Abstract
Objective
Pituitary apoplexy is one of the most serious life-threatening complications of pituitary adenoma. The purpose of this study is to investigate the visual outcome after early transsphenoidal surgery for the patients with pituitary apoplexy.
Methods
We retrospectively reviewed the 31 patients with pituitary apoplexy who were admitted due to acute visual acuity or field impairment and treated by transsphenoidal surgery. Five patients were excluded because of the decreased conscious level. The visual acuity of each individual eye was evaluated by Snellen's chart. Visual fields were also checked using automated perimetry. To compare the visual outcome according to the surgical timing, we divided the patients into 2 groups. The first group, 21 of the patients have been undertaken transsphenoidal approach (TSA) within at least 48 hours after admission. The second group included 8 patients who have been undertaken TSA beyond 48 hours. All patients were monitored at least 12 months after surgery.
Results
Patients were 21 males and 8 females (M : F=2.6 : 1) with the mean age of 42.4 years. Among the enrolled 29 patients, 26 patients presented with decreased visual acuity and 23 patients revealed the defective visual field respectively. Postoperatively, improvement in the visual acuity was seen in 15 patients (83.3%) who underwent surgery within the first 48 hours of presentation, as compared to those in whom surgery was delayed beyond 48 hours (n=5; 62.5%) (p=0.014). Improvement in the visual field deficits was observed in 15 (88.2%) of patients who had been operated on within the first 48 hours of presentation, as compared to those in whom surgery was delayed beyond 48 hours (n=3; 50.0%) (p=0.037).
Conclusion
This study suggests that rapid transsphenoidal surgery is effective to recover the visual impairment in patients with pituitary apoplexy. If there are associated abnormalities of visual acuity or visual fields in patients with hemorrhagic pituitary apoplexy, early neurosurgical intervention within 48 hours should be also required to recover visual impairment.
Keywords: Outcome, Pituitary apoplexy, Transsphenoidal surgery, Vision
INTRODUCTION
Pituitary apoplexy (PA) is a rare but potentially life-threatening clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland6,7,25). It classically presents with a sudden onset of headache, vomiting, visual disturbances, and the decreased consciousness. The rapid worsening of vision is a critical condition, because the tolerance of the optic nerve to ischemia is unpredictable1). Clinically, pituitary apoplexy may be difficult to initially diagnose, and so it was usually diagnosed retrospectively by imaging studies, operative findings, or surgical specimens. Therefore, silent pituitary hemorrhage or subclinical pituitary apoplexy may change the actual incidence19,31).
It is generally accepted that the initial management of pituitary apoplexy consists of careful monitoring of fluid and electrolytes balance coupled with the immediate replacement of deficient hormones, in particular corticosteroids7,16). However, the role and timing of pituitary surgery in this condition remains controversial. Some authors reported that early decompression surgery has provided a good recovery of vision from the deteriorated patients with pituitary apoplexy1,15,23). So, early surgical decompression is the preferential management for patients with PA accompanying visual impairments.
In this study, we focused on patients with pituitary apoplexy who presented with impairment in the visual acuity or field and treated by transsphenoidal approach (TSA) within 48 hours after admission. We have tried to assess visual outcomes after early decompressive surgery in these patients.
MATERIALS AND METHODS
A total of 492 patients who were diagnosed pituitary adenoma and most of them were underwent surgical treatment via TSA for pituitary adenomas in our department from April 1995 to August 2009, and the data was retrospectively reviewed and analyzed. They participated in follow-up after discharge from the hospital. Among these patients, 34 (6.9%) patients who were diagnosed with pituitary apoplexy by specific clinical feature aggravation and/or brain magnetic resonance imaging (MRI). To compare the visual outcome according to the surgical timing, we divided the patients into 2 groups. The first group, 21 of the patients have been undertaken TSA within at least 48 hours after admission. The second group included 8 patients who have been undertaken TSA at 3 days later after admission. We excluded 5 patients with pituitary apoplexy who had varying degrees of visual impairment but who could not cooperate due to the altered mental status.
