Abstract
AIM: To evaluate the clinical outcomes of patients undergoing hepatectomy with hemihepatic vascular occlusion (HHO) compared with total hepatic inflow occlusion (THO).
METHODS: Randomized controlled trials (RCTs) comparing hemihepatic vascular occlusion and total hepatic inflow occlusion were included by a systematic literature search. Two authors independently assessed the trials for inclusion and extracted the data. A meta-analysis was conducted to estimate blood loss, transfusion requirement, and liver injury based on the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Either the fixed effects model or random effects model was used.
RESULTS: Four RCTs including 338 patients met the predefined inclusion criteria. A total of 167 patients were treated with THO and 171 with HHO. Meta-analysis of AST levels on postoperative day 1 indicated higher levels in the THO group with weighted mean difference (WMD) 342.27; 95% confidence intervals (CI) 217.28-467.26; P = 0.00 001; I2 = 16%. Meta-analysis showed no significant difference between THO group and HHO group on blood loss, transfusion requirement, mortality, morbidity, operating time, ischemic duration, hospital stay, ALT levels on postoperative day 1, 3 and 7 and AST levels on postoperative day 3 and 7.
CONCLUSION: Hemihepatic vascular occlusion does not offer satisfying benefit to the patients undergoing hepatic resection. However, they have less liver injury after liver resections.
Keywords: Inflow occlusion, Hemihepatic, Vascular occlusion, Hepatectomy, Pringle maneuver
INTRODUCTION
Liver resection is performed mainly for benign and malignant liver tumors, especially for hepatocellular carcinoma. It is a potential curative treatment option in patients with early stage carcinoma[1]. Intraoperative bleeding is a main concern during liver resections, and mortality and morbidity are clearly correlated with the amount of blood loss and the subsequent blood transfusions[2]. Many methods of hepatic vascular control have been introduced to control intraoperative blood loss. In 1908, Pringle applied inflow vascular occlusion technique (the Pringle maneuver) at the hepatic hilar for the first time. It is a technique of total compression of the hepatoduodenal ligament and the most commonly used and relatively easy method for controlling afferent blood flow[3]. However, the Pringle maneuver also carries the risk of global ischemic damage to the liver and intestinal congestion, especially in patients with chronic liver diseases, the degree of which is likely to be accentuated by a prolonged period of vascular inflow occlusion[4,5]. In 1987, Bismuth and Makuuchi proposed a hemihepatic vascular occlusion (HHO) technique to reduce the severity of visceral congestion and total liver ischemia, especially for the remaining liver[6,7]. By this method, visceral congestion is considered to be limited, because considerable portal blood flow is preserved and only portions of the liver are rendered anoxic[8]. The technique with occlusion of vessels supplying the hemiliver containing the tumor, has been suggested to reduce intraoperative bleeding and postoperative liver functional disturbances because of the interruption of blood flow to the liver[9]. But, portal vein and artery dissection to perform selective clamping is time consuming and may result in another blood loss[10].Many prospective randomized controlled trials (RCTs) and retrospective clinical trials have evaluated the feasibility, safety and efficacy of HHO and total hepatic inflow occlusion (THO), however, the clinical significance between the two vascular control methods remain inconsistent. So, the optimal method of vascular control during hepatic resection continues to be debated.
Up to now, a meta-analysis including all available RCTs is still insufficient. We conducted a systematic review and meta-analysis to evaluate the feasibility, safety and efficacy of HHO and THO in patients undergoing hepatectomy.
MATERIALS AND METHODS
Systematic literature search
A systematic literature search was independently conducted by two authors. They systematically searched the Medline, Embase, Science Citation Index, PubMed and CNKI (China National Knowledge Infrastructure Whole Article Database). The following keywords were used: hemihepatic vascular occlusion, hemihepatic occlusion, selective inflow occlusion, selective clamping or selective portal clamping. The literature search was performed with restriction in languages of English or Chinese and types of randomized controlled trial or controlled clinical trial. The last search was done on November 2, 2010.
Inclusion and exclusion criteria
Type of studies: Only RCTs were considered for this review. Quasi-randomized studies, cohort studies, and case-control studies were excluded.
