Abstract
Two sisters presented with lower limb deformity and difficulty in walking without support. Both had short stature; however, neurodevelopment and secondary sexual characters were normal. Abdominal examination revealed splenomegaly and ophthalmic examination showed presence of Kayser–Fleischer (K–F) rings. Diagnosis of Wilson’s disease was confirmed with low serum copper and ceruloplasmin levels. Further investigations revealed urinary acidification defect with hypercalciuria pointing towards distal renal tubular acidosis. Both patients were started on copper chelation therapy and showed gradual radiographic improvement in osteopaenia.
Background
Wilson’s disease is a rare autosomal recessive disorder of copper metabolism transmitted by mutant (ATP7B) gene on chromosome 13q14-21.1 It may present with acute or chronic neurological or hepatic symptoms. Rare manifestations are renal calculi, haemolytic anaemia and bone pathologies. Renal involvement in Wilson’s disease occurs in the form of renal tubular defects (renal tubular acidosis (RTA) type 1 and 2 and/or Fanconi’s syndrome).2 Here we report an interesting case of metabolic bone disease due to RTA secondary to Wilson’s disease. This is extremely rare presenting feature of Wilson’s disease.
Case presentation
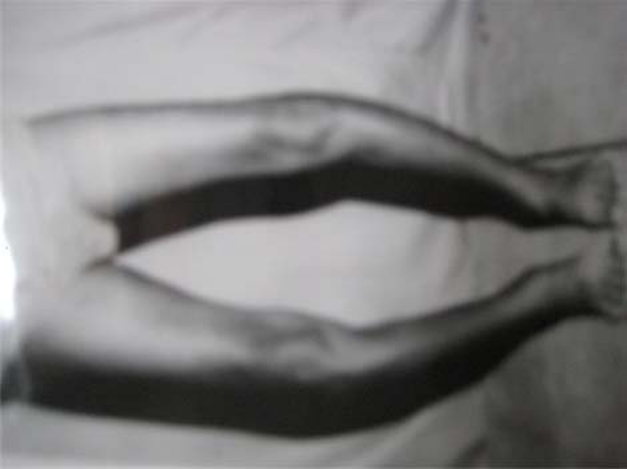
Two Hindu sisters, (A) 15 and (B) 13 years old, third and fifth among five siblings, respectively, school-going and born of a consanguineous marriage, presented with difficulty in walking. Both had bowing of legs (figure 1) which started 1.5 years before and gradually progressed to present status of difficulty walking without support. The elder sister was treated for spontaneous left humerus fracture 2 months earlier. The symptoms were not preceded by fever, bone pains or trauma. There was no history of tuberculosis or exposure to a case of tuberculosis. There was no history of chronic diarrhoea or urinary complaints. There was no history of jaundice or distension of abdomen. There was no history suggestive of delayed milestones, developmental abnormalities or scholastic backwardness. Both of them had history of adequate sun exposure. Family history was not contributory. The elder sister had achieved menarche at the age of 13 years but had secondary amenorrhea. The younger sibling had not achieved menarche yet. Both had short stature. All vital parameters were normal (table 1). There was no pallor, icterus, lymphadenopathy or oedema feet. Secondary sexual characters were well developed. Both had bowing of legs. The elder sister had pathological fracture of shaft of left humerus. There was no rachitic rosary or frontal bossing. Ophthalmic examination showed presence of Kayser–Fleischer (K–F) ring which was later confirmed on slit lamp. Abdominal system examination revealed presence of moderate, non-tender splenomegaly of 10 cm and 8 cm in A and B, respectively. Liver was just palpable in patient A. There was no evidence of free fluid in the abdomen. Cardiac, respiratory and central nervous system examinations were unremarkable. They belonged to lower socioeconomic class with their father, a manual labourer, being the sole earning member of the family.
Figure 1.
Picture showing bowing of the legs in patient B.
Table 1.
Patient characteristics
| Patient A | Patient B | |
|---|---|---|
| Weight (kg) | 28 | 25 |
| Height (cm) | 147 | 132 |
| US (cm) | 67 | 64 |
| LS (cm) | 80 | 74 |
| Ratio of US/LS | 0.827 | 0.864 |
LS, lower segment; US, upper segment.
