Abstract
AIM: To investigate if and how the proinflammatory cytokine interferon γ (IFNγ) affects ghrelin expression in mice.
METHODS: The plasma concentration of ghrelin, and gastric ghrelin and somatostatin expression, were examined in wild-type mice and mice infected with Helicobacter pylori (H. pylori). Furthermore, ghrelin expression was examined in two achlorhydric mouse models with varying degrees of gastritis due to bacterial overgrowth. To study the effect of IFNγ alone, mice were given a subcutaneous infusion of IFNγ for 7 d. Finally, the influence of IFNγ and somatostatin on the ghrelin promoter was characterized.
RESULTS: H. pylori infection was associated with a 50% reduction in ghrelin expression and plasma concentration. Suppression of ghrelin expression was inversely correlated with gastric inflammation in achlorhdyric mouse models. Subcutaneous infusion of IFNγ suppressed fundic ghrelin mRNA expression and plasma ghrelin concentrations. Finally, we showed that the ghrelin promoter operates under the control of somatostatin but not under that of IFNγ.
CONCLUSION: Gastric infection and inflammation is associated with increased IFNγ expression and reduced ghrelin expression. IFNγ does not directly control ghrelin expression but inhibits it indirectly via somatostatin.
Keywords: Ghrelin, Interferon-γ, Somatostatin, Inflammatory diseases, Helicobacter pylori
INTRODUCTION
The gastric peptide hormone ghrelin is, in adults, predominantly produced in P/D1 endocrine cells in humans or in A-like endocrine cells in rats and mice, which are located in the oxyntic glands of the gastric corpus[1-5]. Within the oxyntic glands, ghrelin-containing cells are found from the neck to base in both rats and humans[2,6-8]. Ghrelin-producing cells are also found in the antrum of the stomach and proximal small intestine as well as in other organs[2,9-14], but these sites are of lesser importance as the plasma ghrelin concentrations are reduced by 65% after gastrectomy[13]. Plasma ghrelin consists of two forms; the active acylated ghrelin, which is the ligand for the GH secretagogue (GHS) receptor, and the non-acylated ghrelin, which constitutes greater amounts in the blood than the acylated form[11]. Ghrelin is involved in energy homeostasis and ghrelin plasma concentrations are decreased in obesity and increased in states of negative energy balance such as fasting, anorexia or cachexia[11] as well as being inversely correlated to body mass index (BMI) and insulin secretion[11,13]. Ghrelin plasma concentrations increase before meals and decrease after eating[15,16]. However, to what degree ghrelin is important as a meal initiator or cause of increased caloric ingestion in obesity has not yet been determined[17].
Recently, several studies have found that infection with the gram-negative bacteria Helicobacter pylori (H. pylori) reduces ghrelin concentrations in both humans[7,18,19] and rodents[20]. With regard to various upper gastrointestinal diseases, plasma concentrations of ghrelin were lowest in chronic gastritis and gastric ulcer and highest in acute gastritis[21]. Furthermore, children infected with H. pylori have faltering growth[22,23], which suggests that H. pylori could alter signals from the stomach related to the control of growth and body weight[24].
The inflammation that occurs in the H. pylori-infected host is a Th1-dominated immune reaction which is regulated by, among others, the lymphocyte-derived cytokine interferon-γ (IFNγ)[25]. In the gastrin knockout (KO) mouse, which is another model for chronic gastritis due to bacterial overgrowth, we and others have also found increased gastric production of IFNγ and expression of IFNγ regulated transcripts[26,27]. Furthermore, IFNγ is one of the major cytokines behind the inflammatory response to H. pylori as no inflammation occurs during H. pylori infection without the presence of IFNγ[25]. Finally, infusion of IFNγ triggers inflammation in vivo without H. pylori[26]. Since approximately 50% of the world population is infected with this bacteria[28], knowledge of the factors modulating body weight during H. pylori infection could have great impact on health in general. Since little is known about the factors that regulate ghrelin expression during H. pylori infection and gastric inflammation[29], we examined the effect of IFNγ on ghrelin expression in mice.
MATERIALS AND METHODS
Mice
Groups of wild-type (wt) C57BL/6J mice (aged 12-16 wk), KO mice which are gastrin deficient (aged 12-16 wk or 48-56 wk)[30], histidine decarboxylase (HDC) KO mice (aged 48-56 wk)[31] and matching control mice were used. All mice were male mice that had been backcrossed to the C57BL/6J mouse strain. The mice were kept under specific pathogen-free conditions and monitored according to the Federation of European Laboratory Animal Science Associations recommendations[32] with 12 h light, 12 h dark cycles. The study was approved by the Danish Animal Welfare Committee.
H. pylori infection
C57BL6/J mice (n = 10) were inoculated with a non-mouse-adapted clone of H. pylori strain 67:21, originally isolated from an antral biopsy obtained from a Swedish female with gastric ulcer. The strain is VacA+ and contains the entire Cag pathogenicity island (PAI) with genetic stability in the Cag PAI[33]. The mice were inoculated every second day (three times) during a 5-d period. After the mice had been sacrificed, DNA was extracted and analyzed for the presence of Helicobacter species using a semi-nested polymerase chain reaction-denaturing gradient gel electrophoresis assay, specific for the genus Helicobacter, as described previously[34]. A matched group of uninfected C57BL6/J mice were used as controls. All animal experiments were approved by the Danish Animal Welfare Committee (2005/562-40) and the Danish Forest and Nature Agency (20010077355/6).