Type of participants: Patients who were about to undergo selective liver resection for benign or malignant liver tumor were included, irrespective of age, gender, cirrhosis, tumor size and nodule numbers. Trials in which patients required contralateral hepatic resection or had distant metastasis or synchronous malignancy in other organs were excluded in the study.
Types of interventions: We included trials comparing total hepatic inflow occlusion with hemihepatic vascular occlusion in hepatectomy, irrespective of ischemic preconditioning before vascular occlusion. Trials only comparing other types of vascular occlusion were excluded.
Type of outcome measures: Primary outcomes: Operative blood loss, biochemical markers of liver injury, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and transfusion requirement. Secondary outcomes: Peri-operative mortality, peri-operative morbidity, operating time , ischemic duration and hospital stay.
Selection of studies
Two authors identified and evaluated independently the trials for inclusion in form of abstracts or full text if necessary. Any disagreement in study selection and data extraction was resolved by discussion.
Data extraction
Two authors extracted the data on a standard form that included population characteristics (sex, age, percentage of major liver resections, methods of ischemic preconditioning and the presence of chronic liver disease) the co-interventions and information on the outcome measures in each trial.
Quality assessment
We assessed the methodological quality of the trials independently. The assessment was made based on sample size calculation; sequence generation; allocation concealment; whether blinding method was adopted for the participants of patients and those who performed the trial and evaluate the outcome; efficacy of randomization; deviations, withdrawals and dropouts; and definition of outcome parameters[11,12].
Statistical analysis
We pooled the synchronized extraction results as estimates of overall therapeutic effects in a meta-analysis using Review Manager Version 5.0 for Windows. The estimated effect measures were odds ratio (OR) for dichotomous data and weighted mean difference (WMD) for continuous data, both reported with 95% confidence intervals (CI). We checked all results for clinical and statistical heterogeneity. Clinical heterogeneity was evaluated based on the study populations and interventions, definition of outcome measures, concomitant treatment, and perioperative management. Heterogeneity was determined by Chi-squared test. P value of 0.10 was considered significant difference and I2 values were used for the evaluation of statistical heterogeneity (I2 of 50% or more indicating presence of heterogeneity)[13]. We used a fixed-effects model to synthesize data when heterogeneity was absent, otherwise a random-effects model would be used. Data were presented as forest plot and funnel plot was used to assess publication bias.
RESULTS
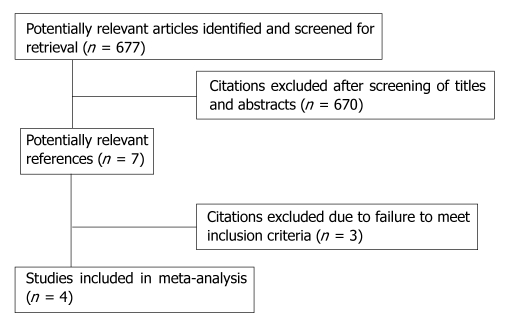
We searched a total of 677 references published between 2002 and 2010. Four RCTs[14-17] including 338 patients met the predefined inclusion criteria (Figure 1). All the trials (Table 1) compared HHO (n = 171) with THO (n = 167). Three trials enrolled cirrhotic and non-cirrhotic patients[14,16,17] and one trial enrolled only cirrhotic patients[15]. In all trials, both major (> 2 segments) and minor (≤ 1 segments) hepatic resections were performed, but one trial exclusively included patients undergoing complex central liver resections. Tables 2, 3, 4 summarize the baseline characteristics and outcomes of the trials. The potential bias of included trials are shown in Table 5. Only one of the trials reported the blinding methods used and the generation of allocation sequence[16].
Figure 1.
Reference flow chart.
Table 1.
Characteristics of randomized controlled trials comparing total hepatic inflow occlusion with hemihepatic vascular occlusion
| Author (yr) | Design | Sample size (n) | THO (n) | HHO (n) | Journal | Comparison |
| Figueras et al (2005) | RCT | 80 | 39 | 41 | Annals of Surgery | Complete vs selective portal triad clamping |
| Wu et al (2002) | RCT | 58 | 28 | 30 | Arch Surg | Hemihepatic vs total hepatic occlusion techniques |
| Yuan et al (2010) | RCT | 120 | 60 | 60 | The American Journal of Surgery | Pringle maneuver vs hemihepatic vascular occlusion |
| Liang et al (2009) | RCT | 80 | 40 | 40 | Hepato-Gastroen-terology | Continuous hemihepatic with intermittent total hepatic inflow occlusion |
| Total | -- | 338 | 167 | 171 | -- | -- |
THO: Total hepatic inflow occlusion; HHO: Hemihepatic vascular occlusion; RCT: Randomized controlled trials.
