Abstract
Background
A lot of pathogens enter the body via the nasal route. The construction of non-toxic mutants of heat labile Escherichia coli enterotoxin (LT), which is a potent mucosal adjuvant, represents a major breakthrough for the development of mucosal vaccines.
Objective
This study was undertaken to critically evaluate the adjuvanticity of the mutant of LT (LTK63) on the cellular immune responses to intranasally co-administered recombinant measles virus nucleoprotein (rMVNP).
Methods
Groups of CBA mice were immunized intranasally with rMVNP with or without LT or LTK63 as adjuvants. Another group was immunized subcutaneously with rMVNP in Freund's adjuvant. rMVNP and measles virus (MV) were used in a proliferation assay to test the LTK63 potentiating ability to induce T cell responses. Subsequently MVNP synthetic peptides spanning the length of the N protein were used with a proliferation assay to identify the T cell epitopes.
Results
Splenocytes from mice immunized intranasally with rMVNP plus LT or LTK63, showed strong dose dependent proliferative responses to both the MVNP and MV. However, proliferative responses from the latter group were significantly lower than the former group (P < 0.05). Splenocytes tested recognized peptides 20, 21, 28, 31, 39, 40 and 50, suggesting these to be among important epitopes. Subcutaneous route was not effective in priming for T cell responses to rMVNP.
Conclusion
These data further demonstrate the great potential of LTK63 as a safe mucosal vaccine adjuvant.
Keywords: Adjuvant, Evaluation, LTK63, Cellular immunity, mice
Introduction
The intranasal route has been shown to be an effective route for immunization with various antigens. However, in many instances it may be necessary to increase the immunogenicity of vaccine antigens by use of adjuvants 1, 2.
Cholera toxin (CT) produced by Vibrio cholerae and the heat-labile (LT) enterotoxin of Escherichia coli have been shown to be potent mucosal immunogens and exert mucosal adjuvanticity to linked or co-administered antigens. These enterotoxins consist of six covalently linked polypeptide chains, comprising of a single A-subunit with NAD-glycohydrolase and ADPribosyltransferase activities responsible for activating adenylcyclase in target eucaryotic cells, and five B-subunits that bind the holotoxin to GM1-ganglioside receptors 3, 4.
The adjuvanticity of these proteins has been a subject of intense research but their toxicity precludes their exploitation in vaccines 5, 6, 7. It is the A-subunit that is toxic; and is also responsible for ADP-ribosylation of the GTP binding protein which leads to activation of the adenylcyclase system, persistent cAMP production, and ultimate loss of electrolytes and water from enterocytes with concomitant diarrhea 8, 9.
One approach being used to resolve the toxicity of CT is the use of the non-toxic B-subunit instead. Apart from being non-toxic, CT-B stimulates good specific immunity when given orally, which has raised hopes for its use as a vaccine adjuvant instead of the holotoxin. In an attempt to overcome the problem of toxicity of LTs and to obtain powerful and safe mucosal adjuvants, a series of mutants of LT have been constructed by site directed mutagenesis, while taking advantage of the known tridimensional structure of LT 8, 10, 11. This is by introducing single substitutions of the conserved amino acids in the active site of the LT. The results of these manipulations are that LT mutants (such as LTK7 and LTK63) devoid of enzymatic activity have been constructed. These mutants have been shown to be effective adjuvants for the induction of strong immune responses to a variety of antigens administered mucosally. These include both cellular and humoral immune responses 5. However, though the LTK63 mutant was shown to exert a strong adjuvant effect, the use of the wild type LT toxin was shown to be a more potent adjuvant for the in vivo induction of CTL responses to intranasally co-administered synthetic peptides 12. This has led to the suggestion that ADP-ribosyltransferase activity may be contributing to the adjuvant activity of the wild type LT toxin 5, 10.
In our previous work 13, we critically evaluated the adjuvanticity of the mutant of Escherichia coli heat-labile enterotoxin (LTK63), on the humoral immune responses to intranasally co-administered recombinant measles virus nucleoprotein. In this paper we evaluate the potential of LTK63 mutant as a mucosal adjuvant for the induction of peptide- and MV-specific cellular immune responses.
