Abstract
The prevalence of osteoporosis has been increasing globally. Recently surgical indications for elderly patients with osteoporosis have been increasing. However, only few strategies are available for osteoporotic patients who need spinal fusion. Osteoporosis is a result of negative bone remodeling from enhanced function of the osteoclasts. Because bone formation is the result of coupling between osteoblasts and osteoclasts, anti-resorptive agents that induce osteoclast apoptosis may not be effective in spinal fusion surgery, necessitating new bone formation. Therefore, anabolic agents may be more suitable for osteoporotic patients who undergo spinal fusion surgery. The instrumentations and techniques with increased pullout strength may increase fusion rate through rigid fixation. Studies on new osteoinductive materials, methods to increase osteogenic cells, strengthened and biocompatible osteoconductive scaffolds are necessary to enable osteoporotic patients to undergo spinal fusion. When osteoporotic patients undergo spinal fusion, surgeons should consider appropriate osteoporosis medication, instrumentation and technique.
Keywords: Osteoporosis, Spine, Fusion, Osteoblast, Osteoclast
INTRODUCTION
Because the degenerative changes in the intervertebral discs and spinal facet joint capsules in people over 50 years of age are associated with spinal instability and there are the increased life expectancy, improved quality of life and the elderly desire to remain physically active, surgical indications for degenerative spinal conditions in elderly patients have been increased2,21,28,33,37). The surgical outcome and perioperative complication of spinal fusion in elderly patients can be negatively affected by co-morbidities, such as cardiopulmonary disease, renal disease, diabetes mellitus, nutritional disorders and osteoporosis18). Because osteoporosis is strongly associated with poor fusion rate and bone stability, it is crucial to understand the pathophysiology of osteoporosis and its treatment, in order to enhance spinal fusion and preserve bone stability. Spinal surgeons must be informed of the appropriate treatment plan for osteoporosis, and formulate appropriate strategies for osteoporotic patients who need to undergo spinal fusion surgery. The objective of this article was to review the prevalence and pathophysiology of osteoporosis, and strategies to facilitate spinal fusion.
PREVALENCE OF OSTEOPOROSIS AND SPINAL FUSION IN ELDERLY PATIENTS
Osteoporosis is a major global problem, because over 10 million people are currently diagnosed with osteoporosis34). Although 80% of osteoporotic patients are women, a considerable number of men are also affected19,25). The age matched prevalence of osteoporosis is 17-20% of women over 50 years old, 26% over 65 years old and 50% over 85 years old in the United States. In the Republic of Korea, the prevalences of osteoporosis in the lumbar spine in women between 50 and 59, 60 and 69 and 70 and 79 years old are 32.8%, 62.2%, 88.9%, respectively34,42,48). In addition, the prevalence of osteoporosis in male and female patients over 50 years old who underwent spinal surgery were 14.5% and 51.3%, respectively21). Due to increasing life expectancy, the number of elderly patients with osteoporosis will increase even further.
Due to an increasingly aged population, degenerative spinal stenosis and spondylolisthesis have become more frequently diagnosed67,72). Up to 10% of women over 60 years may be affected by degenerative spondylolisthesis and one study presented the rates of male and female patients with spondylolisthesis (degenerative or spondylolytic types) at 14.8% and 66.1%, respectively21,34). In elderly patients, iatrogenic cause of instability following spinal surgery may occur because of pre-existing degenerative changes in the facet joints and intervertebral disc. If instability of the spine at the index level is confirmed by preoperative radiological evaluations or when iatrogenic instability occurs, fusion operation should be considered in elderly patients40,67,72). Several reports claimed that decompression and additional fusions in elderly patients who experienced spinal stenosis and instability, such as spondylolisthesis, produced favorable outcomes, because lumbar arthrodesis with spinal instrumentation produce satisfactory outcome in elderly patients20,32,40,65,72). Many studies demonstrated fusion failure which negatively impact on clinical outcomes, and fusion rates ranged from 56% to 100%13,56,71). There exist reports on the outcome of lumbar arthrodesis following instrumentation in patients over 60 years of age, which indicated the prevalence of delayed and collapsed fusion in elderly patients to be higher than that in younger patients. The fusion rates of elderly patients reported were over 90%32,57). In elderly osteoporotic patients who underwent lumbar arthrodesis with instrumentation, the fusion rates were 89.7%, 95.8%20,43). In other words, old age and osteoporosis are not contraindication in spinal arthrodesis. The number of elderly patients who needs spinal surgery will increase and the prevalence of osteoporosis in elderly patients is high. The existence and severity of osteoporosis should be preoperatively assessed in elderly patients, and appropriate strategy to facilitate spinal fusion should be formulated.