Table 2.
Characteristics of patients in randomized controlled trials comparing total hepatic inflow occlusion with hemihepatic vascular occlusion
| Author (yr) | Age | Sex | Cirrhosis | Ischaemic | Resection margin (≤ 1 segments:≥ 2 segments) THO/HHO | Diseases |
| (mean yr) | (male:female) | (n:N) | preconditioning | HCC: Others | ||
| THO/HHO | THO/HHO | THO/HHO | THO/HHO | |||
| Figueras et al (2005) | 61.8/62 | 31:8/28:13 | 18:39/21:41 | IC | 25:14/29:12 | 16:23/17:24 |
| Wu et al (2002) | 57.5/53.2 | 23:5/25:5 | 28:28/30:30 | IC | 5:23/7:23 | 25:3/26:4 |
| Yuan et al (2010) | 48.6/49.3 | 46:14/41:19 | 39:60/35:60 | IC if transaction time > 30 min or CC | 5:55/5:55 | 44:16/43:17 |
| Liang et al (2009) | 49.4/49.55 | 27:13/31:9 | 17:40/19:40 | IC or CC | 6:34/10:30 | 20:20/21:19 |
| Total | -- | 127:40/125:46 | 102:167/105:171 | -- | 41:126/51:120 | 105:62/107:64 |
IC: Intermittent clamping; CC: Continuous clamping; HCC: Hepatocellular carcinoma; N: The number of all patients in one trial; n: The number of patients with cirrhosis; THO: Total hepatic inflow occlusion; HHO: Hemihepatic vascular occlusion.
Table 3.
Outcomes of randomized controlled trials comparing total hepatic inflow occlusion with hemihepatic vascular occlusion
| Author (yr) | Operative time (min) | Ischemic duration (min) | Blood loss (mL) | Transfusion requirements | Complications total (n) | In-hospital stay (d) | In-hospital death (n) |
| THO/HHO | THO/HHO | THO/HHO | THO/HHO | THO/HHO | THO/HHO | THO/HHO | |
| Figueras et al (2005) | 207 ± 48/219 ± 45 | 41 ± 14/47 ± 18 | 671 ± 533/735 ±397 | 4:39/6:41 | 15:39/ 12:41 | 9.38 ± 4.9/8.15 ± 3.8 | 0:39/1:41 |
| Wu et al (2002) | 409 ± 19.2/ 399 ± 15.6 | 96.0 ± 10.9/ 94.2 ± 9.9 | 1685 ± 170/ 1159 ± 221 | 12:28/5:30 | 8:28/10:30 | 14.8 ± 1.4/16.4 ± 1.4 | 0:28/0:30 |
| Yuan et al (2010) | 114.2 ± 37.2/ 133.5 ± 44.6 | 16.6 ± 8.7/ 14.9 ± 4.5 | 339.5 ± 205.1/ 354.4 ± 240.3 | 6:60/4:60 | 19:60/12:60 | 13.7 ± 5.2/10.2 ±4.1 | 1:60/0:60 |
| Liang et al (2009) | 203.98 ± 38.36/ 236.15 ± 49.2 | 40.17 ± 13.30/ 42.38 ± 12.79 | 569.8 ± 285.56/ 649.35 ± 279.05 | 14:40/15:40 | 8:40/9:40 | 9.85 ± 3.55/10.12 ± 2.41 | 0:40/0:40 |
| Total | -- | -- | -- | 36:167/30:171 | 50:167/43:171 | -- | 1:167/1:171 |
THO: Total hepatic inflow occlusion; HHO: Hemihepatic vascular occlusion.
Table 4.