The medical records of these patients were retrospectively analyzed. Data regarding age; sex; clinical symptoms and signs; endocrinologic functioning; visual acuity and visual field status at admission, discharge, and follow-up were collected from the medical records. The intratumoral hemorrhage was investigated by brain MRI showing hypointense to isointense signal compared to surrounding brain tissue on both T1- and T2-weighted images in the acute stage of hematoma, on the other hand, hyperintense signal was identified in the chronic stage of hematoma. All patients received steroids therapy perioperatively. Visual acuity was recorded using the Snellen's chart and visual field was evaluated using Goldman perimetery. Visual functions including acuity and field were assessed before surgery, 1 month after surgery, 3 months after surgery and every 6 months if visual impairment persisted. Finally, all patients followed-up at least 12 months (median, 32 months) and the followed-up interval was 2 weeks to 6 months.
Visual outcomes including acuity and field were graded as 4 categories based on the change of records by the Snellen's chart and Goldman perimeter. Complete resolution included patients with normal vision or no definite visual impairment. Partial resolution included patients with the improved vision comparable to the preoperative status but didn't have normal vision. Unchanged resolution included patients with the unchanged vision compared to the preoperative status. Worsening group include patients with the deteriorated vision compared to the preoperative status. Improvement or deterioration of visual symptom after surgery was defined as when the changed symptom was detected by clinical evaluation at least more than 2 times 1 week interval. These findings were compared with those obtained at the follow-up.
Statistical comparison between two groups, in terms of timing of surgery and improvement in visual outcome, was done using the Mann-Whitney u-test. p-value less than 0.05 was considered significant.
RESULTS
Patient characteristics
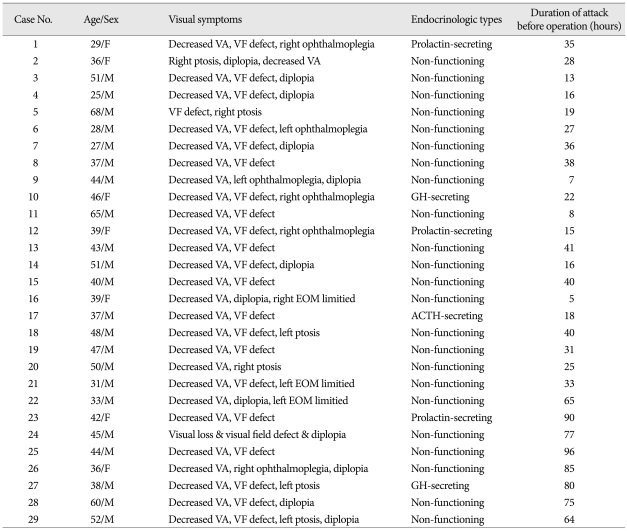
Of the 29 patients with pituitary apoplexy, 21 (72.5%) were men and 8 (27.5%) were women with a mean age of 42.4 years, ranged from 25 to 68 years. All patients had symptoms and signs of pituitary apoplexy. The most common clinical symptom was headache (100%) followed by the decreased visual acuity in 26 (89.6%) patients, and visual field defect in 23 (79.3%) patients. Dysfunction of the extra-ocular movement was also presented in some patients. However, there were no patients with blindness in our series.
Tweny-three (79.3%) patients with pituitary apoplexy had the non-functioning tumors endocrinologically. Only 6 patients had functioning pituitary tumors, which included the prolactin-secreting adenoma in 3 patients, growth hormone (GH)-secreting adenoma in 2 patients, and an adrenocorticotropic hormone (ACTH)-secreting adenoma prior to the apoplectic presentation. Mean duration before surgery was 24.4 hours in the first group (n=21), and 79.0 days in the second group (n=8), respectively (Table 1).
Table 1.
Clinical symptoms and signs in patients with pituitary apoplexy
ACTH : adrenocorticotropic hormone, GH : growth hormone, EOM : extraocular motion, VA : visual acuity, VF : visual field
Visual outcomes
After transsphenoidal decompression for the patients with pituitary apoplexy, 20 (76.9%) of 26 patients who had the decreased visual acuity and 18 (78.2%) of 23 patients who had the decreased visual field before surgery resulted in the improvement. Visual resolution was noted within 24 hours of surgery in most cases who had recovered in the acuity and visual field, and its recovery has been continued during the consistent periods.