IFNγ infusion
Wild-type mice were given a continuous subcutaneous IFNγ infusion (8 μg/kg per hour or 24 μg/kg per hour for 7 d) for each group (n = 6) using osmotic minipumps (Alzet no.2001; Alza Corp., Cupertino, CA). Control mice received a saline infusion instead. The lower dose of IFNγ equals the dose of IFNγ used by Kang et al[26].
Tissue and plasma collection
The mice were anesthetized with intraperitoneal 2,2,2 tribromoethanol (Sigma-Aldrich Corp., St. Louis, MO), blood was collected in EDTA-tubes and the stomachs removed. The stomachs of all mice were dissected into fundus and antrum and immediately placed in liquid nitrogen. Plasma and tissue was subsequently stored at -80°C until further analysis.
Measurement of plasma ghrelin
Plasma ghrelin was measured in EDTA plasma without extraction using RIA no. RK-031-31 (Phoenix Peptides, Belmont, CA). This assay measures the sum of Ser3-octanoyl and Ser3-des-octanoyl ghrelin peptides. The assay has a detection limit of 20 pmol/L, an interassay variation of 13%, and an intra-assay variation of 5%[4].
mRNA extraction and analysis
The stomachs were dissected into fundus and antrum and immediately placed in liquid nitrogen. RNA was extracted using the method described by Chomcynski and Sacchi[35], and quantitative changes in the specific mRNA were determined by real-time PCR using the Lightcycler (Roche, Mannheim, Germany) as described by Chen et al[36]. Quantitations were performed using one of the following primer sets for each analysis: Ghrelin forward primer (FP) 5'-TCTGCAGTTTGCTGCTACTCA-3' and ghrelin reverse primer (RP) 5'-CCTCTTTGACCTCTTCCCAGA-3’; IFNγ FP 5'-CCTTTGGACCCTCTGACTTG-3' and IFNγ RP 5'-CATCCTTTTGCCAGTTCCTC-3'; gastrin FP 5'-CACTTCATAGCAGACCTGTCCA-3' and gastrin RP 5'-CTGGCCTCTGGAAGAGTGTT-3'; somatostatin FP 5'-CCCAGACTCCGTCAGTTTCT-3' and somatostatin RP 5'-TCAGAGGTCTGGCTAGGACAA-3'; iNOS FP 5'-ACCCCTGTGTTCCACCAGGAGATGTTGAA-3' and iNOS RP 5'-TGAAGCCATGACCTTTCGCATTAGCATGG-3' and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) FP 5'-GGTGCTGAGTATGTCGTGGA-3' and GAPDH RP 5'-GTGGTTCACACCCATCACAA-3'. Each run consisted of one negative control, one sample in which the Moloney murine leukemia virus reverse transcriptase had been omitted in the reverse transcription (RT) step, a standard curve generated by 3-fold serial dilution of RT reactions, and seven to nine RT reactions from each of the three strains. Expression of a given transcript was normalized to a GAPDH quantification performed on the same RT reaction as previously described[36].
Immunohistochemical analysis
Stomachs were rinsed in ice-cold PBS, fixed in 4% paraformaldehyde in PBS for 4-6 h and embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin. Immunohistochemistry was performed using the rabbit ghrelin antibody H-031-31 diluted 1:1 000 (Phoenix Peptides) detected with Envision-DAB+ (Dako, Glostrup, Denmark) as previously described[37]. The specificity of the immunostaining was tested by absorbing the primary antibodies with antigen before applying them to the slides or omitting the primary antibody when purified antigen was not available. The morphometrical analysis was performed by cell counting in transversely cut sections as described[38].
Cell lines
NCI-H727 cells were grown in RPMI 1640 media (Invitrogen, Carlsbad, CA), 10% FBS (Biowest, Nuaillé, France), penicillin (100 U/mL) and streptomycin (100 μg/mL) (Invitrogen) and cultured at 37°C in 5% CO2.
