Abstract
Liver transplantation is considered as the most effective treatment for end-stage liver disease. However, serious complications still exist, particularly in two aspects: ischemia and subsequent reperfusion of the liver, causing postoperative hepatic dysfunction and even failure; and acute and chronic graft rejections, affecting the allograft survival. Heme oxygenase (HO), a stress-response protein, is believed to exert a protective function on both the development of ischemia-reperfusion injury (IRI) and graft rejection. In this review of current researches on allograft protection, we focused on the HO-1. We conjecture that HO-1 may link these two main factors affecting the prognosis of liver transplantations. In this review, the following aspects were emphasized: the basic biological functions of HO-1, its roles in IRI and allograft rejection, as well as methods to induce HO-1 and the prospects of a therapeutic application of HO-1 in liver transplantation.
Keywords: Liver transplantation, Heme oxygenase-1, Allograft rejection, Ischemia/reperfusion injury
INTRODUCTION
Transplantation remains the main therapeutic option for patients with end-stage liver disease. Thanks to the clinical use of immunosuppressants, acute rejections have been brought under substantial control. However, the adverse effects of these drugs, such as the development of blood hypertension, hyperlipidemia, diabetes, renal failure, and de novo tumors in transplanted patients, are significant, increasing the postoperative mortality. The severe side effects of the immunosuppressants limit their success in attenuating acute rejection. In addition, surgery and preservation of the allografts result in a cascade of ischemia-reperfusion injury (IRI) in the transplantation, for which there are still no effective therapeutic interventions. Consequently, the strategies to simultaneously attenuate IRI and induce donor-specific tolerance would considerably improve the quality of life and survival of the transplant recipients.
The liver, an immunologically privileged organ, bears inherent tolerogenic properties in the event of orthotopic liver transplantation (OLT). Liver allografts could be established and maintained even without immunosuppressants[1]. In humans, liver transplants can also confer protection on other organ grafts stemming from the same donor[2]. Based on the aforementioned characteristics of the liver, it seems more feasible to induce a donor-specific tolerance in liver transplantations than in the case of transplantations of other solid organs.
More attentions have been paid to heme oxygenase (HO)-1 because of its cytoprotective, antioxidant, maintaining microcirculation, modulating the cell cycle and anti-inflammatory functions[3]. In the process of a liver transplantation, many cell types, including Kupffer cells, endothelial cells, and dendritic cells (DCs), can induce an HO-1 overexpression to prevent IRI and rejections[4-6]. Since HO-1 seems to be involved in both processes, it may act as a linkage between IRI and rejection in liver transplantation in order to induce donor-specific tolerance.
BASIC BIOLOGICAL FUNCTIONS OF HO-1 AND ITS BYPRODUCTS
HOs are rate-limiting enzymes in the heme catabolism. The heme catabolism by HO-1 produces carbon monoxide (CO), free iron, and biliverdin that is subsequently converted to bilirubin by biliverdin reductase[7]. Three HO isozymes have been identified: HO-1, HO-2 and HO-3. HO-1 is an inducible enzyme, while the other two are expressed constitutively[8].
HO-1 is a bona fide 32-kDa stress protein (Hsp32), variously manifested in endothelial, epithelial, smooth muscle and other cell types. HO-1 plays a protective role in many disease models via its anti-inflammatory, anti-apoptotic, and anti-proliferative actions[3]. Three products of the heme metabolism are considered to be beneficial due to their immunomodulatory, anti-apoptotic, and vasoactive properties.
CO, despite its potential toxicity, has recently caused a great interest because of its function as a signaling molecule with vasodilatory effects mediated by cGMP, and its antiapoptotic and anti-inflammatory effects[9,10]. CO can travel freely throughout intracellular and extracellular compartments and exert a wide spectrum of modulating physiological effects on multi-systems[11].
Bilirubin, a byproduct, is found to exert a beneficial influence on many diseases, including atherosclerosis, inflammatory, autoimmune, degenerative diseases, and cancer, in which it serves as a highly lipophilic antioxidant[12]. It can slightly reduce ethanol-induced lipid peroxidative injury by decreasing MDA content[9]. In addition, Takamiya et al[13] have demonstrated that HO-1 stabilizes mast cells (MCs) in order to exercise an anti-inflammatory action through bilirubin.
Furthermore, Fe, the third product, despite its cytotoxic pro-oxidant effects, induces an over-expression of ferritin, which in turn has strong antioxidant effects through the depletion of free iron and also by other less characterized effects that result in the induction of tolerogenic dentritic cells[14].