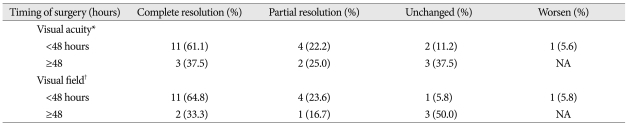
At last follow-up, results of visual outcome were related to the timing of TSA, as shown in Table 2. Improvement in the visual acuity was seen in 15 patients (83.3%) who underwent surgery within the first 48 hours of presentation, as compared to those in whom surgery was delayed beyond 48 hours (n=5; 62.5%)(p=0.014) (Table 2). Eleven (61.1%) patients had the complete resolution and 4 (22.2%) patients had partial resolution in visual acuity. But, 2 (11.2%) patients had no changes and one (5.5%) patient was deteriorated in the visual acuity after surgical intervention.
Table 2.
Relationship between timing of surgery after apoplectic attack and postoperative visual outcome in patients with pituitary apoplexy
*p=0.014, †p=0.037. NA : not available
Similarly, improvement in the visual field deficits was observed in 15 (88.2%) of patients who had been operated on within the first 48 hours of presentation, as compared to those in whom surgery was delayed beyond 48 hours (n=3; 50.0%) (p=0.037). However, there was no change of the visual field in one patient and one worsening in a patient.
Illustrative cases
Case 1
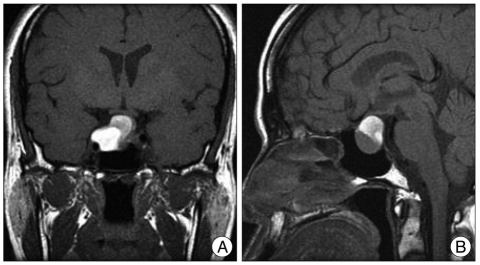
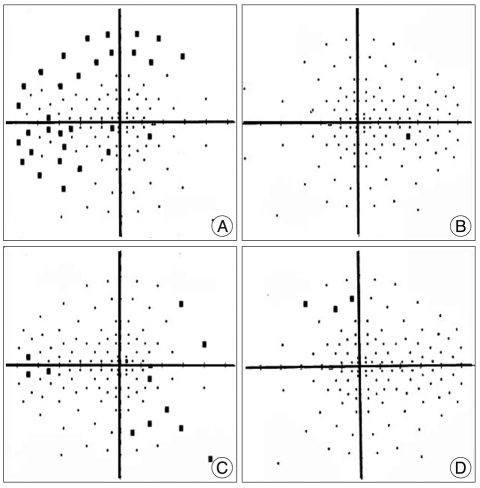
A 29-year-old female patient presented with rapid progression of the visual disturbance. On neurological examination, she was alert and insignificant except visual field defect by the confrontation test. Her visual acuity of the right eye assessed by Snellen's chart was 6/36 in the right eye. Her initial serum sodium level was 141 mmol/L. A basal level of serum prolactin was 26.2 ng/mL, indicating hyperprolactinemia. Levels of serum GH, ACTH, and cortisol were 0.1 ng/mL, 21.2 ps/mL, and 7.1 ug/dL revealing normal ranges, respectively. T1-weighted brain MRI demonstrated a large sellar mass that included portions of mixed-signaled intensity. The mass had a suprasellar extension compressing the optic chiasm (Fig. 1). Goldman perimetry demonstrated the right temporal hemianopsia (Fig. 2A, B). On the 2nd day after admission, TSA was performed to decompress the optic apparatus and to remove the tumor including hemorrhage. Pathological findings of specimens were confirmed as pituitary adenoma accompanying hemorrhage. Comparing preoperative and postoperative visual field tests, visual field defect was partially improved on the Goldmann perimetry obtained at the 17th postoperative day (Fig. 2C, D). Her visual acuity was also partially improved to 6/18 in the right eye.