Postoperative aspartate aminotransferase and alanine aminotransferase levels of patients in randomized controlled trials comparing total hepatic inflow occlusion with hemihepatic vascular occlusion
| Author (yr) | AST (U/L) | AST (U/L) | AST (U/L) | ALT (U/L) | ALT (U/L) | ALT (U/L) |
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | |
| THO/HHO | THO/HHO | THO/HHO | THO/HHO | THO/HHO | THO/HHO | |
| Wu et al (2002) | 420 ± 790/290 ± 770 | 180 ± 320/190 ± 510 | 50 ± 40/30 ± 20 | 370 ± 490/480 ± 510 | 330 ± 320/320 ± 270 | 90 ± 20/70 ± 20 |
| Yuan et al (2010) | 812.6 ± 475.3/447.6 ±210.3 | 423.7 ± 265.4/207.5 ± 79.3 | 143.6 ± 87.5/64.2 ± 29.4 | 1013.6 ± 654.4/369.4 ± 347.2 | 592.2 ± 416.4/218.4 ± 185.3 | 172.4 ± 125.8/ 79.6 ± 55.3 |
| Figueras et al (2005) | NS | NS | NS | 402 ± 258/372 ± 234 | NS | NS |
THO: Total hepatic inflow occlusion; HHO: Hemihepatic vascular occlusion.
Table 5.
Assessment the methodological quality of included studies
| Author (yr) | Sample size calculation | Generation of allocation sequence | Allocation concealment | Deviations, withdrawals and dropouts | Efficacy of randomization | Blinding | Definition of outcome parameters |
| Figueras et al (2005) | Yes | No description | Sealed envelope | Yes | Yes | No description | Yes |
| Wu et al (2002) | No description | No description | Sealed envelope | No description | Yes | No description | Yes |
| Yuan et al (2010) | Yes | Yes | Sealed envelope | Yes | Yes | Single-blinded | No description |
| Liang et al (2009) | No description | No description | No description | Yes | Yes | No description | Yes |
Effects of interventions
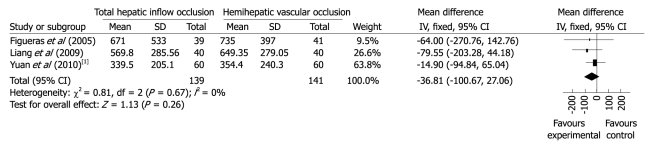
Blood loss. Information on intraoperative blood loss was available in all analyzed trials. The trial by Wu et al[15] reported significantly more blood loss in patients of both groups. Statistical heterogeneity was presented and P = 0.000 001. Funnel plot to evaluate publication bias for outcome of blood loss demonstrated a strong asymmetry, suggesting the existence of severe publication bias. Clinical heterogeneity analysis found that complex central liver resections were performed on cirrhotic patients, and the cut surface area was wider and would increase intraoperative blood loss. Meta-analysis of the other three trials showed no significant difference between THO group and HHO group (WMD -36.81; 95% CI -100.67 to 27.06, P = 0.26, I2 = 0%) (Figure 2).
Figure 2.
Meta-analysis of blood loss in randomized controlled trials comparing total hepatic inflow occlusion with hemihepatic vascular occlusion. 1Blood loss.
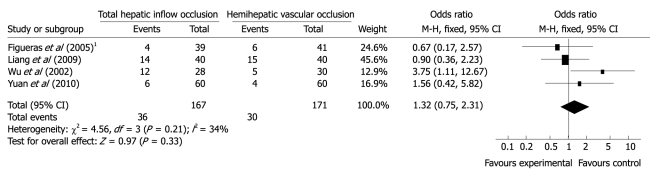
Transfusion requirement. All trials reported the number of patients who needed transfusion in both groups. Funnel plot did not demonstrate a strong asymmetry. Meta-analysis (Figure 3) indicated no difference in postoperative transfusion requirement between the groups (OR 1.32 95% CI 0.75-2.31, P = 0.33, I2 = 34%). Since there was no uniform definition of the average transfusion volume in the trials, we did not compare the transfusion volume in the study.
Figure 3.
Meta-analysis of aspartate aminotransferase levels on postoperative 1st d. 1Transfusion requirements (n).