PATHOGENESIS OF POSTMENOPAUSAL OSTEOPOROSIS
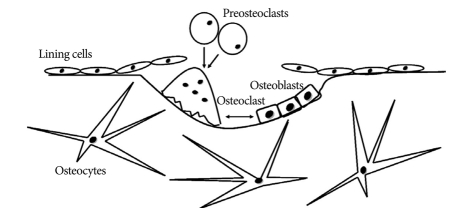
Although the skeletal system appears to be a static structure macroscopically, the bone is a collection of dynamic tissues microscopically. In other words, remodeling, including bone absorption and formation in the microcracks of bone occurs continuously41,50). Bone remodeling is performed by basic multi-cellular unit (BMU) within the bone remodeling cavity, and the BMU is composed of osteoclasts, osteoblasts, bone lining cells and osteocytes (Fig. 1)66). Complete regeneration of adult skeleton through remodeling takes 10 years and remodeling serves to repair damage and prevent aging and fracture41). Remodeling with positive balance occurs in the growing skeleton, and negative remodeling causes reduced bone mineral density (BMD) and osteoporosis. Among several etiologies of osteoporosis, menopause is the most common cause. Bone loss in both women and men begins in the 40s and rapid bone loss in women occurs during the first 5-10 years after menopause46). In addition, women accumulate less bone mass than men during the developmental period. Therefore, the incidence of fracture is higher in women than in men58).
Fig. 1.
This scheme shows the basic mutilcellular units (BMU) in resorption cavity. After the lining cells prepare the damaged bone surface, osteoclasts as fused cell preosteoclasts absorb the bone in a BMU and release several growth factors that promote osteoblasts.
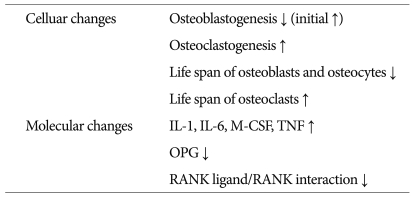
After the loss of estrogen (menopause), several events occur. In terms of molecular changes, several cytokines such as IL-1, IL-6, macrophage colony stimulating factor and tumor necrosis factor increase and these cytokines stimulate osteoclast development50). Osteoprotegerin stimulated by estrogen has very potent inhibitory effects on osteoclastogenesis through blocking receptor activation of nuclear factor-κB (RANK) ligand/RANK interaction - the main stimulator involved in osteoclast differentiation and activation50,64). In terms of cellular changes, although the formation of osteoblast and osteoclast in the bone marrow is up-regulated initially after menopause, the first event as the mesenchyme differentiates toward osteoblasts has decreased and the increased osteoclast formation persists51). In addition, estrogen deficiency leads to shorter osteoblast and osteocytes lifespan, and prolongation of osteoclast lifespan52,60). The longer lifespan of osteoclasts is responsible for deeper absorption cavities27,60). In addition, increase in osteocyte apoptosis may impair the osteocytes-canalicular mechanoreceptor as the skeletal signals of detection of microdamage and repair. Bone loss through negative bone remodeling may be the downstream consequence of these changes (Table 1).
Table 1.