Fig. 1.
Brain T1-weighted magnetic resonance (MR) images showing a sellar tumor with suprasellar extension in the coronal (A) and sagittal (B) planes. MR images demonstrate a mixed-signaled sellar mass compressing the optic chiasm.
Fig. 2.
Goldman perimetry showing the right temporal hemianopsia in the preoperative stage (A and B) and partial improvement at the 17th postoperative day (C and D).
Case 2
A 36-year-old female patient presented with headache and rapid deterioration of the visual impairment. On neurological examination, she revealed the bitemporal hemianopsia by the confrontation test and her visual acuity by Snellen's chart was 6/24 in both eyes. Laboratory tests did not show endocrine abnormalities. Preoperative brain MRI showed a large pituitary tumor which was hyperintense to the adjacent brain parenchyme on T1-weighted image, and extended into the suprasellar portion compressing the optic apparatus and the anterior part of the 3rd ventricle. On the next day after admission, emergent TSA was undertaken to decompress the optic apparatus and to resect a tumor with hemorrhage. Pathological examination confirmed the findings of pituitary adenoma including hemorrhagic components. Postoperative MRI showed a total removal of the pituitary tumor including hematoma, and replacement of the deformed optic apparatus. On the Goldman perimetry taken at the 14th day after surgery, bitemporal hemianopsia was almost recovered. Visual acuity was also improved to 6/6 in both eyes.
DISCUSSION
In this study, we have assessed visual outcome in a series of 21 patients with pituitary apoplexy who have been undertaken TSA within 48 hours after admission. Pituitary apoplexy usually occurs in the presence of a pituitary adenoma, though it has also occasionally been described in a non-adenomatous pituitary gland24,27). The incidence of surgically-treated pituitary apoplexy is variable from 1.9% to 12.8% depending on the definition3,10,11,18). In our series, the incidence is 6.8% (26/383 patients). These inconsistent incidences are caused by the lack of a uniform definition for pituitary apoplexy. However, a clinically silent pituitary apoplexy is much more common. Importantly, 14.4-25.7% of patients with pituitary adenomas have pituitary hemorrhage or necrosis according to sellar imaging or surgical findings and postoperative histological finding, without symptoms of classical pituitary apoplexy5,19,31). Thus, the majorities are clinically silent, and only small part of them present with classical symptoms suggesting pituitary apoplexy.
The pathogenetic mechanisms of pituitary apoplexy have not been fully elucidated. Cardoso et al.7) have suggested that inherent vasculopathy in pituitary adenomas provides them more susceptible to infarction and hemorrhage. This may explain why pituitary adenomas are more susceptible to vascular stress than other brain tumors7,22). The pituitary gland is a hypervascular structure and so, the pituitary adenomas are vulnerable to bleed and necrosis than other intracranial tumors. The etiologies of pituitary apoplexy have suggested that a rapidly growing adenoma that exceeds its blood supply may lead to ischemic necrosis of the gland, direct compression of hypophyseal vessels resulting in necrosis of tumor followed by hemorrhage, or reduced blood flow in the pituitary gland or tumor during major operations4,12).
The clinical presentations are variable, some patients may have only mild symptoms whereas others may present with severe clinical appearance6,20,22,31). Compression of the optic apparatus leads to a decrease in visual acuity and field defects; extension into the cavernous sinus and compression of the extra-ocular nerves develops to oculomotor paresis; destruction of the pituitary gland directs to hypopituitarism; and leakage of blood into the subarachnoid space presents signs of meningism17). Clinical appearance of pituitary apoplexy can mimic other intracranial pathologies including aneurysmal subarachnoid hemorrhage, bacterial meningitis, and less commonly, also midbrain infarction24). Thus, pituitary apoplexy may be clinically difficult to diagnose, and so, it was usually diagnosed retrospectively by neuroimaging, operative findings or surgical specimens. The clinical syndrome of pituitary apoplexy is characterized by the sudden onset of headache, vomiting, visual disturbance, ophthalmoplegia, and altered sensorium. Semple et al.26) reported 62 cases of pituitary apoplexy of which 84% presented with headache, 61% with impaired visual acuity, 43% with visual field changes, 43% with ophthalmoplegia and 13% with altered sensorium. In our series, all patients presented with headache, 85.7% with decreased visual acuity, 80.2% with impaired visual field and nausea or vomiting followed by 42.8% with ophthalmoplegia and 38.1% with diplopia. However, there were no patients with blindness following catastrophic pituitary hemorrhage.