HO-1 ATTENUATES LIVER IRI IN LIVER TRANSPLANTATION
IRI is a continual process that culminates in hepatocellular injury. Clinically, it remains a major obstacle to liver transplantation and can lead to hepatic dysfunction and even post-transplantation failure. As a result, the mechanisms and prevention of cellular injury during hepatic ischemia and subsequent reperfusion needs to be elucidated[15].
Kupffer cells, the resident macrophage population within the liver, play key roles in IRI. They are activated after reperfusion by various stimuli in an autocrine fashion by Toll-like receptor 4 signaling[16], or by complement activation[17,18]. After being activated, they release inflammatory cytokines and free radicals, such as reactive oxygen species (ROS), tumor necrosis factor α (TNFα), interleukin 1 (IL-1), nitric oxide (NO), thromboxanes, and leukotrienes[19], and recruit neutrophils to the liver. TNF and IL-1 can also recruit and activate CD4+T-lymphocytes which maintain Kupffer cell activation by secretion of the granulocyte stimulating factors or interferon (IFN)-γ[20,21]. Of all the hepatic cells, the nonparenchymal sinusoidal endothelial cells (SECs) are most susceptible to IRI[22]. SECs are activated by tissue anoxia that disturbs the intracellular energy metabolism and enzyme function, leading to their apoptosis. These cause marked microcirculatory disturbances, leukocyte and platelet adhesion, diminished blood flow and continuation of the ischemic process, resulting in massive hepatic necrosis[23]. Thus, the inhibition of SEC apoptosis may be a useful therapeutic strategy to reduce the risk of ischemia injury in liver preservation. Yue et al[5] have found that the apoptosis of SECs was attenuated after the TAT-HO-1 was transducted into the liver, which may be associated with an increased expression of Bcl-2 and a reduced expression of Bax.
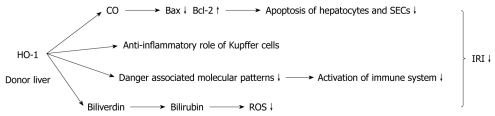
It is well known that it is of critical importance to attenuate IRI in liver transplantation. Both HO-1 and its products of degradation play a role in attenuating IRI (Figure 1). The findings that Hmox-/- animals are more susceptible to IRI injury than the Hmox-/+ and Hmox+/+ animals indicate that HO-1 may play a potent protective role in IRI[24]. A further study has shown that donor livers with an enhanced HO-1 expression lowered the serum ALT/AST levels of the recipient, alleviated allograft injury, and suppressed cytokine release[4]. Luke Devey described a mechanism that HO-1 could drive macrophage differentiation down an “anti-inflammatory” pathway[24]. Therefore, preconditioning the donor liver, especially its Kupffer cells with a strong induction of HO-1, plays a potential protective role. Kupffer cells are not only the main factor associated with liver IRI, but also a major site of expression of the hepatic HO-1. Based on these findings, we can assume that HO-1 in Kupffer cells is induced exclusively to exert a protective function in the event of IRI. Additionally, HO-1 can modulate each stage of the immune activation pathway such as limiting the production of damage-associated molecular patterns, modulating T cell activation, and enhancing immunological tolerance[25].
Figure 1.
Function of heme oxygenase 1 and its degradation product in ischemia and reperfusion injury during liver transplantation. HO-1: Heme oxygenase 1; CO: Carbon monoxide; SEC: Sinusoidal endothelial cell; ROS: Reactive oxygen species; IRI: Ischemia-reperfusion injury.
As previously described, CO mediates a cytoprotective and anti-inflammatory effect in I/R related oxidative injury. It significantly reduces the messenger RNA (mRNA) levels of the proapoptotic Bax, while it up-regulates the anti-apoptotic Bcl-2. Bax and Bcl-2 are both found to be expressed in hepatocytes and SECs at the sinusoidal space. Therefore, CO reduces the IRI-mediated apoptosis through an overexpression of Bcl-2 and diminished Bax expression. This protective role of CO is mediated by an activation of the soluble guanylyl cyclase, as demonstrated by the fact that 1H-(1,2,4)oxadiazole (4,3-α) quinoxaline-1-one (ODQ; a soluble guanylyl cyclase inhibitor), completely reversed its beneficial effect[26].
The oxidation of bilirubin by ROS results in the conversion of bilirubin to biliverdin, the latter being a precursor of bilirubin in the heme degradation that is recycled to bilirubin in mammals by biliverdin reductase. This recycling process between bilirubin and biliverdin is believed to be behind one of the explanations for bilirubin’s powerful antioxidant effects in the redox cycle[27]. However, the exact mechanism of the protective role of HO-1 remains to be fully explained.
