treated rats (McLean et al, 2010), was shown to reverse the asymmetric spine synapse loss in PFC, indicating that PCP-induced dystrophic changes in PFC are not permanent. Unexpectedly, an acute dose of olanzapine, which transiently elevates PFC DA turnover (Jentsch and Roth, 2000) and reverses the cognitive deficit in PCP-treated rats (Abdul-Monim et al, 2006; McLean et al, 2010), also reversed the asymmetric spine synapse loss in the PFC. Whether this remodeling is long lasting or transient, and/or dependent upon brain levels of olanzapine, remains to be determined. To our knowledge, this is the first electron microscopic demonstration that olanzapine is capable of eliciting spine synapse remodeling in the PFC.
The mechanisms responsible for synaptic remodeling were not directly addressed in this study, but because of the pharmacology of PCP, they likely involve alterations in glutamatergic and dopaminergic function (Hajszan et al, 2006). Olanzapine is an atypical antipsychotic drug that is capable of normalizing dopaminergic neurotransmission in PFC (Kuroki et al, 1999; Li et al, 1998), which suggests that prefrontal dopaminergic tone may be a critical component in spine synapse remodeling in the PCP model. It is possible that a similar situation exists in schizophrenia, where there is evidence for loss of DA tone together with dystrophic changes in the PFC (see Introduction and Akil et al, 1999). The cognitive deficits resulting from a lesion-induced dopaminergic dysfunction in PFC is known to be severe (Brozoski et al, 1979), supporting the potential link between DA neurotransmission and cognitive function. The morphological consequences of a disruption to DA transmission in PFC has been demonstrated by a recent study, which showed that selective damage to the dopaminergic innervation of PFC causes a loss of dendritic spines on pyramidal cells in the rat, whereas the restoration of dopaminergic tone with olanzapine reversed this effect (Wang and Deutch, 2008). Our data builds on the data of Wang and Deutch (2008) by demonstrating that that olanzapine treatment counteracts the asymmetric synapse loss in the PFC induced in the PCP model. Furthermore, the ability of olanzapine to restore asymmetric synapse number when given acutely is consistent with a dopaminergic mechanism, as a rapid elevation of DA levels in the PFC is induced by olanzapine, which lasts at least 120 min after i.p. injection at the dose used here (Dazzi et al, 2004). In the PFC, some dopaminergic synapses are part of a synaptic complex in which the dendritic spine of a pyramidal neuron is innervated by both a dopaminergic symmetric synapse and a glutamatergic asymmetric synapse (Goldman-Rakic et al, 1989), an arrangement that allows for direct dopaminergic modulation of the overall excitability of cortical projection neurons by influencing local spine responses to excitatory inputs. Dopaminergic innervation of the PFC is not exclusive to a particular layer, and varies with species and even with subregion of the PFC. In primate PFC, DA innervation favors superficial layers, whereas in rodent PFC, DA terminals are denser in deeper layers (Smiley and Goldman-Rakic, 1993; Van Eden et al, 1987). Dopaminergic terminals usually form symmetrical synapses with the dendritic tree of either pyramidal (glutamatergic) cells or GABAergic interneurons in the PFC (Sesack et al, 1995). The exact changes in PFC circuitry caused by the olanzapine-induced increase in local DA tone are hard to determine, and are further complicated by changes in feedback pathway activity. Nevertheless, the present data demonstrate that repeated PCP treatment, which elicits a decrease in DA tone in the PFC, and olanzapine, which increases DA release in PFC have opposite effects on asymmetric synapse number in layer II/III of rat PFC, a region important for cognitive function. Thus, altered prefrontal dopaminergic neurotransmission in PCP models of schizophrenia may provoke both morphological changes, in the form of prefrontal spine synapse remodeling, and cognitive deficits.