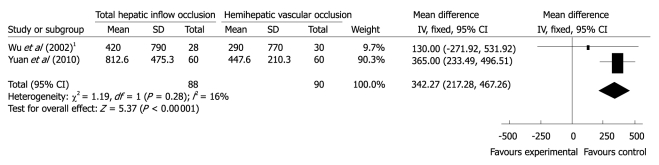
Biochemical markers of liver injury. All the four trials provided AST and ALT levels on postoperative days. However, data on AST and ALT were available in only two studies. We did not draw funnel plots to examine the potential publication bias in this review, because the number of the included trials was small. Wu et al[15] provided the data of ALT and AST levels on postoperative days 1, 3, 5 and 7, and Yuan et al[16] gave the information on postoperative days 1, 3 and 7. The ALT levels on postoperative day 1 in Figueras’s study[14] were also available. Meta-analysis of ALT levels on postoperative days 1, 3 and 7 showed no significant difference between the two groups (WMD on day 1/191.03, 95% CI -239.04 to 621.10, P = 0.38, I2 = 94%; WMD on day 3/192.86, 95% CI -163.66 to 549.37, P = 0.29, I2 = 94%, and WMD on day 7/54.43, 95% CI -16.81 to 125.67, P = 0.13, I2 = 94%). Meta-analysis of AST levels on postoperative days 3 and 7 in the two studies showed no significant difference between THO group and HHO group (WMD on day 3/127.52, 95% CI -88.92 to 343.96, P = 0.25, I2 = 73%; WMD on day 7/49.10, 95% CI -9.1 to 107.3, P = 0.10, I2 = 94%). Meta-analysis of AST levels on postoperative day 1 indicated higher postoperative AST levels in the THO group (WMD 342.27; 95% CI 217.28 to 467.26; P = 0.00 001, I2 = 16%) (Figure 4).
Figure 4.
Meta-analysis of aspartate aminotransferase levels on postoperative Day 1. 1Aspartate aminotransferase (D1).
Peri-operative mortality and morbidity. Four studies provided data on peri-operative mortality and morbidity. In total, two patients died in the four trials. Both died from liver failure, one in THO group and the other in HHO group. Meta-analysis of these studies revealed neither of the two groups showed superiority in overall morbidity (OR 1.28, 95% CI 0.79-2.07, P = 0.31, I2 = 0%) and mortality (OR 1.03, 95% CI 0.14-7.44, P = 0.98, I2 = 0%). Meta-analysis of bile leak (OR 0.92, 95% CI 0.35 -2.44, P = 0.87, I2 = 0%) and hepatic insufficiency (OR 1.02, 95% CI 0.29 - 3.60, P = 0.97, I2 = 35%) showed no statistically significant difference.
Operating time, ischemic duration and hospital stay. There was no statistically significant difference in operating time (WMD -12.44, 95% CI -32.88 to 8.00, P = 0.23, I2 = 86%) between the two groups, also in hospital stay (WMD 0.63, 95% CI -1.60 to 2.85, P = 0.58, I2 = 91%) and in ischemic duration (WMD 0.61, 95% CI -1.40 to 2.61, P = 0.55, I2 = 43%)
DISCUSSION
The key points in hepatectomy are to control intraoperative bleeding and prevent postoperative complications such as liver failure and bile leakage[18]. Intraoperative blood loss has been shown to significantly influence the short-term prognosis of patients undergoing liver resection[19,20]. Hemihepatic vascular clamping selectively interrupts the arterial and venous inflow to the right or left hemiliver and therefore avoids both splanchnic blood stasis and ischemia or ischemia-reperfusion injury to the whole liver[9,21]. A retrospective study[22] indicated that the average bleeding volume and transfusion requirements were less in hemihepatic vascular occlusion group compared with Pringle maneuver group. But, other retrospective studies[8,23] showed no difference between the two groups. Our meta-analysis showed no significant difference in blood loss and transfusion requirements between the two groups. Three[14,16,17] of the four trials in the review showed no difference in the amount of hemorrhage and blood transfusion requirements, but one study[15] reported that the amount of operative blood loss and the incidence of blood transfusion were significantly higher in group THO patients (1685 mL vs 1159 mL, P = 0.049) and the volume of blood loss was much higher than in other studies. It could be explained by the fact that the patients in the study had cirrhosis and underwent complex central liver resections, while other trials included both cirrhotic and non-cirrhotic patients. The procedures presented herein were more difficult and time-consuming than conventional major hepatectomy and transected plane was also wider[15,24-26]. Both factors induced massive bleeding and difficulties in hemostasia.