Contrary to the aforementioned protective role of HO-1 against oxidant-induced injury and the induction of HO-1 as an adaptive response against oxidative damage, Froh et al[28] reported that a cobalt protoporphyrin (CoPP)-induced HO-1 over-expression increases liver injury, as demonstrated by an over-expression of hepatic ALT, aggravation of cell necrosis, and fibrosis. They suggested that high levels of HO-1 may sensitize cells to oxidative stress due to an accumulation of free divalent iron, thereby increasing oxidative injury. Since Kupffer cells are the main source of HO-1 in the liver, an increased expression of HO-1 may also aggravate the activation of Kupffer cells, thus increasing the formation of inflammatory and fibrogenic mediators. The controversial role of the HO-1 expression in human liver allografts of either cytoprotection or increased cytotoxicity ought to be investigated in more detail in the future.
HO-1 REDUCES REJECTIONS IN LIVER TRANSPLANTATION
The spontaneous graft tolerance of the liver is an active process which depends upon the transfer of donor leukocytes in the liver. Migration within the recipient’s lymphoid system results in an early immune activation of recipient lymphocytes, with their subsequent deletion from exhaustion. Regulatory T cells and antigen presenting cells (APCs) are both involved in inducing donor-specific tolerance.
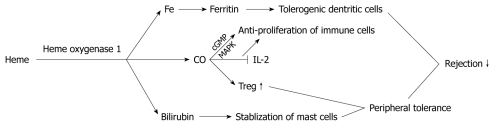
One highly significant tolerance inducing mechanism is the suppression of allogeneic responsiveness by regulatory T cells. Regulatory T cells (Tregs) are a subset of T lymphocytes that play a core role in immunological suppression and the termination of immune responses. Deficiency or dysfunction of these cells may lead to autoimmunity or an aggravated pathogen-induced inflammation[29]. The end-products of HO-1 degradation do not have much organ specificity to reduce rejection (Figure 2). CO could provide one mechanism by which the regulatory capacity of Tregs is generated. CO has been shown to have broad anti-proliferative effects in human CD4+T cells[30]. Generally, CO is thought to perform those anti-proliferative actions by modulating the activity of guanylate cyclase to increase the cellular cGMP levels, and through the MAPK (mitogen activated protein kinase) pathway. More specifically, CO was also shown to block the production of IL-2, a principal cytokine responsible for T cell proliferation[31]. CO, functioning as a nonspecific suppressor, also fits with the observation that Treg cells are capable of suppressing the proliferation of various immune cell types without major histocompatibility complex restriction and in a non-Ag-specific manner.
Figure 2.
Function of heme oxygenase 1 degradation product to reduce rejection. CO: Carbon monoxide; Treg: Regulatory T cells; IL: Interleukin.
Recently, the existence of interactions between Tregs and MCs has been demonstrated[32]. The secretion of immunosuppressive mediators, such as transforming growth factor β, forms the basis of the pivotal role of MCs in inducing allograft tolerance. IL-9 is the functional link by which activated Tregs recruit and activate MCs to mediate regional immune suppression. This is demonstrated by the fact that by neutralizing IL-9, allograft rejection in tolerant mice is greatly accelerated[33]. However, the degranulation of intragraft or systemic MCs causes the loss of Tregs and MCs from the graft, and impairs Treg function. As a result, rejection occurs in the established tolerant allograft[34]. Therefore, it seems important to stabilize the MCs in order to sustain allograft tolerance. As described above, the liver more easily induces tolerance than other organs. Although MCs are not abundant in the liver, an inhibition of MC degranulation seems important, because of the hepatic immunological privilege. Takamiya et al[13] illustrated that an overexpression of HO-1 suppresses compound 48/80-, IgE-induced MC degranulation through bilirubin. Yasui et al[35] demonstrated that the upregulation of HO-1 within the MCs also inhibits their cytokine production associated with a selective suppression of the DNA-binding activity of the AP-1 transcription factors. As a result, to induce HO-1 within MCs may be another target to affect Tregs. On the other hand, because MCs are involved in the fibrosis of chronic rejection, inhibiting MC degranulation and cytokine production may control the progression of chronic rejection. Whether the HO-1 overexpression of the donor’s or the recipient’s MCs is more important, needs to be ascertained.
