Abstract
Background
Malignant transformation in a mature cystic ovarian teratoma is rare. Except in cases with high index of suspicion or overt metastasis, oophorectomy is the mainstay of treatment for ovarian teratoma.
Method
A 46-year-old perimenopausal woman who had salpingo-oophorectomy following a clinical diagnosis of benign ovarian tumour that was subsequently reported histologically as mature cystic ovarian teratoma with malignant transformation is presented.
Results
She was referred to our facility based on the histopathology report and haematuria two weeks after surgery. Cystoscopic biopsy done was reported as metastatic squamous cell carcinoma most probably from the ovary. Patient was thereafter referred for radiotherapy but was lost to follow-up after the first course.
Conclusion
Adequate evaluation prior to surgery in suspected ovarian teratoma with malignant transformation is critical to determine extent of surgery and adjuvant therapy. Prognosis in advanced disease condition such as the case presented is generally poor although radical pelvic surgery with resection of the adjacent involved bladder before radiotherapy would probably have improved her prognosis.
Keywords: ovarian teratoma, malignant, perimenopausal, radiotherapy
Introduction
Mature cystic ovarian teratoma is the commonest germ cell tumour found predominantly in young women with a median age of 30 years1. It accounts for 20% of all ovarian neoplasm2. It is usually a multiloculated cyst containing sebum, keratinous debri and hair. In addition, any of the derivatives from the three germ cell layers could be found in various combinations.
Clinically, it is usually asymptomatic but may be discovered accidentally during gynaecologic investigations for other conditions or due to mass effect2. When it is discovered during childhood, ovarian cystectomy is curative. However, if discovered in women above 50 years of age, the likelihood of malignant transformation of any of the components is very strong3. We present the report of a woman who had salpingo-oophorectomy for a clinically diagnosed benign cystic ovarian tumour that turned out to be mature cystic ovarian teratoma with malignant transformation and infiltration of the bladder wall by the malignant cells.
Case report
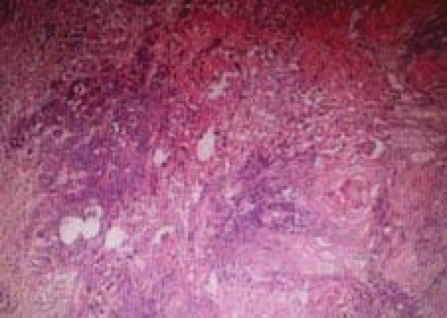
A 46-year old para 4+0, 4 alive, perimenopausal woman presented at a private hospital facility with 5 months history of abdominal pain, lower abdominal mass, and frequency of urination and dysuria. Abdominal examination revealed an abdominopelvic mass of about 16 week's cyesis. The abdominal ultrasound showed a left ovarian mass that was not well delineated from the bladder. She had exploratory laporotomy and important operative findings included a partially cystic left ovarian mass in the midline adherent to the perivesical region with moderate pelvic adhesion. The uterus and the right ovary were grossly normal. Patients had left salpingooophorectomy with adhesiolysis and she was subsequently discharged from the hospital a week after the surgery. The histopathology report showed a partially cystic ovarian mass that weighed 100gm and measured 8×7×5cm. The cut surface is unilocular containing hair and sebum with solid areas. Microscopy showed tissue composed of stratified squamous epithelium with adnexal structures, glandular elements, adipose tissue and areas of malignant squamous epithelial cells (Fig. 1).
Figure 1.
Section from the ovary showing malignant squamous epithelial cells forming keratin pearls admixed with lymphocytes. Haematoxylin & Eosin × 100
She however presented at our hospital facility with haematuria and dysuria three weeks after surgery at the referral hospital. A micturating cystogram was done and this was suggestive of an extensive mass in the bladder. This was followed by cystoscopy that revealed focal grapelike masses within the urinary bladder which were biopsied. Histopathology showed extensively necrotic tissue debri with focal aggregates of malignant squamous epithelial cells forming keratin pearls most probably arising from the ovarian tumour. The patient was thereafter referred for palliative radiotherapy but was lost to follow-up after the first course of radiotherapy.
Discussion
Malignant transformation of a mature cystic ovarian teratoma is exceedingly rare and occurs in only 2% of cases4. The rarity of this condition is generally true for all age groups but in perimenopausal and older women as in this case, the chance of any of the components becoming malignant is higher3. Though the reason for this is not clear, it is postulated that long-term presence of mature cystic ovarian teratoma and squamous metaplasia of the columnar epithelium may be followed by malignant change5,6. Most of the malignant change in mature cystic ovarian teratoma recorded in literatures is predominantly squamous cell carcinoma and this is said to account for 80% of the malignancies7. Other types of malignant transformation includes adenocarcinoma, carcinosarcoma and malignant struma ovarii among others8,9.