The cellular and molecular changes after loss of estrogen
IL : interleukin, M-CSF : macrophage-colony stimulating factor, TNF : tumor necrosis factor increase, OPG : Osteoprotegerin, RANK : receptor activator of nuclear factor-κb
BIOLOGY OF SPINAL FUSION
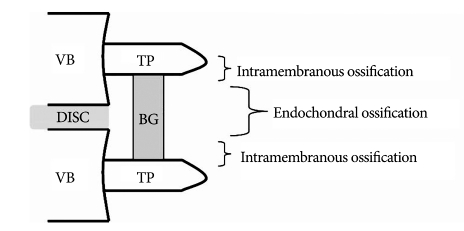
Although instrumentation and technique have been improving, non-union still occurs in 5 to 35% of patients who undergo spinal fusion9,14). Non-union in spinal surgery frequently leads to unsatisfactory clinical outcomes23,30). Therefore, understanding of the histological and biologic events in spinal fusion is crucial to spinal surgeons who treat patients with and without osteoporosis. Clinically relevant lumbar fusion animal model provide information on the methods that facilitate fusion in several articles. Non-decortication of the transverse process did not result in arthrodesis and primary vascular supply to the fusion mass originated from decorticated bone, not from the adjacent muscle10,69). Intra-membranous bone formation occurs in the area near the transverse processes, and endochondral bone formation which involves bone formation through a cartilage intermediate occurs centrally at the interface between the upper and lower halves of the bridging bone73). Cartilage formed through endochondral ossification has poor vascular supply and low oxygen saturation. However, in the mid and late stages of bone formation, extension of bone formation towards the central zone occurs and disappearance of cartilage and bone formation occurs in the central area (Fig. 2)10,11,69). The transient cartilaginous area may explain why many non-unions are found to occur in the central zone of a fusion mass. Considering the previous description and three factors for bone formation as osteoconductive scaffold, osteogenic cell and osteoinductive materials, the characteristics of host bed such as vascularity and quality of bone marrow, the distance of fusion site and the quality of bone graft should be assessed by the surgeon. Although there is no publication that discusses the histological difference between osteoporosis and non-osteoporosis animal models with spinal fusion, reduced osteoblast ability, poor vascularity and lower bone marrow quality in the host bed may contribute to non-union in elderly osteoporotic patients. Therefore, surgeons must consider bone graft quality, proper osteoinductive materials (for example, bone morphogenetic proteins (BMPs) and other growth factors), increasing the ability of osteoblasts [for example, with intermittent administration of parathyroid hormone (PTH)] and preventing factors that may hinder fusion, including long-term use of non-steroidal anti-inflammatory agents and smoking, before performing spinal fusion on elderly osteoporotic patients.
Fig. 2.
The scheme shows the different ossifications in center posterolateral fusion. VB : vertebral body, TP : transverse process, BG : bone graft.
STRATEGIES FOR OSTEOPOROTIC PATIENTS WITH SPINAL FUSION
Osteoporosis reduces bone quality through negative bone remodeling. Low bone quality can reduce the pull-out strength of pedicle screw, and negative bone remodeling can cause delayed bone fusion3,22). Therefore, before performing spinal fusion surgery on osteoporotic patients, we should pursue effective strategies to increase the pull-out strength and facilitate positive bone remodeling.
Pharmacotherapeutic strategies
Osteoporosis secondary to loss of estrogen is the cause of negative bone remodeling through reduced function and life span of osteoblasts, and the reverse for osteoclasts. In addition, bone remodeling depends on communication between the osteoblast lineage, including lining cell, preosteoblasts, osteocytes and the osteoclast lineage66). Thus, in order to obtain good fusion rate in osteoporotic patients, we should be aware of the antiresorptive and anabolic agents.