The urgent treatment of pituitary apoplexy consists of supportive measures, fluid and electrolytes monitoring, and replacement of deficient hormones, in particular corticosteroids7). A prompt surgical decompression of pituitary gland is indicated in pituitary apoplexy with severe neuro-ophthalmic signs resulting in blindness7,27,29). However, the role of urgent surgery in patients without or with milder neuro-ophthalmic signs remains controversial. Some authors give emphasis to rapid surgical decompression of the pituitary fossa3,12,13,22). On the other hand, others prefer more conservative approach, particularly in the absence of progressive neuro-ophthalmic signs.
Pituitary apoplexy guidelines development group was also published 'UK Guidelines for the Management of Pituitary Apoplexy' in 201021). The guideline recommended that a decision regarding the timing of the decompression surgery should be based on the severity and the progression of the signs and symptoms, and surgical decompression should be performed preferably early timing, as soon as possible, within the first seven days of onset of symptom.
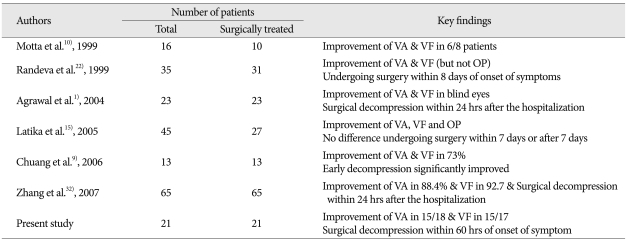
Effectiveness of visual recovery by the early surgery has been verified in the visually-impaired patients with pituitary apoplexy1,23). Patients who received early surgery had a significant improvement of visual acuity compared with those whose surgery was delayed more than 8 days22). It has also been advocated that urgent surgical treatment of pituitary apoplexy may get better outcomes in terms of preservation of pituitary function and recurrence of pituitary adenoma2,8,22). So, rapid surgical decompression has been proposed in many previous studies to be an effective method of treating pituitary apoplexy patients1,8,30). In our series, patients with visual impairment also recovered their visual acuity in 83.3%, and visual field in 88.4% after early transsphenoidal decompression. Finally, the authors emphasize the immediate management of supportive measures including the fluid and electrolytes monitoring, replacement of deficient hormones, and most importantly, early surgical treatment14,26). Bills et al.3) divided into those who underwent surgery within a week of the onset of apoplexy and those who underwent surgery after that time, finally found that significantly better outcome in visual acuity deficits in those patients decompressed within the first week and a similar trend with regard to visual field deficits, which did not reach significance.
Our data also support the importance of the rapid surgical decompression for the patients who have been suffered from the sudden onset of visual impairments in pituitary apoplexy. According to our experiences, the most important factor of a successful treatment for the patient with pituitary apoplexy is an early diagnosis and urgent surgical decompression. Therefore, we advocate early transsphenoidal surgery as the modality of choice in the view of its multifaceted benefits. Rapid surgical decompression relieves the visual deficit as well as provides tissue for the histopathological examination, and prevents a recurrent apoplectic episode and additional tumor growth, besides being the definitive treatment for most patients6).
CONCLUSION
Visual impairment is the serious complication followed by pituitary apoplexy. Surgical decompression provides recovery of visual functions and favorable outcomes. Our results support that early surgical decompression within 48 hours should be undertaken in the patient with pituitary apoplexy accompanying visual dysfunction.
Table 3.