Methods
Recombinant measles virus nucleoprotein
The rMVNP (Edmonton strain) was kindly provided by Professor M. Steward (London School of Hygiene and Tropical Medicine).
Synthetic peptides
The peptide sequences were based on the predicted amino acid sequence of the Edmonton Zagreb strain of measles virus (MV) 14. Fifty overlapping peptides (15 mers with a 5 amino acid overlap) spanning amino acids 1–505 of the MVNP were synthesized at the London School of Hygiene and Tropical Medicine, by the RAMPs (Rapid multiple synthesis, Du Pont) solid-phase method using Fmoc-chemistry and the 4-(2′, 4′-Dimethoxyphenyl-Fmoc-amino-methyl)-phenoxy resin (Novabiochem). Fmoc-protected amino acids were converted to the active ester by treatment with hydroxybenzotriazol and diisopropylcarbodiimide in dimethylformamide (DMF). The subsequent coupling reactions were performed in DMF and the Fmoc groups removed with 20% piperidine followed by a series of washes in DMF. After synthesis, side chain protecting groups were removed and the peptide cleaved in trifluoracetic acid in the presence of scavengers. Following cleavage, the peptides were extracted into diethylether and purified by preparative HPLC.
Mice
Female CBA (H-2k) mice (6–8 weeks old at the start of the experiment) were purchased from the National Institute of Medical Research (Mill Hill, London, UK). They were kept at the Biological Services Unit at The Royal Veterinary College, London, UK. The different groups of mice were kept in separate cages and provided with water and food by attendants. Access was prohibited to the public. Only scientists going to take samples were allowed in after putting on protective clothing, disposable shoes, masks and gloves. Ethical approval to conduct the study was obtained from the Royal Veterinary College Ethics Committee.
Immunization
The immunogenicity of rMVNP was tested after immunization of 4 groups of CBA mice (6 mice per group) with rMVNP. Three groups were immunized intranasally with 20 µg/dose of rMVNP in 30 µl of PBS on day 0 with or without 5 µg/dose of LT (Sigma, Gillingham, UK) or LT mutant, LTK63, which bears a single substitution Ser-63 '! Lys (kindly provided by CHIRON Sienna, Italy) (group A, B and C, respectively). These mice were boosted intranasally on day 7 and 14 with a 20 µg/dose of rMVNP with or without LT or LTK63 (5 µg/dose). The fourth group of mice (group D) was immunized subcutaneously (one of the usual route of vaccine administration) on day 0 with 30 µg/dose of rMVNP in Freund's adjuvant (1:1 emulsion). Each mouse received a 200 µl volume of the (1:1) emulsion.
Lymphocyte proliferation assays
On day 77, mice were sacrificed, spleens from each group removed aseptically, pooled and the mononuclear cells collected by centrifugation following the standard proliferative assay 15. Red blood cells were lysed using ACK buffer [8.29g NH4Cl (0.15M) + 1g KHCO3 (1M) + 37.2 mg Na2EDTA (0.1 mM) in 1 liter of distilled water] (5 ml/spleen) for 5 minutes. Lymphocytes were washed twice in RPMI-1640 medium and then resuspended in complete medium (RPMI-1640 supplemented with 1% autologous serum, 2mM L-glutamine, 10mM HEPES buffer and 100 µg/ml antibiotics). Viable mononuclear cells were counted using eosin stain (0.2% w/v) and the number of cells was adjusted to 2×106/ml. Cell suspension (200 µl) was placed in each well of a 96-well round bottom microtitre plate and incubated in the presence of 10 µl of MVNP overlapping 15 mer peptides (10 µg/ml) or various concentrations of MVNP (0.0005 to 5 µg/well) or of heat inactivated measles virus (0.002–2×102 p.f.u/well) in a humidified 5% CO2 atmosphere at 37°C for 4 or 5 days. The various concentrations of the MVNP (0.0005 to 5 µg/well) were employed to test whether lymphocyte stimulation depended on the quantity of the MVNP used. Eighteen hours before the end of the culture, cells were pulse-labeled with 1 µci of tritiated thymidine. Cells were harvested and [3H]thymidine incorporation was assessed by liquid scintillation spectrometry and the results expressed as stimulation indices (SI), which represent the ratio of the mean counts per minute (c.p.m) from triplicate (overlapping peptides) or hexaplicate (MVNP or MV) cultures containing antigen to the mean counts per minute in hexaplicate wells containing cells and medium only. Values greater than 2.0 were considered positive.