Bisphophonates
Biphosphonates are typical anti-resorptive agents that include alendronate, ibandronate, etidronate and pamidronate. The mechanism of bisphosphonate is to promote apoptosis of mature osteoclasts and result in slow rate of bone remodeling38,50,60). Many animal studies presented the effects of bisphosphonates on the skeletal system. In animal studies that investigated fracture healing and pull-out strength of implants, bisphosphonates did not adversely affect the skeletal system55,61). However, according to recent studies, bisphosphonates inhibit or delay spinal fusion through reduced incorporation between grafted bone and host bone37,45,68). In other words, the anti-fracture effect of bisphosphonates is not proportional to their efficacy on bone fusion. Therefore, when osteoporotic patients are scheduled to undergo spinal fusion, surgeons must consider the need of using other antiresorptive or anabolic agents postoperatively.
Other classes of anti-absorptive agents
There are many studies on new treatment targets of osteoclasts, except bisphosphonate that acts chiefly by inducing the osteoclast apoptosis. The targets of new resorption inhibitors are RANKL, cathepsin K, vacuolar type H+ATPase (V-ATPases) and intergrin ανβ364). RANKL is a main stimulator of osteoclast differentiation. The anti-RANKL antibody, denosumab (AMG 162) was evaluated in Phase I, II and III trials and the effect of denosumab on BMD was superior to that of alendronate6,15,53). Cathepsin K is a cysteine protease expressed by osteoclasts and degrades bone matrix protein. A human cathepsin K inhibitor, odanacatib (MK-0822) is well-tolerated and increases lumbar spine and total-hip BMD of postmenopausal women in Phase I and II studies12,31). V-ATPases acts as a proton pump in the resorptive cavity under osteoclasts and intergrin ανβ3 is the main integrin on osteoclasts. Research on V-ATPases inhibitor and ανβ3 antagonist is ongoing54,64).
PTH
Only one drug acts as anabolic agent to osteoporosis - recombinant human PTH, triparatide. Although high levels of PTH cause decreased BMD through increased bone resorption, low and intermittent PTH elevation increases bone formation secondary to its anti-apoptotic effect on osteoblasts35,38,39,64). Prior studies concluded that PTH treatment did not increase the incidence of bone tumor, such as osteosacroma36,59,70). It must be emphasized that the experience of PTH use is so far limited in the United States and Europe to 2 years and 18 months, respectively. If PTH treatment is not followed by antiresorptive therapy, the increased BMD would be lost7,26). Therefore, it needs to develop additional anabolic agents that can be continuously used in osteoporotic patients. The results of animal studies suggested that PTH enhanced the healing of bone fracture, BMD, mechanical strength and arthrodesis of the spine1,4). As concurrent use of alendronate for increasing positive remodeling reduced the anabolic effect of PTH, the use of PTH on osteoporotic patients taking bisphosphonates may be refrained after spine arthrodesis8).
Other anabolic agents
Other targets for anabolic agents are Wnt signaling pathway and activin. The Wnt signaling pathway has a critical role on bone formation, and sclerostin and Dickkopf (DKK)-1 are the inhibitors of the Wnt signaling pathway26,64). The efficacy of sclerostin and DKK-1 antagonists have been examined in several animal studies and these results are related to clinical studies for osteoporosis patients47,64). Activin is a member of the TGF-β superfamily and activin receptor is one of the BMP receptors64). Activation of BMP receptors causes differentiation of osteoblasts and increased bone formation26). Although there is no clinical report on the outcome of BMP therapy in osteoporotic patients who have undergone lumbar spinal fusion, BMPs increased fusion rate for non-osteoporotic patients with spine arthrodesis according to recent articles. In an animal study using osteoporotic rats, higher dose of BMP-7 increased spinal fusion rate16,49,63). Therefore, agents that exert effects on the BMP pathway, such as BMPs and activin, are promising anabolic agents for osteoporotic patients with or without spine fusion.
Implant based strategies
Cancellous bone is more affected by osteoporosis than cortical bone, therefore lower BMD has been a major factor in poor screw fixation, screw loosening and fixation failure22). Many techniques have been employed to enhance the pullout strength of the pedicle screw29). The preparation for screw hole or minimization of tapping hole can affect the pullout strength in osteoporotic bone and, although the anatomical constraints vary with patients, bigger and longer screws may provide good solution for fragile bones24). The angulation of two screws and screw positioning in areas of higher BMD in the vertebrate may increase pullout strength62,69). Also, to improve the fixation and fatigue strength of instrumentation, screw augmentation with polymethyl methacrylate has yielded favorable outcomes5,17,43). These techniques may enhance bone fusion through stabilization of fusion segments.