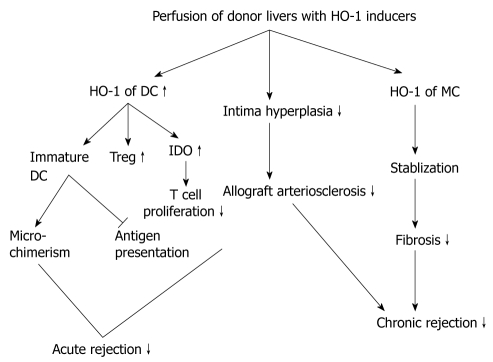
It is well known that the Treg functions depend on the activity of APCs, and the HO-1 of APCs may also influence the Tregs. George et al[36] have demonstrated that lack of HO-1 in the APCs significantly impairs the suppressive function of Treg cells on effector T cells, which indicated the importance of HO-1 within APCs. APCs are involved in initiating rejection and can also be mediated to induce tolerance. Perfusion of donor liver with HO-1 inducers will up-regulate HO-1 expression of many cells in the liver, including hepatic dentritic cells. This pathway contains some mechanism for HO-1 to reduce both acute and chronic rejections (Figure 3). There are two recognition patterns associated with organ transplantation rejection: direct and indirect recognition induced by donor and recipient APCs, DCs are the main APCs in the liver, the only cell type that can activate naïve CD4+T cells. Hepatic DCs differ from those in other organs, because they are less immunostimulatory in response to diverse antigens. Some data have also shown that compared with splenic and blood DCs, freshly isolated mouse liver DCs express lower levels of Toll-like receptor 4 mRNA and are less able to activate allogeneic T cells, or polarize naïve T cells toward Th1 responses to LPS[37]. Immature DCs (imDCs) lack the capability of presenting alloantigen to alloreactive T cells because of a low expression of costimulatory molecules[38]. As it is shown, imDCs express HO-1, but apparently reduce or lose the ability of expression as they become mature[39]. There is expanding evidence that donor hepatic imDCs can downregulate immune responses, thus inducing and maintaining peripheral T-cell tolerance[39]. Furthermore, it has been hypothesized that the presence of large numbers of imDCs within the donor liver that circulate and repopulate the recipient contribute to microchimerism, another mechanism associated with donor-specific tolerance[40]. However, in the event of an acute liver transplant rejection, when the imDCs undergo maturation upon alloantigen stimulation, they induce an acute rejection through direct recognition. Consequently, keeping DCs in their immature state is crucial in order to induce an antigen-specific tolerance in liver transplantation.
Figure 3.
Perfusion of donor liver with heme oxygenase 1 inducers could reduce rejections through many pathways. HO-1: Heme oxygenase 1; DC: Dendritic cell; Treg: Regulatory T cells; IDO: Indoleamine 2,3-dioxygenase; MC: Mast cell.
Many studies have supported the idea that an induction of HO-1 or its products can inhibit DC maturation. Chauveau et al[39] have proven that the induction of HO-1 directs DC refractory to an LPS-induced maturation. Recent studies have also demonstrated that an HO-1 overexpression inhibits the secretion of cytokines critical for DC maturation, such as IL-12[41]. Indoleamine 2,3-dioxygenase (IDO) is a further mechanism associated with the immunosuppressive activity of imDCs, whereby IDO can inhibit T cell proliferation through tryptophan degradation, and induce Tregs as well[42,43]. An upregulation of HO-1 resulted in an IDO overexpression through CO[6]. The above-mentioned information all demonstrates that by inducing HO-1, the development of DCs could be directed selectively toward a tolerogenic DC type. In chronic rejection, graft arterial vasculature remodels after the transplantation. Chronic graft dysfunction is characterized by the development of intimal hyperplasia and narrowing of the vessel lumen[44]. Cheng et al[45] have found that a loss of HO-1 in DCs or HO-1 gene silencing by small interfering RNA upregulated the MHCII expression through CIITA-driven transcriptional regulation and transcription 1 (STAT1) phosphorylation. They have also illustrated that an inhibition of HO-1 in DCs aggravated the development of transplant arteriosclerosis by increasing intimal hyperplasia, and by activating a CD4(+) T cell allograft response mediated by an MHCII upregulation. Therefore, we conclude that the activity of HO-1 is an important regulatory mechanism affecting multiple levels of the immune response to induce tolerance. Elucidating its effects on specific immune cells will aid the development of therapeutic strategies for a variety of inflammatory disorders, including autoimmune diseases and transplant rejection.
UP-REGULATION OF HO-1 AS POTENTIAL THERAPY ON GRAFT PROTECTION IN LIVER TRANSPLANTATION
Based on the aforementioned information, we can conclude that HO-1 plays a potential protective role in both IRI and graft rejection, whereby HO-1 may act as a link between these two events (Table 1). Thus, by upregulating HO-1 within the donor liver allograft, a preconditioned pre-transplantation hepatic status can be provided that may uphold allograft survival and normal function for a long time.
Table 1.