Summary of retrospective studies about visual outcomes after surgery of pituitary apoplexy
VA : visual acuity, VF : visual field, OP : ophthalmoplegia
References
- 1.Agrawal D, Mahapatra AK. Visual outcome of blind eyes in pituitary apoplexy after transsphenoidal surgery : a series of 14 eyes. Surg Neurol. 2005;63:42–46. doi: 10.1016/j.surneu.2004.03.014. discussion 46. [DOI] [PubMed] [Google Scholar]
- 2.Arafah BM, Harrington JF, Madhoun ZT, Selman WR. Improvement of pituitary function after surgical decompression for pituitary tumor apoplexy. J Clin Endocrinol Metab. 1990;71:323–328. doi: 10.1210/jcem-71-2-323. [DOI] [PubMed] [Google Scholar]
- 3.Bills DC, Meyer FB, Laws ER, Jr, Davis DH, Ebersold MJ, Scheithauer BW, et al. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993;33:602–608. doi: 10.1227/00006123-199310000-00007. discussion 608-609. [DOI] [PubMed] [Google Scholar]
- 4.Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry. 2001;71:542–545. doi: 10.1136/jnnp.71.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonicki W, Kasperlik-Zaluska A, Koszewski W, Zgliczynski W, Wislawski J. Pituitary apoplexy : endocrine, surgical and oncological emergency. Incidence, clinical course and treatment with reference to 799 cases of pituitary adenomas. Acta Neurochir (Wien) 1993;120:118–122. doi: 10.1007/BF02112028. [DOI] [PubMed] [Google Scholar]
- 6.Brougham M, Heusner AP, Adams RD. Acute degenerative changes in adenomas of pituitary body; with special reference to pituitary apoplexy. J Neurosurg. 1950;7:421–439. doi: 10.3171/jns.1950.7.5.0421. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso ER, Peterson EW. Pituitary apoplexy : a review. Neurosurgery. 1984;14:363–373. doi: 10.1227/00006123-198403000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Chanson P, Lepeintre JF, Ducreux D. Management of pituitary apoplexy. Expert Opin Pharmacother. 2004;5:1287–1298. doi: 10.1517/14656566.5.6.1287. [DOI] [PubMed] [Google Scholar]
- 9.Chuang CC, Chang CN, Wei KC, Liao CC, Hsu PW, Huang YC, et al. Surgical treatment for severe visual compromised patients after pituitary apoplexy. J Neurooncol. 2006;80:39–47. doi: 10.1007/s11060-006-9148-7. [DOI] [PubMed] [Google Scholar]
- 10.da Motta LA, de Mello PA, de Lacerda CM, Neto AP, da Motta LD, Filho MF. Pituitary apoplexy. Clinical course, endocrine evaluations and treatment analysis. J Neurosurg Sci. 1999;43:25–36. [PubMed] [Google Scholar]
- 11.Deb S. Clinical significance of pituitary apoplexy. J Indian Med Assoc. 1998;96:302–303. 307. [PubMed] [Google Scholar]
- 12.Ebersold MJ, Laws ER, Jr, Scheithauer BW, Randall RV. Pituitary apoplexy treated by transsphenoidal surgery. A clinicopathological and immunocytochemical study. J Neurosurg. 1983;58:315–320. doi: 10.3171/jns.1983.58.3.0315. [DOI] [PubMed] [Google Scholar]
- 13.Epstein S, Pimstone BL, De Villiers JC, Jackson WP. Pituitary apoplexy in five patients with pituitary tumours. Br Med J. 1971;2:267–270. doi: 10.1136/bmj.2.5756.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraioli B, Esposito V, Palma L, Cantore G. Hemorrhagic pituitary adenomas : clinicopathological features and surgical treatment. Neurosurgery. 1990;27:741–747. discussion 747-748. [PubMed] [Google Scholar]
- 15.Kim JK, Park BJ, Cho KT, Lee SK, Cho MK, Kim YJ. Surgical outcomes of pituitary apoplexy. J Korean Neurosurg Soc. 2005;38:450–455. [Google Scholar]