Other strategies
Mesenchymal cells differentiate to osteoblasts are critical for increasing fusion rate. Although the fusion rate achieved by using bone marrow aspiration (BMA) with collagen was inferior to that in autologous iliac crest bone for posterior lumbar interbody fusion, the fusion rate of posterolateral lumbar fusion was comparable to that of autologous bone44). However, as there is low concentration of osteogenic cell in the BMA, it is ineffective as a bone graft substitute. Therefore, investigations for methods of stimulating osteoblast differentiation, expanding the number of osteoblast and new osteoconductive scaffold with structural strength are needed.
CONCLUSION
Osteoporosis results in fragile bone through negative bone remodeling. As such, prior to performing spinal fusion on osteoporosis patients, surgeons should consider multidisciplinary strategies, including the use of the antiresorptive and anabolic agents, proper instrumentations and BMA. Perioperative strategies in osteoporotic patients may affect the radiological and clinical outcomes.
References
- 1.Abe Y, Takahata M, Ito M, Irie K, Abumi K, Minami A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1-34) in a rat spinal arthrodesis model. Bone. 2007;41:775–785. doi: 10.1016/j.bone.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Aebi M. The scoliosis. Eur Spine J. 2005;14:925–948. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 3.Aldini NN, Fini M, Giavaresi G, Giardino R, Greggi T, Parisini P. Pedicular fixation in the osteoporotic spine : a pilot in vivo study on long-term ovariectomized sheep. J Orthop Res. 2002;20:1217–1224. doi: 10.1016/S0736-0266(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 4.Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg Am. 2005;87:731–734. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 5.Aydogan M, Ozturk C, Karatoprak O, Tezer M, Aksu N, Hamzaoglu A. The pedicle screw fixation with vertebroplasty augmentation in the surgical treatment of the severe osteoporotic spines. J Spinal Disord Tech. 2009;22:444–447. doi: 10.1097/BSD.0b013e31818e0945. [DOI] [PubMed] [Google Scholar]
- 6.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2005;20:2275–2282. doi: 10.1359/jbmr.2005.20.12.2274. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 8.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. PaTH Study Investigators. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 9.Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976) 2002;15:S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 10.Boden SD. The biology of posterolateral lumbar spinal fusion. Orthop Clin North Am. 1998;29:603–619. doi: 10.1016/s0030-5898(05)70034-1. [DOI] [PubMed] [Google Scholar]
- 11.Boden SD, Schimandle JH, Hutton WC, Chen MI. The use of an osteoinductive growth factor for lumbar spinal fusion. Part I : Biology of spine fusion. Spine (Phila Pa 1976) 1995;20:2626–2632. doi: 10.1097/00007632-199512150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, et al. Odanacatib, a cathesin-K inhibitor for osteoporosis : a two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25:937–947. doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 13.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1991;18:2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 14.Bridwell KH, Sedgewick TA, O'Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–472. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 15.Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass : a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;14:1–34. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 16.Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91:1181–1189. doi: 10.2106/JBJS.G.01485. [DOI] [PubMed] [Google Scholar]