Statistics
The data were compiled in Microsoft Excel and evaluated using GraphPad 5.0 statistical package (GraphPad Software, Inc., San Diego, CA). The effect of treatment and concentration on the results was analyzed using a one way ANOVA. Comparison between the treatments and mong the different concentrations was done using Newman-Keuls multiple comparison test set a significance level of P < 0.05.
Results
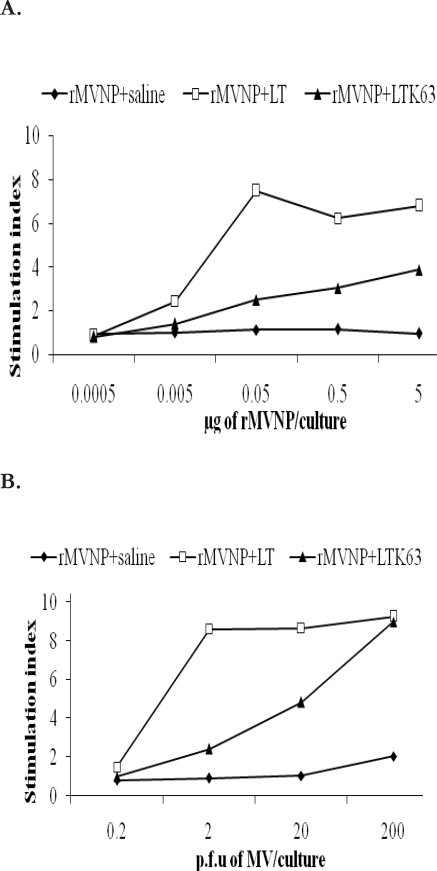
Proliferative responses of splenocytes from mice immunized intranasally with rMVNP with or without adjuvants (4 days incubation of cells with antigens)
The adjuvanticity of the LT and LTK63 was tested by measuring in vitro proliferative responses of rMVNP primed splenocytes following culture with MV or MVNP. When splenocytes from mice immunized intranasally with rMVNP alone were tested, no responses were detected. However, splenocytes from mice immunized with rMVNP plus LT or LTK63, showed strong proliferative responses to the MVNP and these responses were dose dependent (Fig 1A). In groups of mice immunized with rMVNP+LT maximum responses were observed at 0.5 µg/ml concentration of MVNP whereas in groups of mice immunized with rMVNP+LTK63, higher responses were seen at concentration of 5 µg/ml of MVNP, the highest concentration tested in this study. Similarly splenocytes from mice immunized with rMVNP plus LT or LTK63, showed strong proliferative responses to the MV and these responses were also dose dependent (Fig 1B). For MV stimulation, both groups of mice immunized with rMVNP+LT and rMVNP+LTK63, showed maximum responses at a concentration of 2 × 102 plaque forming units (p.f.u) / well while those immunized with rMVNP+LTK63 showed higher responses at 2 × 102 p.f.u / well Overall, proliferative responses of splenocytes from the rMVNP+LTK63 primed group of mice were significantly lower than those from the rMVNP+LT group (p < 0.05).
Figure 1.
Proliferative responses of splenocytes from CBA mice immunized intranasally with rMVNP in saline, with LT or LTK63.
The splenocytes were stimulated in vitro with various dilutions of (A) MVNP (0.0005 to 5 µg/culture) or (B) heat inactivated MV (0.002×102 to 2×102 p.f.u/culture) or medium from mock infected cells for 4 days. Data are presented as stimulation indices (ratio of mean c.p.m of triplicate cultures with antigen to the mean c.p.m of six replicates of control cultures without antigens).
Proliferative responses of splenocytes from mice immunized intranasally with rMVNP with or without adjuvants (5 days incubation of cells with antigens)
In order to optimize the duration of incubation of the spleen cells with antigens, a number of plates were incubated for 5 days as opposed to 4 days above. Proliferative responses observed (Fig. 2) were basically similar to those obtained after 4 days incubation. When splenocytes from mice immunized intranasally with rMVNP alone were tested, no responses were detected. Proliferative responses of splenocytes from groups of mice immunized with rMVNP+LTK63 were not significantly different from those of mice immunized with rMVNP+LT (P > 0.05). .