Investigations
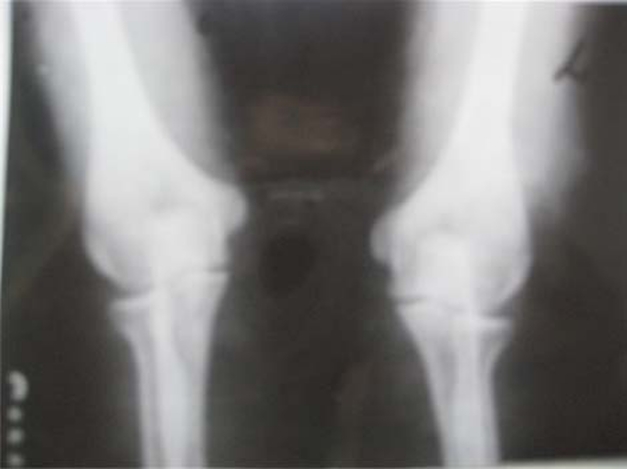
Complete haemogram, random blood sugar, renal function and liver function tests were normal in both. Both were hepatitis B surface antigen, anti-hepatitis C virus (anti-HCV) and PCR HCV negative. Twenty-five hydroxyl cholecalciferol levels were normal. However, arterial blood gases revealed mild non-anion gap metabolic acidosis. X-ray chest and bones revealed marked osteopaenia (figure 2). Patient A had presence of a healing fracture of shaft of right humerus. Ultrasound of the abdomen confirmed moderate splenomegaly. Mild hepatomegaly was present in both with coarse echotexture. Portal hypertension was present (portal vein 12 mm and partially recanalized thrombus in patient B). Oesophagogastroduodenoscopy showed presence of grade I varices in patient A. Varices were absent in patient B. However, echotexture of liver was not suggestive of cirrhosis on liver scan. Serum copper and ceruloplasmin levels were found to be low in both (table 2). Presence of K–F ring, low serum copper, low ceruloplasmin levels, altered liver echotexture and portal hypertension confirmed the diagnosis of Wilson’s disease. In view of bone deformities, minimal metabolic acidosis and generalised osteopaenia, patients were worked up to rule out RTA as shown in (table 3). Urinary pH was inappropriately high for the degree of metabolic acidosis (tables 2 and 3). There was evidence of hypercalciuria. Fractional excretion of bicarbonate was <5% with high copper excretion confirming distal (type1) RTA.
Figure 2.
X-ray around the elbow joint of patient A, showing osteopaenia.
Table 2.
Table showing the blood investigations of both patients
| Tests | Patient A | Patient B |
|---|---|---|
| Serum alkaline phosphatase (IU/l) | 493 | 1092 |
| Serum calcium (mg%) | 8.2 | 8.4 |
| Serum inorganic phosphate (mg%) | 3.3 | 2.6 |
| Serum uric acid (mg%) | 1.8 | 2.0 |
| Serum sodium (mEq/l) | 141 | 140 |
| Serum potassium (mEq/l) | 4.2 | 3.8 |
| Serum chloride (mEq/l) | 114 | 116 |
| Blood pH | 7.28 | 7.28 |
| Bicarbonate (mmol/l) | 16.7 | 15.2 |
| Plasma anion gap | 10.3 | 8.8 |
| Serum copper levels (OD) | 0.018 | 0.027 (NR=2–5) |
| Serum ceruloplasmin (mg/dl) | 12 | 15 (NR=20–50) |
| 25 hydroxy vitamin D (ng/ml) | 21.9 | 10 (NR=9.9–41.5) |
Table 3.
Table comparing the urine analysis of both patients
| Urine | Patient A | Patient B |
|---|---|---|
| pH | 7.4 | 6.5 |
| Proteins | Absent | Absent |
| Sugar | Absent | Absent |
| Sodium (mEq/l) | 260 | 202 |
| Potassium (mEq/l) | 15.6 | 21.2 |
| Chlorides (mEq/l) | 162 | 186 |
| Urinary anion gap | 113.6 | 37.2 |
| Bicarbonate (mEq/l) | 13.5 | 27.2 |
| Fractional excretion of bicarbonate (%) | <5 | <5 |
| Citrate | Absent | Absent |
| Amino acids | Absent | Absent |
| Calcium (mEq/l) | 135.6 | 157.5 |
| 24 h urinary copper (µg/day) | 103.87 | 120 (N=10–30 µg/day) |
Differential diagnosis
-
▶
Nutritional vitamin D deficiency – osteomalacia, rickets.
-
▶
Hypophosphatemic rickets – autosomal dominant or X linked dominant.
-
▶
RTA.