- 17.Burval DJ, McLain RF, Milks R, Inceoglu S. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae : biomechanical analysis of pedicle fixation strength. Spine (Phila Pa 1976) 2007;32:1077–1083. doi: 10.1097/01.brs.0000261566.38422.40. [DOI] [PubMed] [Google Scholar]
- 18.Carreon LY, Puno RM, Dimar JR, 2nd, Glassman SD, Johnson JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85:2089–2092. doi: 10.2106/00004623-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 20.Cavagna R, Tournier C, Aunoble S, Bouler JM, Antonietti P, Ronai M, et al. Lumbar decompression and fusion in elderly osteoporotic patients : a prospective study using less rigid titanium rod fixation. J Spinal Disord Tech. 2008;21:86–91. doi: 10.1097/BSD.0b013e3180590c23. [DOI] [PubMed] [Google Scholar]
- 21.Chin DK, Park JY, Yoon YS, Kuh SU, Jin BH, Kim KS, et al. Prevalence of osteoporosis in patients requiring spine surgery : incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007;18:1219–1224. doi: 10.1007/s00198-007-0370-8. [DOI] [PubMed] [Google Scholar]
- 22.Coe JD, Warden KE, Herzig MA, McAfee PC. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine (Phila Pa 1976) 1990;15:902–907. doi: 10.1097/00007632-199009000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Conaty JP, Mongan ES. Cervical fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1981;63:1218–1227. [PubMed] [Google Scholar]
- 24.Cook SD, Barbera J, Rubi M, Salkeld SL, Whitecloud TS., 3rd Lumbosacral fixation using expandable pedicle screws : an alternative in reoperation and osteoporosis. Spine J. 2001;1:109–114. doi: 10.1016/s1529-9430(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 25.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 26.Deal C. Future therapeutic targets in osteoporosis. Curr Opin Rheumatol. 2009;21:380–385. doi: 10.1097/BOR.0b013e32832cbc2a. [DOI] [PubMed] [Google Scholar]
- 27.Eriksen EF, Landgdahl B, Vesterby A, Rungby J, Kassem M. Hormone replacement therapy prevents osteoclastic hyperactivity : a histomorphometric study in early postmenopausal women. J Bone Miner Res. 1999;14:1217–1221. doi: 10.1359/jbmr.1999.14.7.1217. [DOI] [PubMed] [Google Scholar]
- 28.Fraizer DD, Lipson SJ, Fossel AH, Katz JN. Associations between spinal deformity and outcomes after decompression for spinal stenosis. Spine (Phila Pa 1976) 1997;22:2025–2029. doi: 10.1097/00007632-199709010-00017. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson SJ, Winkler F, Nolte LP. Anterior fixation in the osteoporotic spine : cut-out and pullout characteristics of implant. Eur Spine J. 2002;11:527–534. doi: 10.1007/s00586-002-0449-z. [DOI] [PubMed] [Google Scholar]
- 30.Farey ID, McAfee PC, Gurr KR, Randolph MA. Quantitative histologic study of the influence of spinal instrumentation on lumbar fusions : a canine model. J Orthop Res. 1989;7:709–722. doi: 10.1002/jor.1100070512. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong le T, Falgueyret JP, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–928. doi: 10.1016/j.bmcl.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Glassman SD, Polly DW, Bono CM, Burkus K, Dimar JR. Outcome of lumbar arthrodesis in patients sixty-five years of age or older. J Bone Joint Surg Am. 2009;91:783–790. doi: 10.2106/JBJS.H.00288. [DOI] [PubMed] [Google Scholar]
- 33.Greenfield RT, 3rd, Capen DA, Thomas JC, Jr, Nelson R, Nagelberg S, Rimoldi RL, et al. Pedicle screw fixation for arthrodesis of the lumbosacral spine in the elderly : an outcome study. Spine (Phila Pa 1976) 1998;23:1470–1475. doi: 10.1097/00007632-199807010-00008. [DOI] [PubMed] [Google Scholar]
- 34.Hart RA, Prendergast MA. Spine surgery for lumbar degenerative disease in elderly and osteoporotic patients. Instr Course Lect. 2007;56:257–272. [PubMed] [Google Scholar]