Upregulation of heme oxygenase-1 in donor liver to alleviate ischemia-reperfusion injury and rejection
| Effective product | Targets | Results | Ref. |
| HO-1 | KC | Preventing IRI | [4] |
| HO-1 | Attenuating apoptosis of SEC | Alleviating IRI | [5] |
| HO-1 | Inhibiting DC maturation | Reducing rejection | [6] |
| HO-1 | Anti-inflammatory differentiation of KC | Preventing IRI | [24] |
| HO-1 | Modulating oxidative stress and proinflammatory mediators | Alleviating IRI | [30] |
| CO | Suppressing T cell proliferation | Reducing rejection | [31] |
| HO-1 | Microchimerism | Inducing allograft tolerance | [41] |
| CO | Inhibiting TLR-induced DC maturation | Reducing rejection | [42] |
| HO-1 | Suppressing intragraft infiltration of KC and neutrophils, preventing proinflammatory cytokine and chemokine expression | Alleviating IRI | [50] |
| HO-1 | Inducing Treg | Inducing allograft tolerance | [56] |
| Biliverdin | Decreasing P-selectin, ICAM-1, iNOS and IL-6 | Alleviating IRI | [60] |
HO-1: Heme oxygenase 1; CO: Carbon monoxide; KC: Kupffer cell; SEC: Sinusoidal endothelial cell; DC: Dendritic cell; TLR: Toll-like receptor; Treg: Regulatory T cells; ICAM-1: Intercellular adhesion molecule-1; iNOS: Inducible nitric oxide synthase; IL: Interleukin; IRI: Ischemia-reperfusion injury.
HO-1 is highly inducible by a variety of stimuli including heme, NO, cadmium, growth factors, and hyperoxia. These diverse stimuli act via a similarly broad range of signaling pathways. The nuclear factor (NF)-κB and activator proteins-1 and -2 lie in the promoter region of HO-1.The transcription factor NF-E2-related factor-2 is recognized as the key mediator of HO-1 induction and the protective functions of HO-1, as seen in experimental models both in vivo and in vitro[46]. Due to the potential toxicity of the conventional HO-1 inducers, such as hemin and CoPP, a number of new strategies, including protein transduction, traditional Chinese medicine, adenoviral transduction and others, have attracted substantial interest as potential HO-1 inducers[5,47,48].
Protoporphyrines are prototypic HO-1 inducers in vitro. Depending on the metal atom of the porphyrines, the enzymatic HO-1 function is activated (e.g. iron or cobalt atom). However, since porphyrines are heavy metals, their clinical usage has many limitations.
Simvastatin, clinically used as a lipid-lowering drug, is another inducer of HO-1. Lee et al[49] have shown that the protective effect of statins on vessels is produced by HO-1. Uchiyama et al[50] further demonstrated that simvastatin increases the HO-1 expression by inducing a nuclear translocation of the heat shock factor 1 in vascular endothelial cells. However, there are still several unresolved problems. Simvastatin is administered orally in clinical applications. Uchiyama et al[50], however, induced HO-1 by an intraperitoneal injection of a high-dose of simvastatin in their experiments. In view of its potential for a clinical application, a pilot study is necessary to evaluate whether simvastatin administered orally also induces HO-1.
In recent years, traditional Chinese herbal medicine has become popular in inducing HO-1. Sinomenine, a pure alkaloid extracted from the Chinese medical plant Sinomenium acutum, has been investigated for its protective effect on hepatic cells affected by an overexpression of HO-1 to attenuate IRI[5]. Isodon Serra (I. Serra) is another Chinese medicinal herb that has been found to possess the capacity to induce HO-1. It is a perennial herb that has been used widely for the treatment of arthritis, enteritis, jaundice, hepatitis, lepromatous leprosy, ascariasis and acute cholecystitis[51]. Moreover, it has been used in China to treat esophageal cancer. It has an anti-proliferative effect on melanoma cells and many other kinds of malignant cells[52,53]. However, our studies focused on other functions, besides its anti-tumorigenic activities[54,55]. Matsushima et al[56] have proven that crassin acetate, a coral-derived cembrane diterpenoid, can effectively induce HO-1 mRNA/protein expression and HO-1 enzymatic activity in DCs. Nodosin and Oridonin, extracts obtained from I. Serra, also belong to a type of diterpenoid. Our previous studies elucidated that Oridonin has an immunosuppressive effect by regulating the cell mitosis cycle and modulating the signal mechanisms of four cytokines (IL-2, IFN-γ, IL-12 and TNFα)[54]. Oridonin, a potent HO-1 inducer, is a promising immunosuppressive drug. Hu et al[55] have shown that Oridonin upregulated the HO-1 expression at both the transcriptional and translational levels, and accordingly promoted HO-1 activity in vitro in their experiments. However, the exact mechanisms of our findings remain to be further investigated. Nodosin, another I. serra extract, also induces HO-1. We used a Nodosin solution in vitro to perfuse the isolated liver, while lactic Ringer’s solution was used as control. The results showed that the expression of HO-1 in both the mRNA and the protein is higher in the perfused group than in the control group[57]. The potential role of I. Serra extract in the upregulation of HO-1 suggested that it is a novel nontoxic drug candidate for liver allograft protection. More models and methods used for the study of I. Serra extracts need to be defined in the future investigations. We believe that in the near future, the full pharmacological activity and detailed mechanisms of I. Serra will be further described.