Liver injury due to ischemia and subsequent reperfusion are major concerns in inflow vascular occlusion[27-29] and are usually monitored after surgery by measuring aminotransferase levels[30]. We found no significant difference on ALT levels on postoperative days 1, 3 and 7 in the two groups, also on AST levels on postoperative days 3 and 7. Three RCTs[14,15,17] and one retrospective study[18] drew the same conclusion. Theoretically, the blood flow in one lobe of the liver in group HHO is preserved and the liver function damage may be less than that in group THO[31].Yuan et al[16] indicated that the Pringle maneuver group was associated with a significantly higher peak in ALT and AST levels (P = 0.01). Meta-analysis showed that AST levels on postoperative day 1 were also higher in the THO group (WMD 342.27, 95% CI 217.28-467.26, P = 0.00001, I2 = 16%). Chau et al[23] concluded that patients subjected to HHO responded better than those subjected to the Pringle maneuver in terms of earlier recovery of postoperative liver function. Therefore, HHO resulted in less liver injury and was advantageous in the recovery of postoperative liver function.
Unfortunately, only two trials in our analysis included data on ALT and AST levels. There were no significant differences in patients’ general characteristics, resection margin, and ratio of cirrhotic to non-cirrhotic patients (P = 0.05). However, intermittent clamping was used in the trial by Wu et al[15], whereas Yuan et al[16] did continuous clamping if transaction time was ≤ 30min, otherwise intermittent clamping would be used. A RCT[32] comparing intermittent portal triad clamping with continuous clamping showed no statistically significant differences, although the peak AST level was lower in the intermittent portal triad clamping. Belghiti et al[33] suggested that in chronic patients, the transaminase levels were significantly higher in the continuous portal triad clamping than in the intermittent portal triad clamping. Cirrhotic liver and pre-existing liver were less able to tolerate ischemia than normal liver in clinical observations or animal experiments[28,34,35].The proportion of chronic patients in the two RCTs were 100% and 61.7% respectively, which may influence the ALT and AST levels in HHO and THO groups and account for the lack of difference between the two groups on postoperative days 3 and 7. Due to the limited number and non-available data in the trials, no subgroup analysis was performed in patients with cirrhosis, which is known to increase the sensitivity of the livers to ischemia[30].
There was one death in Figueras’ trials[14] in HHO group as a result of hepatic insufficiency in a patient with hepatitis C virus (HCV) cirrhosis . His blood loss during the operation was 2120 mL and 5 units of red blood cell transfusion were required. Yuan et al[16] reported one patient in the Pringle maneuver group who died of liver failure on the 26th d after a right hepatectomy. The total mortality was 0.51% and total peri-operative morbidity was 27.51%. But no statistically significant difference was found in the peri-operative mortality, peri-operative morbidity, operating time, ischemic duration and hospital stay. Complications included ascites, bile leak, hepatic insufficiency, portal thrombosis, pleural effusion, wound infection, hemorrhage and so on. Meta-analysis of bile leak and hepatic insufficiency showed no significant difference between THO group and HHO group.
This review has some limitations. First, our literature search might have not detected all relevant evidences and the number of RCTs included in this review is small. Second, incomplete reporting of important methodological issues, such as sample size calculation, randomization process and blinding assessment of trial quality, might raise doubts on the adequate power of these studies[36]. Third, the heterogeneity of the patients in the included trials may influence the conclusions as some trials included major and complex central liver resections and some included normal and cirrhotic livers.
In conclusion, the current evidence shows no advantage of hemihepatic vascular occlusion over the total hepatic inflow occlusion in terms of blood loss, transfusion requirement, mortality and morbidity, operating time and hospital stay. However, HHO results in less liver injury after liver resections. Further trials are required to assess optimal technique of hepatic vascular control for the patients hepatectomy especially for the patients with chronic cirrhosis.
COMMENTS
Background
Possibility of life-threatening hemorrhage always exists in patients with liver resection, so liver vascular control to reduce blood loss is important. Since the Pringle maneuver technique was successfully applied by Pringle in 1908, many methods of hepatic vascular control have been introduced to accelerate the development of hepatic surgery. Bismuth and Makuuchi proposed the hemihepatic vascular occlusion technique, which attracted much attention among surgeons.