- 35.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis : a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz M, Stewart A, Greenspan SL. Sequential parathyroid hormone/alendronate therapy for osteoporosis-robbing Peter to pay Paul? J Clin Endocrinol Metab. 2000;85:2127–2128. doi: 10.1210/jcem.85.6.6658. [DOI] [PubMed] [Google Scholar]
- 37.Huang RC, Khan SN, Sandhu HS, Metzl JA, Cammisa FP, Jr, Zheng F, et al. Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976) 2005;30:2516–2522. doi: 10.1097/01.brs.0000186470.28070.7b. [DOI] [PubMed] [Google Scholar]
- 38.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 39.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with PTH. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine (Phila Pa 1976) 1986;11:107–110. doi: 10.1097/00007632-198603000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fracture. J Clin Invest. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim CH, Kim YI, Choi CS, Park JY, Lee MS, Lee SI, et al. Prevalence and risk factors of low quantitative ultrasound values of calcaneus in Korean elderly women. Ultrasound Med Biol. 2000;26:35–40. doi: 10.1016/s0301-5629(99)00126-x. [DOI] [PubMed] [Google Scholar]
- 43.Kim KH, Lee SH, Lee DY, Shim CS, Maeng DH. Anterior bone cement augmentation in anterior lumbar interbody fusion and percutaneous pedicle screw fixation in patients with osteoporosis. J Neurosurg Spine. 2010;12:525–532. doi: 10.3171/2009.11.SPINE09264. [DOI] [PubMed] [Google Scholar]
- 44.Kitchel SH. A preliminary comparative study of radiographic results using mineralized collagen and bone marrow aspirate versus autologous bone in the same patients undergoing posterior lumbar interbody fusion with instrumented posterolateral lumbar fusion. Spine J. 2006;6:405–411. doi: 10.1016/j.spinee.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Lehman RA, Jr, Kuklo TR, Freedman BA, Cowart JR, Mense MG, Riew KD. The effect of alendronate sodium on spinal fusion : a rabbit model. Spine J. 2004;4:36–43. doi: 10.1016/s1529-9430(03)00427-3. [DOI] [PubMed] [Google Scholar]
- 46.Lindsay R. Sex steroids in the pathogenesis and prevention of osteoporosis. In: Riggs BL, editor. Osteoporosis : Etiology, Diagnosis and Management. New York: Raven Press; 1988. pp. 333–358. [Google Scholar]
- 47.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 48.Looker AC, Johnston CC, Jr, Wahner HW, Dunn WL, Calvo MS, Harris TB, et al. Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res. 1995;10:796–802. doi: 10.1002/jbmr.5650100517. [DOI] [PubMed] [Google Scholar]
- 49.Lu J, Bhargav D, Wei AQ, Diwan A. Posterolateral intertransverse spinal fusion possible in osteoporotic rats with BMP-7 in a higher dose delivered on a composite carrier. Spine (Phila Pa 1976) 2008;33:242–249. doi: 10.1097/BRS.0b013e318162451b. [DOI] [PubMed] [Google Scholar]
- 50.Manolagas SC. Birth and death of bone cells : basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 51.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling-emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 52.Manolagas SC, Weinstein RS, Bellido T, Bodenner DL. Opposite effects of estrogen on the life span of osteoblasts / osteocytes vs. ostoclasts in vivo and in vitro : an explanation of the imbalance between formation and resorption in estrogen deficiency. J Bone Miner Res 14. 1999;14:S169. [Google Scholar]
- 53.Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy : a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Murphy MG, Cerchio K, Stoch SA, Gottesdiener K, Wu M, Recker R. Effect of L-000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2005;90:2022–2028. doi: 10.1210/jc.2004-2126. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura Y, Hayashi K, Abu-Ali S, Naito M, Fotovati A. Effect of preoperative combined treatment with alendronate and calcitriol on fixation of hydroxyapatite-coated implants in ovariectomized rat. J Bone Joint Surg Am. 2008;90:824–832. doi: 10.2106/JBJS.G.00635. [DOI] [PubMed] [Google Scholar]