Plasmids and transient transfections
A 2.5 Kb fragment containing the mouse ghrelin promoter and exon one was amplified from C57BL6/J genomic DNA using the Expand kit (Roche, Mannheim, Germany) using mGhrMluI primer 5'-ATATACGCGTGTAGAACACTCACCCTAAATCTG-3' and mGhrXhoI primer 5'-ATATCTCGACTGCCTGGGGATGTGGTGCCTG-3'. The fragment was ligated into the pGL3 Bacic reporter vector (Promega, Madison, WI). The promoter sequence was confirmed by sequencing. One day before transfection, 500 000 NCI-H727 cells were seeded in 6-well dishes coated with 0.01% poly-L-lysine. The NCI-H727 cells were transfected using 6 μL TurboFectTM in vitro Transfection Reagent (Fermentas, Burlington, Canada); 2 μg ghrelin promoter plasmid and 1 μg pRL-0 (Promega, Madison, WI) were mixed with 200 μL GIBCOTM Opti-MEM I (Invitrogen, Carlsbad, CA) and incubated for 20 min before application to the cells. Twenty-four hours later, the cells were FBS starved in RPMI1640 media containing 0.5% FBS (Biowest, Nuaillé, France) for 24 h before treatment with forskolin (10 mg, Sigma-Aldrich, St. Louis, MO), IBMX (≥ 99.9%, Sigma-Aldrich, St. Louis, MO), octreotide (200 μg/mL, Mayne Pharma, Melbourne, Australia), or IFNγ (0.2 mg/mL, Immukine, Boehringer Ingelheim, Ingelheim, Germany), for 24 h alone or in combination. All treatments were performed in triplicate. IBMX and forskolin were dissolved in 99.9% DMSO (Merck, Darmstadt, Germany). Cells were then harvested and assayed for luciferase activity using the Dual-Luciferase Reporter Assay System according to the instructions by the manufacturer (Promega, Madison, WI) and normalized to Renilla luciferase activity.
Statistical analysis
Student’s unpaired t-test statistics were used and differences with a P ≤ 0.05 were considered significant. Unless otherwise stated, results are given as mean ± SD.
RESULTS
H. pylori infection is associated with an IFNγ inflammatory response and with suppression of ghrelin expression
The mice were sacrificed 2 mo after inoculation, as earlier studies had shown that a cag-dependent inflammation of the corpus mucosa develops at this time and results in a severe active and chronic gastritis[39,40]. At 2 mo, seven out of ten mice tested positive for H. pylori using semi-nested PCR with primers for H. pylori CagA and urease genes. The non-infected mice were subsequently excluded. All control mice tested negative.
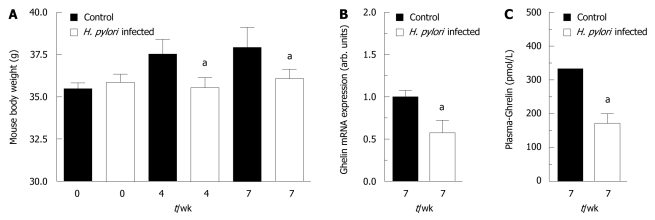
The infection of the mouse stomach by H. pylori caused a 2- to 3-fold increase in the fundic expression of IFNγ and of inducible nitric oxide synthase (iNOS) (Table 1). Furthermore, during the 2 mo infection, the H. pylori-infected mice did not gain weight in contrast to wild-type mice (Figure 1A). Ghrelin mRNA expression was reduced to 55% in the H. pylori-infected mice (Figure 1B). This was associated with a 49% decrease in the plasma ghrelin concentration (Figure 1C). The reduced ghrelin mRNA expression presumably reflects a reduced expression in each cell as opposed to cell atrophy, as the density of fundic ghrelin cells was unaffected (Table 1). In the infected mice the expression of antral somatostatin was suppressed, whereas the fundic somatostatin expression was increased (Table 1).
Table 1.
Fundic somatostatin mRNA increases after 2 mo of Helicobacter pylori infection in wild-type mice (mean ± SE)
|
Fundus |
Antrum |
|||||
| Control | H. pylori | P | Control | H. pylori | P | |
| IFNγ mRNA | 1.0 ± 0.2 | 1.8 ± 0.3 | < 0.05 | 1.0 ± 0.1 | 4.1 ± 0.9 | < 0.05 |
| iNOS mRNA | 1.0 ± 0.1 | 1.5 ± 0.1 | < 0.05 | 1.0 ± 0.2 | 2.2 ± 0.5 | < 0.05 |
| Somatostatin mRNA | 1.0 ± 0.2 | 1.4 ± 0.2 | < 0.05 | 1.0 ± 0.2 | 0.4 ± 0.1 | < 0.05 |
| Ghrelin cells (#/mm mucosa) | 25 ± 3 | 27 ± 5 | NS | 8 ± 2 | 7 ± 3 | NS |
The expression of interferon γ (IFNγ), iNOS and somatostatin mRNA in arbitrary units in H. pylori-infected mice (n = 7) 2 mo after inoculation or in uninfected control mice (n = 7). Ghrelin cell density was unchanged. H. pylori: Helicobacter pyloriNS: Non-significant.
Figure 1.
Mice infected with Helicobacter pylori have reduced ghrelin expression and do not gain weight. C57BL6/J mice were infected with Helicobacter pylori (H. pylori) strain 67:21 [this strain is VacA+ and contains a complete genetically stable Cag pathogenicity island (PAI)]. While the control mice gained weight the infected mice did not (A). Mice infected with H. pylori had reduced ghrelin expression in the stomach (B) and reduced ghrelin plasma concentrations (C). aP < 0.05 vs control.