Figure 2.
Proliferative responses of splenocytes from CBA mice immunized intranasally with rMVNP in saline, with LT or LTK63.
The splenocytes were stimulated in vitro with various dilutions of (A) rMVNP (0.0005 to 5 µg/culture) or (B) heat inactivated measles virus (0.002×102 to 2×102 p.f.u/culture) or medium from mock infected cells for 5 days. Data are presented as stimulation indices (ratio of mean c.p.m of triplicate cultures with antigen to the mean c.p.m of six replicates of control cultures without antigens).
Fine specificity of proliferative responses of splenocytes from mice immunized intranasally with rMVNP+LT
In order to identify the regions from the MVNP which were responsible for proliferative responses, overlapping 15 mer peptides were used for in vitro stimulation of splenocytes primed intranasally with rMVNP+LT. As shown in Fig. 3, proliferative responses were observed upon stimulation with peptides 20, 21, 28, 31, 39, 40 and 50. Of these, relatively stronger proliferative responses were seen with peptides 20, 21 and 40.
Figure 3.
Fine specificity of the proliferative responses of rMVNP primed spleen cells from CBA mice to synthetic MVNP peptides.
Spleen cells from CBA mice immunized with rMVNP+LT were tested for in vitro proliferation to 50 overlapping 15 mer peptides (10 µg/culture) or medium plus cells alone. Data are presented as stimulation indices (ratio of mean c.p.m of triplicate cultures with antigen to the mean c.p.m of six replicates of control cultures without antigens).
Proliferative responses of splenocytes from mice immunized subcutaneously with rMVNP in FA
To test the in vitro proliferative responses of splenocytes from mice immunized subcutaneously with rMVNP in FA, the cells were cultured with the same concentrations of MVNP and MV as it was with cells obtained from mice immunized intranasally (4 days of incubation of cells). No proliferation was observed when cells were stimulated with rMVNP (Fig. 4A). A weak proliferative response was observed when cells were incubated with concentration of MV of 2 × 102 p.f.u/well for 4 days (Fig. 4B).
Figure 4.
Proliferative responses of splenocytes from CBA mice immunized subcutaneously with rMVNP in FA.
The splenocytes were incubated in vitro with various dilutions of (A) MVNP (0.0005 to 5 µg/culture) or (B) heat inactivated measles virus (0.002×102 to 2×102 p.f.u/culture) for 4 or 5 days. Data are presented as stimulation indices (ratio of mean c.p.m of triplicate cultures with antigen to the mean c.p.m of six replicates of control cultures without antigens).
Fine specificity of the proliferative responses of splenocytes from mice immunized subcutaneously with rMVNP in FA
In order to identify the regions from the MVNP which were responsible for proliferative responses, overlapping 15 mer peptides were used for in vitro stimulation of splenocytes primed subcutaneously with rMVNP+FA. No positive proliferative responses were observed when the splenocytes were incubated with any of the 50 overlapping 15 mer peptides (data not shown).
Discussion
The upper respiratory tract is one of the most important routes of the entry of pathogens into the body. The development of vaccines administered via the nasal route offers several advantages to reckon 12, 13, 16, 17. Notwithstanding, in many situations it may be necessary to increase the immunogenicity of vaccine antigens by use of an appropriate adjuvant. The construction of the non-toxic mutants of CT and LT, potent mucosal immunogens and adjuvants 8, 10, 11, 18, represents an enormous contribution to the development of mucosal vaccines.
Both antibody and cellular responses play a critical role against viral infections. In the case of MV, the role of antibodies in protection of humans from re-infection is well known 19. In our previous work we evaluated the adjuvanticity of non-toxic mutant of LT, LTK63, when co-administered intranasally with the rMVNP, on the humoral responses 13. We found LTK63 greatly enhanced the systemic antibody responses to the intranasally co-administered rMVNP. Moreover these responses were similar to responses observed when the rMVNP was co-administered intransally with the wild type LT toxin.