It has to be noted, however, that modulation of dopaminergic neurotransmission in PFC is not the only way for olanzapine to exert its restorative effects on asymmetric spine synapses in the PCP model. For example, it has been reported that in addition to increasing prefrontal DA turnover, chronic administration of olanzapine also elevates brain-derived neurotrophic factor (BDNF) levels in PFC (Czubak et al, 2009; Pillai, 2008). BDNF is a key neurotrophic factor in the brain, and has been shown to have an important role in the formation, maturation, and plasticity of glutamatergic and GABAergic synapses and likely functions as a synaptic morphogen (Carvalho et al, 2008; Gottmann et al, 2009). However, it is not clear whether the effects of olanzapine on BDNF levels in PFC are direct, or indirect, such as by its modulation of dopaminergic tone. Lesions and other manipulations of mesocortical dopaminergic function cause significant deficits in cognitive performance (Pioli et al, 2008) and also alter BDNF gene expression in frontal cortical regions (Fumagalli et al, 2003). It is relevant to the current study that BDNF expression and release can change rapidly (minutes to hours) in response to synaptic activity (Poo, 2001). In addition, there are some interesting and relevant parallels between the present results with the PCP model and a chronic stress model in rodents. Thus, chronic stress has been shown to result in a lowered basal DA tone and a deficient dopaminergic response to stimulation in the PFC together with impaired cognition, a decrease in BDNF in the PFC, and a decrease in the number of dendritic spines in PFC (Mizoguchi et al, 2000; Radley et al, 2006; Ueyama et al, 1997). More importantly, there is evidence that DA directly regulates BDNF expression in striatal cells in vitro (Kuppers and Beyer, 2001), suggesting the involvement of DA in the regulation of prefrontal BDNF levels. Microdialysis studies have shown that olanzapine elicits a rapid and sustained increase in DA release in the PFC (Dazzi et al, 2004). Thus, direct DA regulation of BDNF in the PFC might explain the rapid effects of olanzapine in reversing the spine synapse loss induced by PCP. It is relevant, therefore, that accumulating preclinical and clinical data indicate a potential involvement of neurotrophins in the pathogenesis and therapy of schizophrenia (Buckley et al, 2007; Green et al, 2010; Shoval and Weizman, 2005). In fact, alterations in BDNF levels have been reported in PFC and CSF of schizophrenic patients, even in antipsychotic-naive first-episode subjects (Durany et al, 2001; Hashimoto et al, 2005; Issa et al, 2010; Weickert et al, 2003). Thus, olanzapine-induced increases in DA turnover and BDNF levels may function individually or in concert to contribute to the effectiveness of olanzapine in reversing the enduring dystrophic changes in dendrites and asymmetric spine synapses seen following mesocortical lesions (Wang and Deutch, 2008) or chronic PCP treatment.
In summary, our findings indicate that an enduring loss of asymmetric spine synapses in PFC may, together with the decreased DA function, in part underlie the cognitive deficits observed in the PCP model of schizophrenia (Abdul-Monim et al, 2006; Jentsch, 1999; Jentsch et al, 1997a, 1997b). Our finding that olanzapine given acutely or chronically in a dosage schedule that increases DA turnover and release, and also reverses the PCP-induced cognitive deficit, acts to normalize asymmetric spine synapse number in PFC. The observed PCP- and olanzapine-induced spine synapse remodeling in PFC may involve modulation of glutamatergic and dopaminergic tone, as well as changes in BDNF levels. These findings suggest that dystrophic changes in PFC pyramidal cell dendrites associated with schizophrenia, may not be permanent, but might be reversed with appropriate treatment. The animal PCP model provides a useful paradigm to investigate and modulate plasticity in the PFC with chemical or biological agents.
Acknowledgments
This work was supported by grant MH-57483 from NIMH. We thank Klara Szigeti-Buck and Dorothy Cameron for their excellent technical work.
The authors declare no conflict of interest.