Research frontiers
Both Pringle maneuver and hemihepatic vascular occlusion techniques can reduce blood loss during transaction of the hepatic parenchyma,but Pringle maneuver produces ischemic injury to the remaining liver and intestinal congestion. Hemihepatic vascular occlusion technique has become very popular in recent years, because it is thought to limit visceral congestion and can protect the remaining liver. Many studies including randomized controlled trials (RCTs) have been designed to evaluate the safety, feasibility and efficiency of the two methods.
Innovations and breakthroughs
The authors searched and assessed all the RCTs comparing the two techniques and drew a conclusion by a systematic review and meta-analysis. They found that hemihepatic vascular occlusion did not offer benefit to the patients except for reducing ischemic liver injury after liver resections.
Applications
Hemihepatic vascular occlusion technique should be recommended for hepatectomy to reduce peri-operative blood loss and protect the remaining liver after the surgery.
Terminology
Hemihepatic vascular occlusion is a method which selectively interrupts the arterial and portal inflow to the part of the liver (right or left hemiliver) ipsilateral to the lesion that requires resection. It can be achieved after placing a curved renal pedicle clamp across the right or left portal structures. And Pringle maneuver involves compression of the hepatoduodenal ligament to interrupt all arterial and portal inflow to the whole liver.
Peer review
The manuscript is methodologically well designed and is concise in its data and conclusion. However, it should be subjected to linguistic revision to improve several mistakes in grammar and style.
Footnotes
Supported by a Grant from the National Science and Technology Major Project of China, No. 2008ZX10002-025, 2008ZX10002-026
Peer reviewer: Thilo Hackert, MD, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Bergoc RM, Caro RA. The competitive nature of reticuloendothelial "blockade". Int J Nucl Med Biol. 1975;2:33–36. doi: 10.1016/0047-0740(75)90018-2. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541–549. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Yu WC, Wong J. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999;134:533–539. doi: 10.1001/archsurg.134.5.533. [DOI] [PubMed] [Google Scholar]
- 5.Wu CC, Hwang CR, Liu TJ, P’eng FK. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. Br J Surg. 1996;83:121–124. doi: 10.1002/bjs.1800830139. [DOI] [PubMed] [Google Scholar]
- 6.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 7.Lau WY, Lai EC, Lau SH. Methods of vascular control technique during liver resection: a comprehensive review. Hepatobiliary Pancreat Dis Int. 2010;9:473–481. [PubMed] [Google Scholar]
- 8.Tanaka K, Shimada H, Togo S, Nagano Y, Endo I, Sekido H. Outcome using hemihepatic vascular occlusion versus the pringle maneuver in resections limited to one hepatic section or less. J Gastrointest Surg. 2006;10:980–986. doi: 10.1016/j.gassur.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Makuuchi M, Mori T, Gunvén P, Yamazaki S, Hasegawa H. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155–158. [PubMed] [Google Scholar]
- 10.Gotoh M, Monden M, Sakon M, Kanai T, Umeshita K, Nagano H, Mori T. Hilar lobar vascular occlusion for hepatic resection. J Am Coll Surg. 1994;178:6–10. [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 12.Bañares R, Albillos A, Rincón D, Alonso S, González M, Ruiz-del-Arbol L, Salcedo M, Molinero LM. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology. 2002;35:609–615. doi: 10.1053/jhep.2002.31354. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueras J, Llado L, Ruiz D, Ramos E, Busquets J, Rafecas A, Torras J, Fabregat J. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Ann Surg. 2005;241:582–590. doi: 10.1097/01.sla.0000157168.26021.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CC, Yeh DC, Ho WM, Yu CL, Cheng SB, Liu TJ, P’eng FK. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients: a randomized comparison of hemihepatic and total hepatic occlusion techniques. Arch Surg. 2002;137:1369–1376. doi: 10.1001/archsurg.137.12.1369. [DOI] [PubMed] [Google Scholar]