FUTURE PROSPECTS
In a clinical setting, however, the inducible HO-1 system still has several limitations. The different effects of HO-1, which are neither exclusively cytoprotective nor exclusively cytotoxic, should be further investigated. The HO-1 induced cytoprotection might be restricted to a narrow threshold of overexpression. Besides, there are no available reagents that can specifically induce HO-1. Therefore, the unintended effects of treatment with non-specific HO-1 inducers would likely present a disadvantage[10]. Although an adenoviral-based HO-1 gene transfer has been attempted in vivo, the efficiency of viral transfection is organ dependent. CO may represent a candidate for the treatment of transplanted patients against IRI. However, its therapeutic window must be carefully considered, because the inhalation of high levels can be toxic or even be lethal. Biliverdin and reduced bilirubin may also represent possible candidates for clinical application. We have recently demonstrated that biliverdin had a protective effect in stringent rat liver models of IRI, as evidenced by an improved portal blood flow/bile production and a reduction in hepatocellular damage. It also improved the survival rate in a syngeneic rat OLT model after prolonged cold ischemia[58]. However, because bilirubin in excess can cause neurotoxicity and can act as a lytic agent binding to erythrocyte membranes, the therapeutic window of biliverdin must be examined in detail prior to its clinical use.
From the above, the question arises if HO-1 or its products can be used clinically. Although CO is toxic, beneficial results can be obtained with relatively low doses for appropriate length of time. In rodents, the administration of biliverdin or bilirubin in the first few weeks of life did not reveal much toxicity. Recent evidence indicates that they are not only non-toxic at physiological concentrations in normal cells, they may also have important anti-oxidant, anti-inflammatory, or anti-apoptotic properties[59,60].
Based on this review, which reveals that HO-1 is associated with both processes of IRI and acute rejection, we can conclude that the preconditioning of the donor liver with an upregulation of HO-1 not only attenuates IRI, but also reduces rejection after liver transplantation. HO-1, therefore, seems to stand out as a potential key therapeutic target to maintain graft function and improve the recipients’ prognosis in liver transplantation. However, due to its limitations, the therapeutic role of HO-1 must undergo further critical analysis.
Footnotes
Supported by The grants for Young Scientist Project, National Natural Science Foundation of China, No. 30600598; “Qi Ming Star for Young Scientist” Project, Science and Technology Commission of Shanghai Municipality, No. 10QH1401800; “Shu Guang Scholar” Project, Shanghai Municipal Educational Commission, No. 10SG20; the Key Medical Project of Science and Technology Commission of Shanghai Municipality, No. 09411952500; Nano-specific Project of Science and Technology Commission of Shanghai Municipality, Project No. 0952nm03800; and Research and Innovation Project of Shanghai Municipal Education Commission, Project No. 09YZ103
Peer reviewer: Yasuhiko Sugawara, MD, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine University of Tokyo, Tokyo, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
References
- 1.Calne RY. Immunosuppression in liver transplantation. N Engl J Med. 1994;331:1154–1155. doi: 10.1056/NEJM199410273311711. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 3.McDaid J, Yamashita K, Chora A, Ollinger R, Strom TB, Li XC, Bach FH, Soares MP. Heme oxygenase-1 modulates the allo-immune response by promoting activation-induced cell death of T cells. FASEB J. 2005;19:458–460. doi: 10.1096/fj.04-2217fje. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J Gastroenterol. 2010;16:1285–1292. doi: 10.3748/wjg.v16.i10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue LH, Zhao YL, Chen J, Lu DR. Effect of fusion protein TAT and heme oxygenase-1 on liver sinusoidal endothelial cells apoptosis during preservation injury. Chin Med J (Engl) 2010;123:68–73. [PubMed] [Google Scholar]