In this report we have shown the potential of the non-toxic mutant of LT (LT63) to potentiate systemic T cell responses to rMVNP. These findings extend previous studies that have highlighted the potential of the LT63 mutant as an adjuvant for induction of T-cell responses 12, 19. Although the LTK63 mutant was shown to exert a strong adjuvant effect, the use of LT was shown to be a more potent mucosal adjuvant for the in vitro induction of T-cell proliferative responses. The finding that proliferative responses of splenocytes from mice immunized with rMVNP+LTK63 were significantly lower than those from mice immunized with rMVNP+LT, after 4 days of incubation suggest that LT is a better potentiator of memory than LTK63. These findings agree with and extend previous observations that LT is a superior mucosal adjuvant to the LTK63 mutant for the induction of cellular responses 20, 21. These data also suggest that ADP-ribosylation may be contributing to the adjuvant activity of LT 22. Nonetheless, our findings of no significant different between proliferative responses of splenocytes from groups of mice immunized with rMVNP+LTK63 from those of mice immunized with rMVNP+LT (P > 0.05) following 5 days of incubation with antigens suggest that LTK63 is as potent as LT for mucosal adjuvanticity. Our study was limited in that it did not explore beyond 5 days incubation thus it could not precisely determine the adjuvant potential of LTK63 relative to LT. Contrary to intranasal priming, splenocytes from mice immunized subcutaneously with rMVNP in FA showed very weak proliferation when stimulated with 2×102 p.f.u of MV, suggesting that MVNP gives poor memory subcutaneously, however, these mice were not boosted.
Measles nucleoprotein has an established role in both humoral and cellular immune responses to measles virus. Splenocytes tested in this study gave proliferative responses to 15 mer overlapping peptides, underscoring the role of the nucleoprotein as a key immunogen. T-cell determinants of the MVNP were distributed along most of the last two thirds of the molecule. Splenocyte proliferation identified seven peptides (peptides 28, 31, 20, 21, 39, 40 and 50), but relatively stronger responses were obtained with peptides 20, 21 and 40. Interestingly, two of these peptides; 20 and 40 were also recently greatly recognized by vaccinated and naturally infected humans 23, indicating that these determinants might be important in maintaining measles cellular immunity and could hold a potential for peptide vaccines. Subcutaneous priming with rMVNP in FA resulted in the induction of no T-cell proliferative responses against overlapping peptides further suggesting that poor memory is obtained when this antigen is given via this route. Additionally, contrary to B-cell epitopes which may be mostly conformational, results here highlight the presence of many linear Th epitopes of the MVNP.
Conclusion
These observations highlight the potential use of LTK63 mutant as a mucosal adjuvant for induction of specific cellular responses to intranasally co-administered rMVNP. This non-toxic mutant has shown a great potential as a safe mucosal adjuvant that could in future be used in human mucosal vaccines.
Acknowledgements
We are indebted to Prof. M. Steward (London School of Hygiene and Tropical Medicine) for kindly providing the measles virus nucleoprotein and CHIRON (Sienna, Italy) for donating LTK63.