Treatment
Patients were diagnosed as Wilson’s disease with distal (type1) RTA. They were treated with tablet penicillamine 250 mg/day which was increased gradually to 750 mg/day given in divided doses along with 20mg/day of tablet pyridoxine. Elemental zinc 50 mg three times a day was also started.
Outcome and follow-up
On follow-up after 3 months, x-rays revealed improvement in osteopaenia. Serial follow-up of urinary copper levels was not feasible because of financial constraints. However, patients continue to do well on routine neurological evaluation and liver function tests.
Discussion
Skeletal abnormalities may be found in Wilson’s disease, but has attracted little attention. Spontaneous fractures, osteoarthritis, osteomalacia, osteochondritis dissecans, chondrocalcinosis, subchondral cyst formation and azure lunulae of the fingernails have been described.3 Radiographic abnormalities described include osteoporosis/osteopaenia, osteomalacia (rickets in children) and some distinct changes like marginal bone fragments, angulation of carpal bones, squaring of metacarpal heads and calcification of the joint capsule or tendon insertion.4 Radiographic evidence of osteoporosis is present in up to 88% of persons with Wilson’s disease.3 5 Joint involvement, particularly knees and spine, is also common, and joint pain may be the presenting symptom of Wilson’s disease.5 A sole case of Wilson’s disease presenting with RTA and osteomalacic osteodystrophy has been reported.6 The osteomuscular syndrome of Wilson’s disease seems to be peculiar to the Indian subcontinent as 13 out of 17 cases so far reported come from India.7 8
Our patients presented unusually with skeletal symptoms like pathological fracture and bowing of legs. They had normal vitamin D levels despite having osteopaenia on plain radiograph. Wilson’s disease was suspected in both because of the presence of splenomegaly suggesting portal hypertension and K–F rings on slit lamp examination. It was further confirmed with low serum copper, ceruloplasmin levels and high 24 h urinary copper levels. Liver biopsy was not performed. Renal involvement in the form of distal RTA (dRTA) was proven because of non-anion gap metabolic acidosis, positive urinary anion gap, hypercalciuria and low fractional excretion of bicarbonate. Osteopaenia in these patients was as a result of dRTA causing acidosis and urinary calcium loss.
This highlights the fact that metabolic bone disease may be caused by underlying disorders of renal tubules causing impairment of reabsorption of calcium, phosphate or 1,25 dihydroxy vitamin D3 synthesis. The cause for renal tubular dysfunction like Wilson’s disease can easily be missed because of its subtle manifestations unless sought for.
Learning points.
-
▶
Wilson’s disease may not always have classic neuropsychiatric and/or hepatic manifestations.
-
▶
Rickets- and osteomalacia-like features may be due to causes other than vitamin D deficiency.
-
▶
Wilson’s disease can rarely involve renal tubules and bone.
-
▶
RTA causing osteomalacia/rickets can be a rare presenting feature of Wilson’s disease.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Ala A, Walker AP, Ashkan K, et al. Wilson’s disease. Lancet 2007;369:397–408 [DOI] [PubMed] [Google Scholar]
- 2.Kalra V, Mahajan S, Kesarwani PK. Rare presentation of Wilson’s disease: a case report. Int Urol Nephrol 2004;36:289–91 [DOI] [PubMed] [Google Scholar]
- 3.Golding DN, Walshe JM. Arthropathy of Wilson’s disease. Study of clinical and radiological features in 32 patients. Ann Rheum Dis 1977;36:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie YZ, Zhang XZ, Xu XH, et al. Radiologic study of 42 cases of Wilson disease. Skeletal Radiol 1985;13:114–9 [DOI] [PubMed] [Google Scholar]
- 5.Canelas HM, Carvalho N, Scaff M, et al. Osteoarthropathy of hepatolenticular degeneration. Acta Neurol Scand 1978;57:481–7 [DOI] [PubMed] [Google Scholar]
- 6.Rais N, Dalal D, Bhandarkar SD, et al. Wilson’s disease (a case report). J Postgrad Med 1980;26:149–51 [PubMed] [Google Scholar]
- 7.Dastur DK, Manghani DK, Wadia NH. Wilson’s disease in India. I. Geographic, genetic, and clinical aspects in 16 families. Neurology 1968;18(1 Pt 1):21–31 [DOI] [PubMed] [Google Scholar]
- 8.Joshua GE. Hepatolenticular degeneration (Wilson’s disease) and rickets in children. Indian J Med Res 1973;61:1876–84 [PubMed] [Google Scholar]