Ghrelin expression was reduced in old gastrin knockout mice, but not in young gastrin and old histidine decarboxylase knockout mice
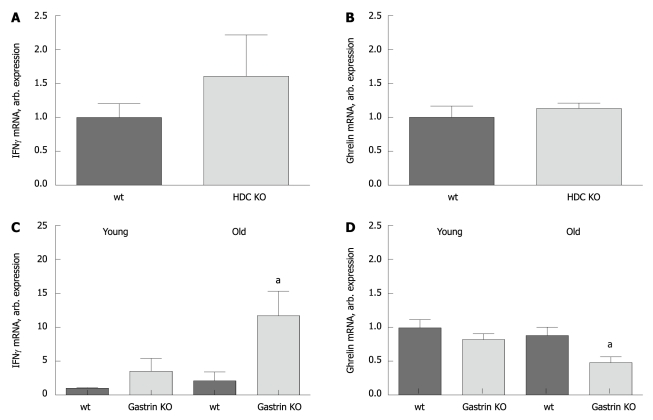
To test whether the altered ghrelin expression could also be found in other mouse models with gastric inflammation, we examined the expression of IFNγ and ghrelin in two other mouse models; the achlorhydric gastrin KO mice and the hypochlorhydric histidine decarboxylase (HDC) KO mice[41,42].
IFNγ expression was not induced in the old HDC KO mice (Figure 2A), and the ghrelin expression was unchanged in these mice (Figure 2B). Young gastrin KO mice only had moderate inflammation, while the old mice had more inflammation when evaluated by higher IFNγ expression (Figure 2C). Ghrelin expression was unaffected in young gastrin KO mice but reduced in old gastrin KO mice (Figure 2D).
Figure 2.
Interferon γ expression is increased and ghrelin expression is reduced in old but not young gastrin knockout mice and histidine decarboxylase knockout mice. The expression of interferon γ (IFNγ) and ghrelin mRNA in young (12-16 wk) and old (48-56 wk) achlorhydric gastrin KO mice and old (48-56 wk) hypochlorhydric histidine decarboxylase (HDC) KO mice (n = 6 in each group) is shown. There is no change in either IFNγ (A) or ghrelin (B) expression in HDC KO mice as compared to wt mice. While the gastric inflammation evaluated by expression of IFNγ increases when the gastrin KO mice get older (C), the expression of ghrelin decreases (D). aP < 0.05.
IFNγ suppresses fundic ghrelin mRNA expression and plasma ghrelin concentrations
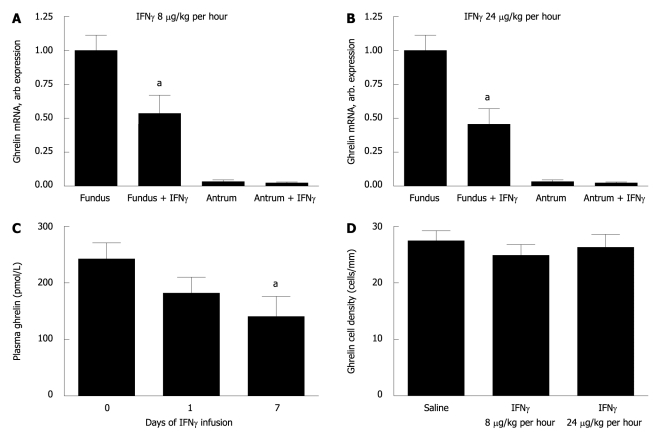
Since both the H. pylori infection and the bacterial overgrowth in the gastrin KO mice were associated with increased expression of IFNγ and reduced ghrelin expression, we examined the effect of IFNγ on ghrelin expression. The fundic ghrelin expression was approximately 30 times higher than the antral (Figure 3A and B). The fundic expression of ghrelin mRNA was halved at both infusion rates of IFNγ (8 μg/kg per hour and 24 μg/kg per hour) examined compared to expression levels in wt mice (Figure 3A and B). In contrast, antral ghrelin expression did not alter significantly under IFNγ infusion at either dose. The reduction in ghrelin expression presumably reflects a reduced expression in each cell as opposed to cell atrophy, as infusion of IFNγ did not change the density of fundic ghrelin cells (Figure 3D). The reduced ghrelin expression was correlated with a 40% reduction in plasma ghrelin concentrations at 7 d of IFNγ infusion (Figure 3C). Furthermore, IFNγ infusion induced the expression of fundic somatostatin, whereas the antral somatostatin expression did not change under the influence of IFNγ (Table 2).
Figure 3.
Interferon γ suppresses ghrelin expression in the gastric fundus and ghrelin concentrations in plasma independently of loss of ghrelin-producing cells. Mice infused with interferon γ (IFNγ) as compared to saline infused mice (n = 6 in each group). IFNγ was infused at low dose (LD) = 8 μg/kg per hour for 7 d or high dose (HD) = 24 μg/kg per hour for 7 d. Low (A) and high (B) dose IFNγ infusion decreases the expression of ghrelin mRNA to a similar degree. HD IFNγ represses ghrelin concentrations in plasma gradually over time (C). The density of fundic ghrelin cells is not altered by IFNγ infusion (D). aP < 0.05.
Table 2.