References
- 1.Baudner BC, Giuliani MM, Verhoef JC, Rappuoli R, Junginger HE, Giudice GD. The concomitant use of the LTK63 mucosal adjuvant and of chitosan-based delivery system enhances the immunogenicity and efficacy of intranasally administered vaccines. Vaccine. 2003;21(25–26):3837–3844. doi: 10.1016/s0264-410x(03)00305-0. [DOI] [PubMed] [Google Scholar]
- 2.Kende M, Tan X, Wlazlowski C, Williams R, Lindsey C, Del Giudice G. Enhancement of intranasal vaccination with recombinant chain A ricin vaccine (rRV) in mice by the mucosal adjuvants LTK63 and LTR72. Vaccine. 2007;25(16):3219–3227. doi: 10.1016/j.vaccine.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 4.Moss J, Richardson SH. Activation of adenylate cyclase by heat-labile Escherichia coli enterotoxin. Evidence for ADP-ribosyltransferase activity similar to that of choleragen. J Clin Invest. 1978;62(2):281–285. doi: 10.1172/JCI109127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci U S A. 1995;92(5):1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peppoloni S, Ruggiero P, Contorni M, Morandi M, Pizza M, Rappuoli R, Podda A, Del Giudice G. Mutants of the Escherichia coli heat-labile enterotoxin as safe and strong adjuvants for intranasal delivery of vaccines. Expert Rev Vaccines. 2003;2(2):285–293. doi: 10.1586/14760584.2.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Fingerut E, Gutter B, Meir R, Eliahoo D, Pitcovski J. Vaccine and adjuvant activity of recombinant subunit B of E. coli enterotoxin produced in yeast. Vaccine. 2005;23(38):4685–4696. doi: 10.1016/j.vaccine.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappouli R, Pizza M. Structure and evolutionary aspects of ADP-ribosylating toxins. In: Alouf J, Freer J, editors. Source book of Bacterial Protein Toxins. London: Academic Press Ltd; 1991. [Google Scholar]
- 10.Pizza M, Fontana MR, Giuliani MM, Domenighini M, Magagnoli C, Giannelli V, Nucci D, Hol W, Manetti R, Rappuoli R. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J Exp Med. 1994;180(6):2147–2153. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tritto E, Muzzi A, Pesce I, Monaci E, Nuti S, Galli G, Wack A, Rappuoli R, Hussell T, De Gregorio E. The acquired immune response to the mucosal adjuvant LTK63 imprints the mouse lung with a protective signature. J Immunol. 2007;179(8):5346–5357. doi: 10.4049/jimmunol.179.8.5346. [DOI] [PubMed] [Google Scholar]
- 12.Partidos CD, Pizza M, Rappuoli R, Steward MW. The adjuvant effect of a non-toxic mutant of heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology. 1996;89(4):483–487. doi: 10.1046/j.1365-2567.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erume J, Partidos H. Evaluation of the adjuvant effect of Escherichia coli heat-labile enterotoxin mutant (LTK63) on the systemic immune responses to intranasally co-administered measles virus nucleoprotein. Part I: antibody responses. Afr Health Sci. 2001;1(1):3–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Rozenblatt S, Eizenberg O, Ben-Levy R, Lavie V, Bellini WJ. Sequence homology within the morbilliviruses. J Virol. 1985;53(2):684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im0312s60. Chapter 3: Unit 3.12. [DOI] [PubMed] [Google Scholar]
- 16.O'Hagan DT, Illum L. Absorption of peptides and proteins from the respiratory tract and the potential for development of locally administered vaccine. Crit Rev Ther Drug Carrier Syst. 1990;7(1):35–97. [PubMed] [Google Scholar]
- 17.Baudner BC, Balland O, Giuliani MM, Von Hoegen P, Rappuoli R, Betbeder D, Del Giudice G. Enhancement of protective efficacy following intranasal immunization with vaccine plus a nontoxic LTK63 mutant delivered with nanoparticles. Infect Immun. 2002;70(9):4785–4790. doi: 10.1128/IAI.70.9.4785-4790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dertzbaugh MT, Elson CO. Cholera toxin as a mucosal adjuvant. In: Springs D R, Koh W C, editors. Topics in vaccine adjuvant Research. Michigan: CRC Press; 1990. [Google Scholar]
- 19.Olszewska W, Erume J, Ripley J, Steward MW, Partidos CD. Immune responses and protection induced by mucosal and systemic immunisation with recombinant measles nucleoprotein in a mouse model of measles virus-induced encephalitis. Arch Virol. 2001;146(2):293–302. doi: 10.1007/s007050170176. [DOI] [PubMed] [Google Scholar]
- 20.Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187(7):1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partidos CD, Salani BF, Pizza M, Rappuoli R. Heat-labile enterotoxin of Escherichia coli and its site-directed mutant LTK63 enhance the proliferative and cytotoxic T-cell responses to intranasally co-immunized synthetic peptides. Immunol Lett. 1999;67(3):209–216. doi: 10.1016/s0165-2478(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 22.Giannelli V, Fontana MR, Giuliani MM, Guangcai D, Rappuoli R, Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997;65(1):331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickman CJ, Khan AS, Rota PA, Bellini WJ. Use of synthetic peptides to identify measles nucleoprotein T-cell epitopes in vaccinated and naturally infected humans. Virology. 1997;235(2):386–397. doi: 10.1006/viro.1997.8678. [DOI] [PubMed] [Google Scholar]