- 16.Lee JH, Kim JH, Moon KS, Joo SP, Lee JK, Kim SH. Pituitary apoplexy : surgical experience with 16 patients. J Korean Neurosurg Soc. 2007;42:83–88. [Google Scholar]
- 17.Maccagnan P, Macedo CL, Kayath MJ, Nogueira RG, Abucham J. Conservative management of pituitary apoplexy : a prospective study. J Clin Endocrinol Metab. 1995;80:2190–2197. doi: 10.1210/jcem.80.7.7608278. [DOI] [PubMed] [Google Scholar]
- 18.McFadzean RM, Doyle D, Rampling R, Teasdale E, Teasdale G. Pituitary apoplexy and its effect on vision. Neurosurgery. 1991;29:669–675. doi: 10.1097/00006123-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Mohanty S, Tandon PN, Banerji AK, Prakash B. Haemorrhage into pituitary adenomas. J Neurol Neurosurg Psychiatry. 1977;40:987–991. doi: 10.1136/jnnp.40.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy : presentation, surgical management, and outcome in 21 patients. Neurosurgery. 1990;26:980–986. [PubMed] [Google Scholar]
- 21.Rajasekaran S, Vanderpump M, Baldeweg S, Drake W, Reddy N, Lanyon M, et al. UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (Oxf) 2011;74:9–20. doi: 10.1111/j.1365-2265.2010.03913.x. [DOI] [PubMed] [Google Scholar]
- 22.Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy : clinical features, management and outcome. Clin Endocrinol (Oxf) 1999;51:181–188. doi: 10.1046/j.1365-2265.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 23.Robinson JL. Sudden blindness with pituitary tumors. Report of three cases. J Neurosurg. 1972;36:83–85. doi: 10.3171/jns.1972.36.1.0083. [DOI] [PubMed] [Google Scholar]
- 24.Rolih CA, Ober KP. Pituitary apoplexy. Endocrinol Metab Clin North Am. 1993;22:291–302. [PubMed] [Google Scholar]
- 25.Rovit RL, Fein JM. Pituitary apoplexy : a review and reappraisal. J Neurosurg. 1972;37:280–288. doi: 10.3171/jns.1972.37.3.0280. [DOI] [PubMed] [Google Scholar]
- 26.Semple PL, De Villiers JC, Bowen RM, Lopes MB, Laws ER., Jr Pituitary apoplexy : do histological features influence the clinical presentation and outcome? J Neurosurg. 2006;104:931–937. doi: 10.3171/jns.2006.104.6.931. [DOI] [PubMed] [Google Scholar]
- 27.Shenkin HA. Relief of amblyopia in pituitary apoplexy by prompt surgical intervention. J Am Med Assoc. 1955;159:1622–1624. doi: 10.1001/jama.1955.02960340042010. [DOI] [PubMed] [Google Scholar]
- 28.Sibal L, Ball SG, Connolly V, James RA, Kane P, Kelly WF. Pituitary Apoplexy : a Review of Clinical Presentation, management and Outcome in 45 Cases. Pituitary. 2004;7:157–163. doi: 10.1007/s11102-005-1050-3. [DOI] [PubMed] [Google Scholar]
- 29.Symon L, Mohanty S. Haemorrhage in pituitary tumours. Acta Neurochir (Wien) 1982;65:41–49. doi: 10.1007/BF01405440. [DOI] [PubMed] [Google Scholar]
- 30.Verrees M, Arafah BM, Selman WR. Pituitary tumor apoplexy : characteristics, treatment, and outcomes. Neurosurg Focus. 2004;16:E6. doi: 10.3171/foc.2004.16.4.7. [DOI] [PubMed] [Google Scholar]
- 31.Wakai S, Fukushima T, Teramoto A, Sano K. Pituitary apoplexy : its incidence and clinical significance. J Neurosurg. 1981;55:187–193. doi: 10.3171/jns.1981.55.2.0187. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Fei Z, Zhang W, Cao WD, Liu WP, Zhang JN, et al. Emergency transsphenoidal surgery for hemorrhagic pituitary adenomas. Surg Oncol. 2007;16:115–120. doi: 10.1016/j.suronc.2007.06.001. [DOI] [PubMed] [Google Scholar]