- 6.Jung ID, Lee JS, Lee CM, Noh KT, Jeong YI, Park WS, Chun SH, Jeong SK, Park JW, Son KH, et al. Induction of indoleamine 2,3-dioxygenase expression via heme oxygenase-1-dependant pathway during murine dendritic cell maturation. Biochem Pharmacol. 2010;80:491–505. doi: 10.1016/j.bcp.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T, Shimizu H, Morimatsu H, Maeshima K, Inoue K, Akagi R, Matsumi M, Katayama H, Morita K. Heme Oxygenase-1 is an Essential Cytoprotective Component in Oxidative Tissue Injury Induced by Hemorrhagic Shock. J Clin Biochem Nutr. 2009;44:28–40. doi: 10.3164/jcbn.08-210-HO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong P, Cederbaum AI, Nieto N. Heme oxygenase-1 protects HepG2 cells against cytochrome P450 2E1-dependent toxicity. Free Radic Biol Med. 2004;36:307–318. doi: 10.1016/j.freeradbiomed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 11.Doré S. Decreased activity of the antioxidant heme oxygenase enzyme: implications in ischemia and in Alzheimer’s disease. Free Radic Biol Med. 2002;32:1276–1282. doi: 10.1016/s0891-5849(02)00805-5. [DOI] [PubMed] [Google Scholar]
- 12.Vítek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem. 2007;43:1–57. doi: 10.1016/s0065-2423(06)43001-8. [DOI] [PubMed] [Google Scholar]
- 13.Takamiya R, Murakami M, Kajimura M, Goda N, Makino N, Takamiya Y, Yamaguchi T, Ishimura Y, Hozumi N, Suematsu M. Stabilization of mast cells by heme oxygenase-1: an anti-inflammatory role. Am J Physiol Heart Circ Physiol. 2002;283:H861–H870. doi: 10.1152/ajpheart.00740.2001. [DOI] [PubMed] [Google Scholar]
- 14.Gray CP, Arosio P, Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood. 2002;99:3326–3334. doi: 10.1182/blood.v99.9.3326. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112–1116. doi: 10.3748/wjg.v8.i6.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 18.Heijnen BH, Straatsburg IH, Padilla ND, Van Mierlo GJ, Hack CE, Van Gulik TM. Inhibition of classical complement activation attenuates liver ischaemia and reperfusion injury in a rat model. Clin Exp Immunol. 2006;143:15–23. doi: 10.1111/j.1365-2249.2005.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 20.Le Moine O, Louis H, Demols A, Desalle F, Demoor F, Quertinmont E, Goldman M, Devière J. Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology. 2000;31:1266–1274. doi: 10.1053/jhep.2000.7881. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Cao H, Wu ZY. Isolation of Kupffer cells and their suppressive effects on T lymphocyte growth in rat orthotopic liver transplantation. World J Gastroenterol. 2007;13:3133–3136. doi: 10.3748/wjg.v13.i22.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Florman S, Roayaie S, Basile J, Zhang ZY, Machac J, Boros P, Miller CM. Differential in vivo recovery of sinusoidal endothelial cells, hepatocytes, and Kupffer cells after cold preservation and liver transplantation in rats. Transplantation. 1998;66:573–578. doi: 10.1097/00007890-199809150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Qiu Y, Shi M, Ding Y. Matrine protects sinusoidal endothelial cells from cold ischemia and reperfusion injury in rat orthotopic liver transplantation. Ann Clin Lab Sci. 2003;33:216–225. [PubMed] [Google Scholar]
- 24.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, Wigmore SJ. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther. 2009;17:65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, Shao J, Li YZ, Yu SC, Zhang ZL. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor- 1. Am J Pathol. 2007;171:1904–1914. doi: 10.2353/ajpath.2007.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, et al. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol. 2003;163:1587–1598. doi: 10.1016/S0002-9440(10)63515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Froh M, Conzelmann L, Walbrun P, Netter S, Wiest R, Wheeler MD, Lehnert M, Uesugi T, Scholmerich J, Thurman RG. Heme oxygenase-1 overexpression increases liver injury after bile duct ligation in rats. World J Gastroenterol. 2007;13:3478–3486. doi: 10.3748/wjg.v13.i25.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer OJ, van der Loos CM, Teeling P, van der Wal AC, Teunissen MB. Immunohistochemical analysis of regulatory T cell markers FOXP3 and GITR on CD4+CD25+ T cells in normal skin and inflammatory dermatoses. J Histochem Cytochem. 2007;55:891–898. doi: 10.1369/jhc.6A7119.2007. [DOI] [PubMed] [Google Scholar]
- 30.Yun N, Eum HA, Lee SM. Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid Redox Signal. 2010;13:1503–1512. doi: 10.1089/ars.2009.2873. [DOI] [PubMed] [Google Scholar]
- 31.Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 32.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 33.Boerma M, Fiser WP, Hoyt G, Berry GJ, Joseph L, Joseph J, Wang J, Crew MD, Robbins RC, Hauer-Jensen M. Influence of mast cells on outcome after heterotopic cardiac transplantation in rats. Transpl Int. 2007;20:256–265. doi: 10.1111/j.1432-2277.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 34.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasui Y, Nakamura M, Onda T, Uehara T, Murata S, Matsui N, Fukuishi N, Akagi R, Suematsu M, Akagi M. Heme oxygenase-1 inhibits cytokine production by activated mast cells. Biochem Biophys Res Commun. 2007;354:485–490. doi: 10.1016/j.bbrc.2006.12.228. [DOI] [PubMed] [Google Scholar]