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J Psychopharmacol. 2003;17:57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169:263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Aravagiri M, Teper Y, Marder SR. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos. 1999;20:369–377. doi: 10.1002/1099-081x(199911)20:8<369::aid-bdd200>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Mahadik S, Pillai A, Terry A., Jr Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153 (Suppl 1:S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay SH, Herman JL. [Disposition of the novel anti-schizophrenic drug [14C]olanzapine in male Fischer 344 and female CD rats following single oral dose administration] Arzneimittelforschung. 1998;48:446–454. [PubMed] [Google Scholar]
- Cosgrove J, Newell TG. Recovery of neuropsychological functions during reduction in use of phencyclidine. J Clin Psychol. 1991;47:159–169. doi: 10.1002/1097-4679(199101)47:1<159::aid-jclp2270470125>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- Czubak A, Nowakowska E, Kus K, Burda K, Metelska J, Baer-Dubowska W, et al. Influences of chronic venlafaxine, olanzapine and nicotine on the hippocampal and cortical concentrations of brain-derived neurotrophic factor (BDNF) Pharmacol Rep. 2009;61:1017–1023. doi: 10.1016/s1734-1140(09)70163-x. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Seu E, Cherchi G, Biggio G. Inhibition of stress-induced dopamine output in the rat prefrontal cortex by chronic treatment with olanzapine. Biol Psychiatry. 2004;55:477–483. doi: 10.1016/j.biopsych.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- Eccles J. The Physiology of Synapses. Springer-Verlag: Heidelberg; 1964. [Google Scholar]
- Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol Psychiatry. 2003;8:898–899. doi: 10.1038/sj.mp.4001370. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Lippincott-Raven: Philadelphia; 1997. [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ.2010Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis Mol Psychiatrye-pub ahead of print 24 August 2010; doi: 10.1038/mp.2010.88(in press). [DOI] [PubMed]
- Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry. 2006;60:639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Issa G, Wilson C, Terry AV, Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis. 2010;39:327–333. doi: 10.1016/j.nbd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Jentsch JD.1999Neurochemical substrates of cognitive dysfunction produced by the psychotomimetic drug phencyclidinePh.D. thesis, Yale University, New Haven, CT.
- Jentsch JD, Dazzi L, Chhatwal JP, Verrico CD, Roth RH. Reduced prefrontal cortical dopamine, but not acetylcholine, release in vivo after repeated, intermittent phencyclidine administration to rats. Neurosci Lett. 1998;258:175–178. doi: 10.1016/s0304-3940(98)00879-9. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997a;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH.2000Effects of antipsychotic drugs on dopamine release and metabolism in the central nervous systemIn: Lidow L (ed).Neurotransmitter Receptors in Actions of Antipsychotic Medications CRC Press: Boca Raton, Florida; 31–41. [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Shahid M, Wong EHF, Roth RH. Asenapine improves cognition function in monkeys repeatedly exposed to the psychostimulant phencyclidine. Biol Psychiatry. 2006;59:145S. [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997b;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther. 1999;288:774–781. [PubMed] [Google Scholar]
- Li XM, Perry KW, Wong DT, Bymaster FP. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl) 1998;136:153–161. doi: 10.1007/s002130050551. [DOI] [PubMed] [Google Scholar]
- McLean SL, Neill JC, Idris NF, Marston HM, Wong EH, Shahid M. Effects of asenapine, olanzapine, and risperidone on psychotomimetic-induced reversal-learning deficits in the rat. Behav Brain Res. 2010;214:240–247. doi: 10.1016/j.bbr.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115:745–753. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PJ, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response in acutely ill schizophrenic patients. J Clin Psychopharmacol. 1997;17:472–477. doi: 10.1097/00004714-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A. Decreased expression of Sprouty2 in the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder: a correlation with BDNF expression. PLoS One. 2008;3:e1784. doi: 10.1371/journal.pone.0001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioli EY, Meissner W, Sohr R, Gross CE, Bezard E, Bioulac BH. Differential behavioral effects of partial bilateral lesions of ventral tegmental area or substantia nigra pars compacta in rats. Neuroscience. 2008;153:1213–1224. doi: 10.1016/j.neuroscience.2008.01.084. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. J Comp Neurol. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. 2005;15:319–329. doi: 10.1016/j.euroneuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereb Cortex. 1993;3:223–238. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Buchanan RW, Gold JM. The role of negative symptoms and cognitive dysfunction in schizophrenia outcome. Int Clin Psychopharmacol. 1998;13 (Suppl 3:S21–S26. doi: 10.1097/00004850-199803003-00004. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Tone S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997;28:103–110. doi: 10.1016/s0168-0102(97)00030-8. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Hoorneman EM, Buijs RM, Matthijssen MA, Geffard M, Uylings HB. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- Wang CC, Shyu BC. Differential projections from the mediodorsal and centrolateral thalamic nuclei to the frontal cortex in rats. Brain Res. 2004;995:226–235. doi: 10.1016/j.brainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]