Fundic expression of somatostatin mRNA increases during subcutaneous interferon γ infusion (mean ± SE)
|
Fundus |
Antrum |
|||||
| Saline | + IFNγ | P | Saline | + IFNγ | P | |
| Low Dose IFNγ | ||||||
| Somatostatin mRNA | 1.0 ± 0.2 | 1.9 ± 0.2 | < 0.05 | 1.0 ± 0.2 | 0.9 ± 0.1 | NS |
| IFNγ mRNA | 1.0 ± 0.1 | 1.1 ± 0.2 | NS | 1.0 ± 0.1 | 1.2 ± 0.2 | NS |
| High Dose IFNγ | ||||||
| Somatostatin mRNA | 1.0 ± 0.1 | 1.6 ± 0.1 | < 0.05 | 1.0 ± 0.1 | 1.1 ± 0.1 | NS |
| IFNγ mRNA | 1.0 ± 0.2 | 1.3 ± 0.1 | NS | 1.0 ± 0.1 | 1.2 ± 0.1 | NS |
The fundic and antral expression of somatostatin mRNA and endogenous interferon γ (IFNγ) mRNA in arbitrary units in mice infused with either IFNγ or saline (n = 6 in each group). Low dose IFNγ = 8 μg/kg per hour for 7 d and high dose IFNγ = 24 μg/kg per hour for 7 d. NS: Non-significant.
The ghrelin promoter operates under the control of somatostatin but not under that of IFNγ
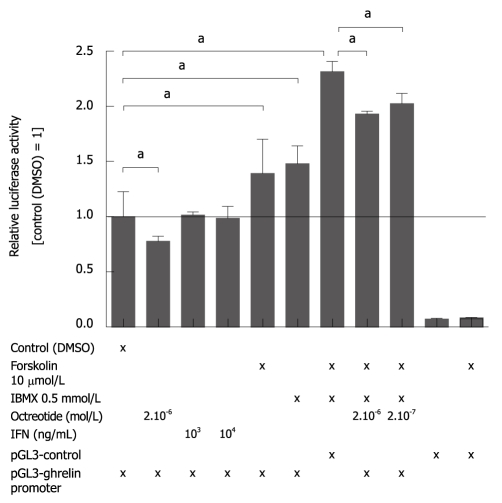
We next examined the effect of IFNγ and somatostatin on the transcriptional regulation of ghrelin using a 2 kb ghrelin promoter construct. The experiments were carried out in NCI-H727 cells since these are carcinoid cells expressing both somatostatin receptor 2 (SSTR2) and SSTR5. This indicates that they could be a good model for A-like cells in the stomach (Døssing, unpublished data). Treatment with IFNγ did not affect the activity of the ghrelin promoter construct. In contrast, forskolin and IBMX both independently and together activated the 2 kb promoter (Figure 4). Moreover, treatment with octreotide (somatostatin analog) reduced the basal ghrelin promoter activity (Figure 4) as well as forskolin/IBMX-induced ghrelin promoter activation in a dose-dependent manner.
Figure 4.
The somatostatin receptor agonist octreotide inhibits activation of the ghrelin promoter. Pulmonary carcinoid NCI-H727 cells were transfected with a 2 kb mouse ghrelin promoter construct and treated for 24 h with forskolin, IBMX, octreotide, and interferon γ (IFNγ) alone or in combination, after which the ghrelin promoter response was measured by the dual-luciferase reporter assay system. Octreotide inhibited both basal and IBMX and/or forskolin-induced ghrelin expression. Forskolin and IBMX were dissolved in dimethyl sulfoxide (DMSO). aP < 0.05.
DISCUSSION
Our results show that the gastric expression of ghrelin mRNA and the plasma concentration of ghrelin are reduced during gastric infection, either due to bacterial overgrowth in general or to H. pylori infection specifically. Both types of infection are associated with an IFNγ inflammatory response. Furthermore, infusion of IFNγ alone could mimic the changes in ghrelin expression and plasma concentration seen during H. pylori infection and bacterial overgrowth.
The observation of reduced ghrelin expression in H. pylori-infected mice is in agreement with several studies that found reduced ghrelin concentrations in both humans[7,18,19] and rodents[20] infected with H. pylori. However, others have reported ghrelin plasma concentration to be unaffected[43,44] or even to increase during H. pylori infection[45]. These discrepancies could be due to differences in the severity of the infection. We found gastric ghrelin expression unaffected in young gastrin KO mice and HDC KO mice, both with only mild inflammation as evaluated by the IFNγ response. However, as the gastrin KO mice got older and developed a more severe gastric inflammation, the ghrelin expression decreased. Similar observations have also been observed in humans, where a correlation between increasing degree of chronic inflammation, severity of glandular atrophy and metaplasia and decreasing ghrelin expression was found[8,19].
Reduced gastric ghrelin expression could either be due to a reduced number of ghrelin cells or reduced expression within each cell. Ghrelin-producing cells are primarily found in the oxyntic glands in the stomach[2,3]. The expression of ghrelin mRNA was more than 30 times higher in the fundus than antrum in control mice. Reduced numbers of ghrelin cells in fundic mucosa has indeed been seen in H. pylori-infected humans[7] and this correlates with a reduction in plasma ghrelin concentrations.