- 56.Okuda S, Miyauchi A, Oda T, Haku T, Yamamoto T, Iwasaki M. Surgical complications of posterior lumbar interbody fusion with total laminectomy in 251 patients. J Neurosurg Spine. 2006;4:304–309. doi: 10.3171/spi.2006.4.4.304. [DOI] [PubMed] [Google Scholar]
- 57.Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2006;88:2714–2720. doi: 10.2106/JBJS.F.00186. [DOI] [PubMed] [Google Scholar]
- 58.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 59.Palmer M, Adami HO, Krusemo UB, Ljunghall S. Increased risk of malignant disease after surgery for primary hyperparathyroidism : a nation-wide cohort study. Am J Epidemiol. 1988;127:1031–1040. doi: 10.1093/oxfordjournals.aje.a114879. [DOI] [PubMed] [Google Scholar]
- 60.Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF. A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res. 1996;11:150–159. doi: 10.1002/jbmr.5650110203. [DOI] [PubMed] [Google Scholar]
- 61.Peter CP, Cook WO, Nunamaker DM, Provost MT, Seedor JG, Rodan GA. Effect of alendronate on fracture healing and bone remodeling in dogs. J Orthop Res. 1996;14:74–79. doi: 10.1002/jor.1100140113. [DOI] [PubMed] [Google Scholar]
- 62.Reinhold M, Schwieger K, Goldhahn J, Linke B, Knop C, Blauth M. Influence of screw positioning in a new anterior spine fixator on implant loosening in osteoporotic vertebrae. Spine (Phila Pa 1976) 2006;31:406–413. doi: 10.1097/01.brs.0000199894.63450.70. [DOI] [PubMed] [Google Scholar]
- 63.Rihn JA, Makda J, Hong J, Patel R, Hilibrand AS, Anderson DG, et al. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion : a clinical and radiographic analysis. Eur Spine J. 2009;18:1629–1636. doi: 10.1007/s00586-009-1046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roux B. New treatment targets in osteoporosis. Joint Bone Spine. 2010;77:222–228. doi: 10.1016/j.jbspin.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Sienkiewicz PJ, Flatley TJ. Postoperative spondylolisthesis. Clin Orthop Relat Res. 1987;221:172–180. [PubMed] [Google Scholar]
- 66.Sims NA, Gooi JH. Bone remodeling : multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 67.Szpalski M, Gunzburg R. Lumbar spinal stenosis in the elderly : an overview. Eur Spine J. 2003;12:S170–S175. doi: 10.1007/s00586-003-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahata M, Ito M, Abe Y, Abumi K, Minami A. The effect of anti-resorptive therapies on bone graft healing in an ovariectomized rat spinal arthrodesis model. Bone. 2008;43:1057–1066. doi: 10.1016/j.bone.2008.08.124. [DOI] [PubMed] [Google Scholar]
- 69.Toribatake Y, Hutton WC, Tomita K, Boden SD. Vascularization of the fusion mass in a posterolateral intertransverse process fusion. Spine (Phila Pa 1976) 1998;23:1149–1154. doi: 10.1097/00007632-199805150-00015. [DOI] [PubMed] [Google Scholar]
- 70.Wermers RA, Khosla S, Atkinson EJ, Grant CS, Hodgson SF, O'Fallon WM, et al. Survival after the diagnosis of hyperparathyroidism : a population-based study. Am J Med. 1998;104:115–122. doi: 10.1016/s0002-9343(97)00270-2. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto T, Ohkohchi T, Ohwada T, Kotoku H, Harada N. Clinical and radiological results of PLIF for degenerative spondylolisthesis. J Musculoskelet Res. 1998;2:181–195. [Google Scholar]
- 72.Yone K, Sakou T, Kawauchi Y, Yamaguchi M, Yanase M. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine (Phila Pa 1976) 1996;15:242–248. doi: 10.1097/00007632-199601150-00016. [DOI] [PubMed] [Google Scholar]
- 73.Zipfel GJ, Guiot BH, Fessler RG. Bone grafting. Neurosurg Focus. 2003;14:e8. doi: 10.3171/foc.2003.14.2.9. [DOI] [PubMed] [Google Scholar]