- 16.Si-Yuan FU, Yee LW, Guang-Gang L, Qing-He T, Ai-Jun LI, Ze-Ya PA, Gang H, Lei Y, Meng-Chao WU, Eric LA, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201:62–69. doi: 10.1016/j.amjsurg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Liang G, Wen T, Yan L, Li BO, Wu G, Yang J, Lu B, Chen Z, Liao Z, Ran S, et al. A prospective randomized comparison of continuous hemihepatic with intermittent total hepatic inflow occlusion in hepatectomy for liver tumors. Hepatogastroenterology. 2009;56:745–750. [PubMed] [Google Scholar]
- 18.Nakai T, Koh K, Funai S, Kawabe T, Okuno K, Yasutomi M. Comparison of controlled and Glisson’s pedicle transections of hepatic hilum occlusion for hepatic resection. J Am Coll Surg. 1999;189:300–304. doi: 10.1016/s1072-7515(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 19.Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33–40. doi: 10.1097/00000658-198701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada M, Matsumata T, Akazawa K, Kamakura T, Itasaka H, Sugimachi K, Nose Y. Estimation of risk of major complications after hepatic resection. Am J Surg. 1994;167:399–403. doi: 10.1016/0002-9610(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 21.Belghiti J, Marty J, Farges O. Techniques, hemodynamic monitoring, and indications for vascular clamping during liver resections. J Hepatobiliary Pancreat Surg. 1998;5:69–76. doi: 10.1007/pl00009953. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Miao X, Xiong L, Xiong G, Hu J, Zhong D, Li Q, Liu W. Application of hemihepatic vascular occlusion with hanging maneuver in hepatectomy. Hepatogastroenterology. 2009;56:442–446. [PubMed] [Google Scholar]
- 23.Chau GY, Lui WY, King KL, Wu CW. Evaluation of effect of hemihepatic vascular occlusion and the Pringle maneuver during hepatic resection for patients with hepatocellular carcinoma and impaired liver function. World J Surg. 2005;29:1374–1383. doi: 10.1007/s00268-005-7766-4. [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Ho WL, Chen JT, Tang CS, Yeh DC, Liu TJ, P’eng FK. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J Am Coll Surg. 1999;188:508–515. doi: 10.1016/s1072-7515(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 25.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711; discussion 711-713. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco D, Borgonovo G. Liver resection in cirrhosis of the liver. In: Blumgart LH, Fong Y, editors. Surgery of the Liver and Biliary Tract. 3rd ed. Philadelphia, Pa: WB Saunders Co; 2000. pp. 1725–1742. [Google Scholar]
- 27.Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636–1640. doi: 10.1046/j.1365-2168.1998.00917.x. [DOI] [PubMed] [Google Scholar]
- 28.Ezaki T, Seo Y, Tomoda H, Furusawa M, Kanematsu T, Sugimachi K. Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg. 1992;79:224–226. doi: 10.1002/bjs.1800790311. [DOI] [PubMed] [Google Scholar]
- 29.Rüdiger HA, Kang KJ, Sindram D, Riehle HM, Clavien PA. Comparison of ischemic preconditioning and intermittent and continuous inflow occlusion in the murine liver. Ann Surg. 2002;235:400–407. doi: 10.1097/00000658-200203000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, Schmidt J, Weitz J, Büchler MW. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg. 2008;95:424–432. doi: 10.1002/bjs.6141. [DOI] [PubMed] [Google Scholar]
- 31.Takayama T, Makuuchi M, Inoue K, Sakamoto Y, Kubota K, Harihara Y. Selective and unselective clamping in cirrhotic liver. Hepatogastroenterology. 1998;45:376–380. [PubMed] [Google Scholar]
- 32.Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921–928; discussion 928-930. doi: 10.1097/01.sla.0000246834.07130.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–375. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huguet C, Nordlinger B, Galopin JJ, Bloch P, Gallot D. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689–93. [PubMed] [Google Scholar]
- 35.Nishimura T, Nakahara M, Kobayashi S, Hotta I, Yamawaki S, Marui Y. Ischemic injury in cirrhotic livers: an experimental study of the temporary arrest of hepatic circulation. J Surg Res. 1992;53:227–233. doi: 10.1016/0022-4804(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 36.Rahbari NN, Koch M, Mehrabi A, Weidmann K, Motschall E, Kahlert C, Büchler MW, Weitz J. Portal triad clamping versus vascular exclusion for vascular control during hepatic resection: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:558–568. doi: 10.1007/s11605-008-0588-6. [DOI] [PubMed] [Google Scholar]