In our study, the density of ghrelin cells did not change indicating that our observations are due to reduced expression within the cells. The mechanism leading to reduced ghrelin expression during H. pylori infection is poorly understood. We found that IFNγ had no direct effect on the ghrelin promoter, suggesting that the IFNγ inhibition of ghrelin expression is mediated via other transmitters. In our study, fundic somatostatin was increased both during gastric inflammation and during infusion of IFNγ. Somatostatin is the universal inhibitor of secretion from endocrine cells[46] and also inhibits ghrelin secretion[47]. We show that octreotide (a somatostatin analog) inhibits the activity of the ghrelin promoter. Increased corpus somatostatin has also been found during H. pylori infection[48]. However, reduced fundic somatostatin expression has been found in other studies[49,50]. One explanation for these differences is the degree of fundic atrophy, which affects both ghrelin and somatostatin expression[7].
Immunoregulation of somatostatin has also been demonstrated in in vitro studies[51]. These showed that TNFα and IL-1β stimulated somatostatin secretion. IL-4 also stimulated somatostatin secretion and together these changes could explain the hypochlorhydria seen in mice infected with H. felis[52]. However, in that study, infusion of IFNγ resulted in a reduction of fundic somatostatin. We have no explanation for the difference in response to IFNγ. The proinflammatory cytokine IL-1β also influences ghrelin levels and seems to suppress excess ghrelin secretion in H. pylori-infected mice[53]. Thus, not only IFNγ but other cytokines as well are associated with reduced ghrelin expression.
We have shown that gastric infections either due to H. pylori or bacterial overgrowth are associated with reduced fundic ghrelin expression and increased IFNγ production. Infusion of IFNγ in mice alone mimics the changes seen in the mice with gastric infections. Stimulation with IFNγ does not directly inhibit the ghrelin promoter; instead the inhibition is mediated through somatostatin.
COMMENTS
Background
Ghrelin is involved in energy homeostasis and ghrelin plasma concentrations are decreased in obesity and increased during fasting, anorexia or cachexia. Recently, several studies have found that infection with Helicobacter pylori (H. pylori) reduces ghrelin concentrations in both humans and rodents. Furthermore, children infected with H. pylori have faltering growth, suggesting that H. pylori may alter signals from the stomach related to the control of growth and body weight. The mechanism(s) through which inflammation modulates ghrelin expression are, however, poorly understood.
Research frontiers
Chronic gastritis induced by H. pylori is a Th1-dominated immune reaction which is regulated by, among others, the lymphocyte-derived cytokine interferon-γ (IFNγ). In the gastrin knockout (KO) mouse which is a model for chronic gastritis due to bacterial overgrowth, increased gastric production of IFNγ has been found. Since little is known about the factors that regulate ghrelin expression during H. pylori infections and gastric inflammation, the authors examined if, and through which mechanisms, IFNγ modulates ghrelin expression in mice.
Innovations and breakthroughs
H. pylori-infected mice and old gastrin KO mice with inflammation due to bacterial overgrowth of the stomach display an increased expression of IFNγ and a decreased expression of ghrelin. The changes in ghrelin and somatostatin expression can be duplicated by infusion of IFNγ alone. IFNγ does not directly suppress ghrelin expression but inhibits it indirectly by increasing somatostatin secretion.
Applications
A better understanding of the mechanisms that control ghrelin expression during inflammation by either H. pylori alone or by gastric bacterial infections in general aids in the understanding of factors modulating growth and body weight during infection. This could have great impact on general health in the population.
Terminology
IFNγ is a cytokine that is important for innate and adaptive immunity against bacterial infections and for tumor control. The most important functions of IFNγ come from its immunostimulatory and immunomodulatory effects. Ghrelin is a hormone produced in the oxyntic glands of the gastric corpus. It is a growth hormone promoting intestinal cell proliferation, and is involved in energy homeostasis. Ghrelin expression was, in this study, found to be inhibited by octreotide, which is an analog of somatostatin. Somatostatin acts as a general inhibitor of secretion from, and growth of, endocrine cells. Somatostatin is widely distributed throughout the body including several locations in the digestive system such as the stomach, intestine and delta cells of the pancreas.
Peer review
This is the first experimental study on the effect of IFNγ on ghrelin expression. It is a very well designed study, using a careful combination of several animal models and different techniques, and the discussion is well structured. The study is valuable in the context of providing evidence on the indirect regulation of ghrelin expression and secretion by IFNγ mediated through somatostatin.
Acknowledgments
Bo Lindberg is thanked for expert technical assistance.