The presentation may be silent or overt depending on the stage of the disease but usually abdominal mass and pain are the presenting complaints as in the case presented. These features however do not distinguish benign from malignant tumour1. The large size of the ovarian mass in our patient tends to suggest long standing disease process which could probably explain the development of malignant transformation observed histologically. The histology showed that the derivatives of all the three germ layers were present which is typical of mature cystic ovarian teratoma. The malignant transformation to squamous cell carcinoma occurred in the skin (ectodermal) component and it was well differentiated. Mature cystic ovarian teratoma with malignant transformation is known to spread by direct local invasion and peritoneal seeding, and this may probably explain the spread to the bladder in this patient3.
The initial urinary symptoms in this patient were thought to be due to pressure effects of the ovarian tumour on the bladder and unfortunately the bladder mass was missed at ultrasound. However presentation of haematuria three weeks following surgery aroused our suspicion of possible local infiltration of the bladder in view of the histopathological results. The bladder biopsy taken at cystoscopy confirmed our suspicion and she was thereafter referred for palliative radiotherapy. It is most likely that our patient already had bladder wall involvement at the time of presentation at the referral hospital although this was missed both at ultrasonography and during the intraoperative procedure. Typically, in situation such as in our patient, radical surgery with resection of the tumour and the involved viscera is advocated10.
Conclusion
The prognosis in patients with malignant transformation of mature cystic ovarian teratoma is poor but worse in those with metastasis. Complete patient work-up to include urinalysis might have revealed microhaematuria which may be a pointer to the bladder lesion prior to surgery and proper staging of the disease.
References
- 1.Comerci JT, Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet gynecol. 1994;84:22–28. [PubMed] [Google Scholar]
- 2.Peterson WF, Prevost EC, Edmunds FT, Huntley JM, Jr, Morris FK. Benign cystic teratomas of the ovary. A clinicostatistical study of 1007 cases with review of the literature. Am J Obstet Gynecol. 1955;70:368–372. doi: 10.1016/s0002-9378(16)37681-5. [DOI] [PubMed] [Google Scholar]
- 3.Stamp GWH, McConnell EM. Malignancy arising in cystic ovarian teratomas. A report of 24 cases. Br J Obst Gyn. 1983;90:671–675. doi: 10.1111/j.1471-0528.1983.tb09289.x. [DOI] [PubMed] [Google Scholar]
- 4.Ayhan A, Bukulmez O, Genc C, Karamursel BS, Ayhan A. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol. 2000;88:153–157. doi: 10.1016/s0301-2115(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 5.Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian mature cystic teratoma. Int J Gynecol Cancer. 2006;16:140–144. doi: 10.1111/j.1525-1438.2006.00285.x. [DOI] [PubMed] [Google Scholar]
- 6.Maeyama M, Miyazaki K, Oka M, Higashi K, Nakayama M, Iwamasa T. Malignant degeneration of benign cystic teratoma of the bilateral ovaries: adenosquamous carcinoma in the right tumor and squamous carcinoma in the left tumor. Nippon Sanka Fujinka Gakkai Zasshi. 1983;35:331–334. [PubMed] [Google Scholar]
- 7.Filipakis GM, Lgoudianakis EE, Genetzakis M, et al. Squamous cell carcinoma arising in a mature cystic teratoma of the ovary with synchronous invasive lobular breast cancer: case report. Eur J Gynaecol Oncol. 2006;27:537–540. [PubMed] [Google Scholar]
- 8.Guney M, Oral B, Demir F, Ozsoy M, Kapucuoglu N. Mucinous adenocarcinoma arising from the gastrointestinal epithelium in benign cystic teratoma of the ovary-case report. Eur J Gynaecol Oncol. 2006;27:304–306. [PubMed] [Google Scholar]
- 9.Kano H, Inoue M, Nishino T, Yoshimoto Y, Arima R. Malignant struma ovarii with Graves' disease. Gynecol Oncol. 2000;79:508–510. doi: 10.1006/gyno.2000.5966. [DOI] [PubMed] [Google Scholar]
- 10.Pantoja E, Rodriguez-Ibanez I, Axtmayer RW, Noy MA, Pelegrina I. Complications of dermoid tumors of the ovary. Obstet Gynecol. 1975;45:89–94. [PubMed] [Google Scholar]