- 36.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazariegos GV, Zahorchak AF, Reyes J, Chapman H, Zeevi A, Thomson AW. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant. 2005;5:314–322. doi: 10.1111/j.1600-6143.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 39.Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 40.Pons Miñano JA, Ramírez Romero P, Robles Campos R, Sánchez Bueno F, Parrilla Paricio P. [Tolerance and chimerism in liver transplantation] Rev Esp Enferm Dig. 2007;99:343–350. doi: 10.4321/s1130-01082007000600007. [DOI] [PubMed] [Google Scholar]
- 41.Rémy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, et al. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182:1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 42.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee HJ, Jeong YI, Lee TH, Jung ID, Lee JS, Lee CM, Kim JI, Joo H, Lee JD, Park YM. Rosmarinic acid inhibits indoleamine 2,3-dioxygenase expression in murine dendritic cells. Biochem Pharmacol. 2007;73:1412–1421. doi: 10.1016/j.bcp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Joosten SA, van Kooten C, Sijpkens YW, de Fijter JW, Paul LC. The pathobiology of chronic allograft nephropathy: immune-mediated damage and accelerated aging. Kidney Int. 2004;65:1556–1559. doi: 10.1111/j.1523-1755.2004.05410.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheng C, Noorderloos M, van Deel ED, Tempel D, den Dekker W, Wagtmans K, Duncker DJ, Soares MP, Laman JD, Duckers HJ. Dendritic cell function in transplantation arteriosclerosis is regulated by heme oxygenase 1. Circ Res. 2010;106:1656–1666. doi: 10.1161/CIRCRESAHA.110.216945. [DOI] [PubMed] [Google Scholar]
- 46.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O’Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 47.Song S, Shen X, Tang Y, Wang Z, Guo W, Ding G, Wang Q, Fu Z. Sinomenine pretreatment attenuates cold ischemia/reperfusion injury in rats: the role of heme oxygenase-1. Int Immunopharmacol. 2010;10:679–684. doi: 10.1016/j.intimp.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase -1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther. 2007;7:89–108. doi: 10.2174/156652307780363134. [DOI] [PubMed] [Google Scholar]
- 49.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 50.Uchiyama T, Atsuta H, Utsugi T, Ohyama Y, Nakamura T, Nakai A, Nakata M, Maruyama I, Tomura H, Okajima F, et al. Simvastatin induces heat shock factor 1 in vascular endothelial cells. Atherosclerosis. 2006;188:265–273. doi: 10.1016/j.atherosclerosis.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Liu J, Jia W, Zhao A, Li T. Distinct immunosuppressive effect by Isodon serra extracts. Int Immunopharmacol. 2005;5:1957–1965. doi: 10.1016/j.intimp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Chen S, Gao J, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. The cytostatic and cytotoxic effects of oridonin (Rubescenin), a diterpenoid from Rabdosia rubescens, on tumor cells of different lineage. Int J Oncol. 2005;26:579–588. [PubMed] [Google Scholar]
- 53.Ren KK, Wang HZ, Xie LP, Chen DW, Liu X, Sun J, Nie YC, Zhang RQ. The effects of oridonin on cell growth, cell cycle, cell migration and differentiation in melanoma cells. J Ethnopharmacol. 2006;103:176–180. doi: 10.1016/j.jep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Yang F, Zhang Y, Li J. Studies on the cell-immunosuppressive mechanism of Oridonin from Isodon serra. Int Immunopharmacol. 2007;7:945–954. doi: 10.1016/j.intimp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Hu AP, Du JM, Li JY, Liu JW. Oridonin promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance and induces HO-1 in rat splenic lymphocytes. Inflamm Res. 2008;57:163–170. doi: 10.1007/s00011-007-7193-0. [DOI] [PubMed] [Google Scholar]
- 56.Matsushima H, Tanaka H, Mizumoto N, Takashima A. Identification of crassin acetate as a new immunosuppressant triggering heme oxygenase-1 expression in dendritic cells. Blood. 2009;114:64–73. doi: 10.1182/blood-2009-02-204297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du JM, Sun LJ, Li JY, Liu JW, Quan ZW. Effect of Nodosin perfusion on the expression of HO-l in hepar tissue of SD rat. Huadong Ligong Daxue Xuebao. 2009;35:373–377. [Google Scholar]
- 58.Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. [DOI] [PubMed] [Google Scholar]
- 59.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 60.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]