Footnotes
Supported by The Danish MRC Grant 271-08-0378 (LFH), the ALF grant (TW), The Lundbeck foundation (KBVD)
Peer reviewer: Guida Portela-Gomes, MD, PhD, Professor, Faculty of Medicine, University of Lisbon, Rua Domingos Sequeira-128, Estoril 2765-525, Portugal
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 3.Tomasetto C, Karam SM, Ribieras S, Masson R, Lefèbvre O, Staub A, Alexander G, Chenard MP, Rio MC. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology. 2000;119:395–405. doi: 10.1053/gast.2000.9371. [DOI] [PubMed] [Google Scholar]
- 4.Dornonville de la Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Håkanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- 5.Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol. 2002;117:511–519. doi: 10.1007/s00418-002-0415-1. [DOI] [PubMed] [Google Scholar]
- 6.Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99:2121–2127. doi: 10.1111/j.1572-0241.2004.30291.x. [DOI] [PubMed] [Google Scholar]
- 7.Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10–16. doi: 10.1210/jc.2004-1330. [DOI] [PubMed] [Google Scholar]
- 8.Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S, et al. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711–1720. doi: 10.1111/j.1572-0241.2005.41492.x. [DOI] [PubMed] [Google Scholar]
- 9.Locatelli V, Bresciani E, Bulgarelli I, Rapetti D, Torsello A, Rindi G, Sibilia V, Netti C. Ghrelin in gastroenteric pathophysiology. J Endocrinol Invest. 2005;28:843–848. doi: 10.1007/BF03347579. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka-Shintani M, Watanabe M. Distribution of ghrelin-immunoreactive cells in human gastric mucosa: comparison with that of parietal cells. J Gastroenterol. 2005;40:345–349. doi: 10.1007/s00535-004-1550-3. [DOI] [PubMed] [Google Scholar]
- 11.Ukkola O. Ghrelin and the metabolic balance. J Endocrinol Invest. 2005;28:849–852. doi: 10.1007/BF03347580. [DOI] [PubMed] [Google Scholar]
- 12.Shiotani A, Miyanishi T, Uedo N, Iishi H. Helicobacter pylori infection is associated with reduced circulating ghrelin levels independent of body mass index. Helicobacter. 2005;10:373–378. doi: 10.1111/j.1523-5378.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 13.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 14.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 15.Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 16.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 17.Kirchner H, Tong J, Tschöp MH, Pfluger PT. Ghrelin and PYY in the regulation of energy balance and metabolism: lessons from mouse mutants. Am J Physiol Endocrinol Metab. 2010;298:E909–E919. doi: 10.1152/ajpendo.00191.2009. [DOI] [PubMed] [Google Scholar]
- 18.Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637–640. doi: 10.1136/gut.52.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117:429–432. doi: 10.1016/j.amjmed.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Masaoka T, Hosoda H, Ota T, Minegishi Y, Nomura S, Kangawa K, Ishii H. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187–194. doi: 10.1136/gut.2003.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isomoto H, Ueno H, Nishi Y, Yasutake T, Tanaka K, Kawano N, Ohnita K, Mizuta Y, Inoue K, Nakazato M, et al. Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Dig Dis Sci. 2005;50:833–838. doi: 10.1007/s10620-005-2648-z. [DOI] [PubMed] [Google Scholar]
- 22.Bravo LE, Mera R, Reina JC, Pradilla A, Alzate A, Fontham E, Correa P. Impact of Helicobacter pylori infection on growth of children: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2003;37:614–619. doi: 10.1097/00005176-200311000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Dale A, Thomas JE, Darboe MK, Coward WA, Harding M, Weaver LT. Helicobacter pylori infection, gastric acid secretion, and infant growth. J Pediatr Gastroenterol Nutr. 1998;26:393–397. doi: 10.1097/00005176-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ. Helicobacter pylori infection and endocrine disorders: is there a link? World J Gastroenterol. 2009;15:2701–2707. doi: 10.3748/wjg.15.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 26.Kang W, Rathinavelu S, Samuelson LC, Merchant JL. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest. 2005;85:702–715. doi: 10.1038/labinvest.3700260. [DOI] [PubMed] [Google Scholar]
- 27.Friis-Hansen L, Rieneck K, Nilsson HO, Wadström T, Rehfeld JF. Gastric inflammation, metaplasia, and tumor development in gastrin-deficient mice. Gastroenterology. 2006;131:246–258. doi: 10.1053/j.gastro.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Czinn SJ. Helicobacter pylori infection: detection, investigation, and management. J Pediatr. 2005;146:S21–S26. doi: 10.1016/j.jpeds.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Hibi T. Does Helicobacter pylori attack ghrelin-producing cells? J Gastroenterol. 2005;40:437–439. doi: 10.1007/s00535-005-1568-1. [DOI] [PubMed] [Google Scholar]
- 30.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 32.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim. 2002;36:20–42. doi: 10.1258/0023677021911740. [DOI] [PubMed] [Google Scholar]
- 33.Björkholm B, Lundin A, Sillén A, Guillemin K, Salama N, Rubio C, Gordon JI, Falk P, Engstrand L. Comparison of genetic divergence and fitness between two subclones of Helicobacter pylori. Infect Immun. 2001;69:7832–7838. doi: 10.1128/IAI.69.12.7832-7838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsson HO, Ouis IS, Stenram U, Ljungh A, Moran AP, Wadström T, Al-Soud WA. High prevalence of Helicobacter Species detected in laboratory mouse strains by multiplex PCR-denaturing gradient gel electrophoresis and pyrosequencing. J Clin Microbiol. 2004;42:3781–3788. doi: 10.1128/JCM.42.8.3781-3788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Zhao CM, Håkanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L. Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology. 2004;126:476–487. doi: 10.1053/j.gastro.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Friis-Hansen L, Wierup N, Rehfeld JF, Sundler F. Antral G-cell in gastrin and gastrin-cholecystokinin knockout animals. Cell Tissue Res. 2005;321:141–146. doi: 10.1007/s00441-005-1110-z. [DOI] [PubMed] [Google Scholar]
- 38.Sundler F, Andersson K, Mattsson H. Administration of omeprazole to rats for one year produces reciprocal effects on antral gastrin and somatostatin cells and no effect on endocrine cells in the colon. Digestion. 1995;56:194–198. doi: 10.1159/000201242. [DOI] [PubMed] [Google Scholar]
- 39.Konturek PC, Brzozowski T, Konturek SJ, Stachura J, Karczewska E, Pajdo R, Ghiara P, Hahn EG. Mouse model of Helicobacter pylori infection: studies of gastric function and ulcer healing. Aliment Pharmacol Ther. 1999;13:333–346. doi: 10.1046/j.1365-2036.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 40.Wiedemann T, Loell E, Mueller S, Stoeckelhuber M, Stolte M, Haas R, Rieder G. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS One. 2009;4:e4754. doi: 10.1371/journal.pone.0004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furutani K, Aihara T, Nakamura E, Tanaka S, Ichikawa A, Ohtsu H, Okabe S. Crucial role of histamine for regulation of gastric acid secretion ascertained by histidine decarboxylase-knockout mice. J Pharmacol Exp Ther. 2003;307:331–338. doi: 10.1124/jpet.103.052019. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka S, Hamada K, Yamada N, Sugita Y, Tonai S, Hunyady B, Palkovits M, Falus A, Watanabe T, Okabe S, et al. Gastric acid secretion in L-histidine decarboxylase-deficient mice. Gastroenterology. 2002;122:145–155. doi: 10.1053/gast.2002.30312. [DOI] [PubMed] [Google Scholar]
- 43.Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S. Influence of H pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol. 2007;13:1595–1598. doi: 10.3748/wjg.v13.i10.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, Guvener N. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423–426. doi: 10.1530/eje.0.1480423. [DOI] [PubMed] [Google Scholar]
- 45.Campana D, Nori F, Pagotto U, De Iasio R, Morselli-Labate AM, Pasquali R, Corinaldesi R, Tomassetti P. Plasma acylated ghrelin levels are higher in patients with chronic atrophic gastritis. Clin Endocrinol (Oxf) 2007;67:761–766. doi: 10.1111/j.1365-2265.2007.02959.x. [DOI] [PubMed] [Google Scholar]
- 46.Low MJ. Clinical endocrinology and metabolism. The somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract Res Clin Endocrinol Metab. 2004;18:607–622. doi: 10.1016/j.beem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Nørrelund H, Hansen TK, Ørskov H, Hosoda H, Kojima M, Kangawa K, Weeke J, Møller N, Christiansen JS, Jørgensen JO. Ghrelin immunoreactivity in human plasma is suppressed by somatostatin. Clin Endocrinol (Oxf) 2002;57:539–546. doi: 10.1046/j.1365-2265.2002.01649.x. [DOI] [PubMed] [Google Scholar]
- 48.Zavros Y, Paterson A, Lambert J, Shulkes A. Expression of progastrin-derived peptides and somatostatin in fundus and antrum of nonulcer dyspepsia subjects with and without Helicobacter pylori infection. Dig Dis Sci. 2000;45:2058–2064. doi: 10.1023/a:1005659104779. [DOI] [PubMed] [Google Scholar]
- 49.Götz JM, Veenendaal RA, Biemond I, Muller ES, Veselic M, Lamers CB. Serum gastrin and mucosal somatostatin in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1995;30:1064–1068. doi: 10.3109/00365529509101608. [DOI] [PubMed] [Google Scholar]
- 50.Milutinovic AS, Todorovic V, Milosavljevic T, Micev M, Spuran M, Drndarevic N. Somatostatin and D cells in patients with gastritis in the course of Helicobacter pylori eradication: a six-month, follow-up study. Eur J Gastroenterol Hepatol. 2003;15:755–766. doi: 10.1097/01.meg.0000059153.68845.1a. [DOI] [PubMed] [Google Scholar]
- 51.Beales I, Calam J, Post L, Srinivasan S, Yamada T, DelValle J. Effect of transforming growth factor alpha and interleukin 8 on somatostatin release from canine fundic D cells. Gastroenterology. 1997;112:136–143. doi: 10.1016/s0016-5085(97)70228-2. [DOI] [PubMed] [Google Scholar]
- 52.Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci USA. 2003;100:12944–12949. doi: 10.1073/pnas.2135193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abiko Y, Suzuki H, Masaoka T, Nomura S, Kurabayashi K, Hosoda H, Kangawa K, Hibi T. Enhanced plasma ghrelin levels in Helicobacter pylori-colonized, interleukin-1-receptor type 1-homozygous knockout (IL-1R1-/-) mice. World J Gastroenterol. 2005;11:4148–4153. doi: 10.3748/wjg.v11.i27.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]