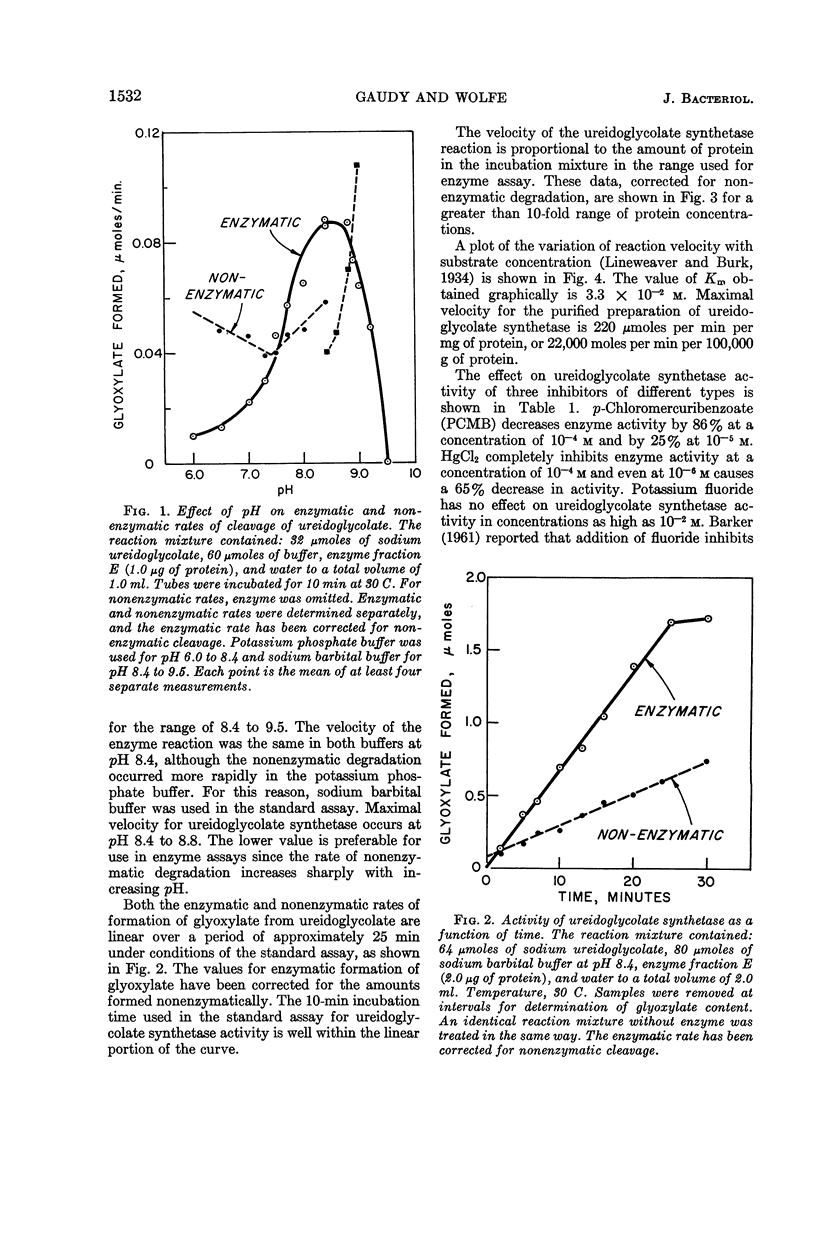
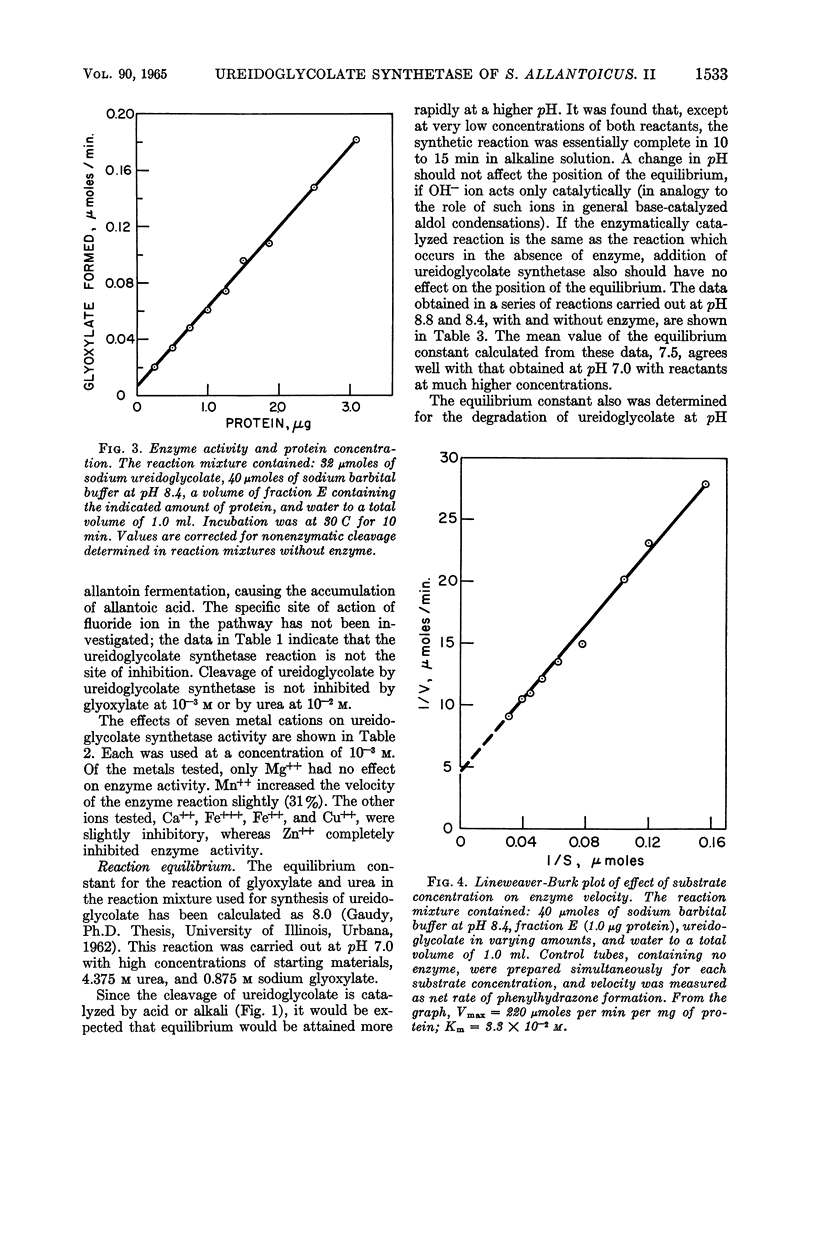
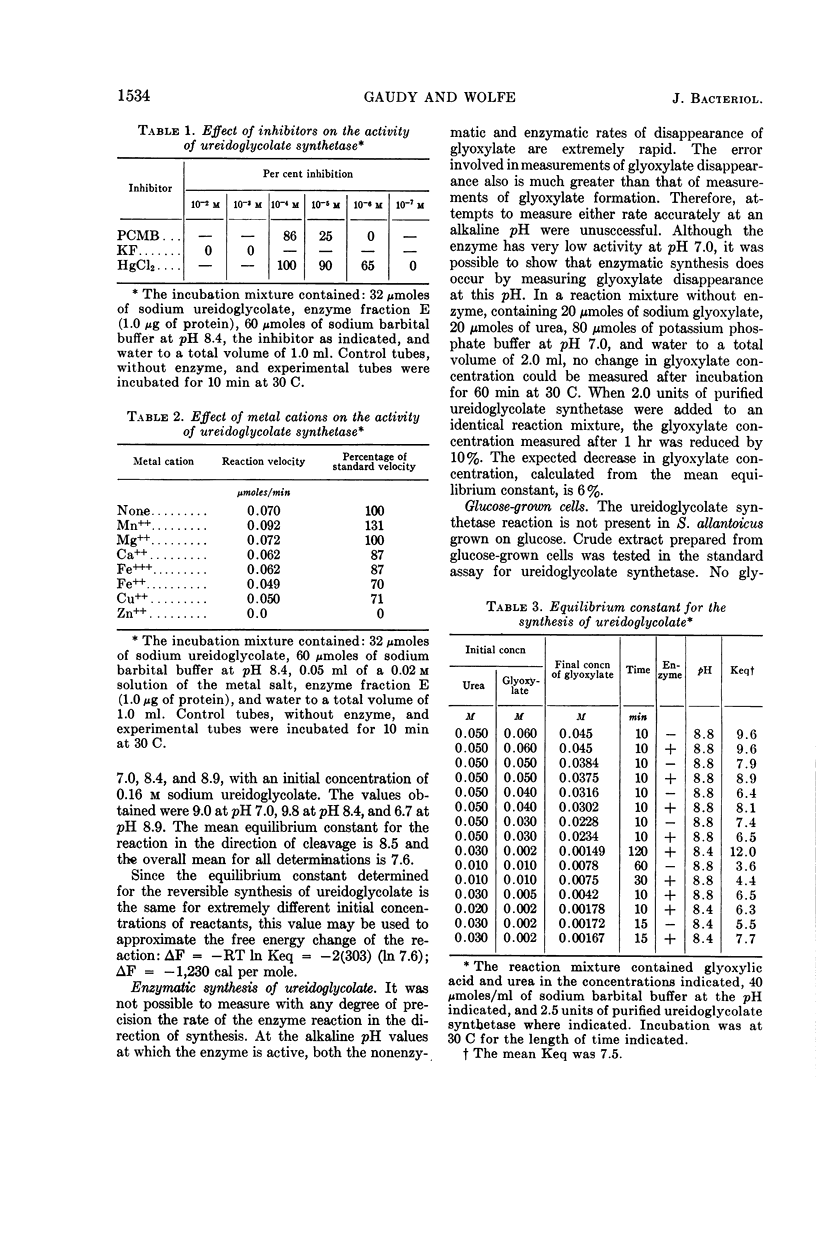
Abstract
Gaudy, Elizabeth T. (University of Illinois, Urbana), and R. S. Wolfe. Ureidoglycolate synthetase of Streptococcus allantoicus. II. Properties of the enzyme and reaction equilibrium. J. Bacteriol. 90:1531–1536. 1965.—The properties of ureidoglycolate synthetase from Streptococcus allantoicus grown on allantoin were studied, by use of the purified enzyme preparation and crystalline sodium ureidoglycolate. Ureidoglycolate synthetase activity was maximal over the pH range of 8.4 to 8.8. No cofactors were required for the reaction. Enzyme activity was inhibited by p-chloromercuribenzoate at relatively high concentrations, by Hg++ or Zn++ ions, and to a lesser extent by several other metal cations. The maximal velocity for the purified ureidoglycolate synthetase, determined graphically from a Lineweaver-Burk plot, was 220 μmoles of glyoxylate formed per min per mg of protein. The substrate concentration required for half-maximal velocity was 3.3 × 10−2m. The equilibrium constant for the synthesis of ureidoglycolate was determined in a series of reaction mixtures covering a wide range of initial concentrations of reactants. The position of the equilibrium was not affected by a change in pH or by the presence of enzyme. The equilibrium constant for the reaction in the direction of synthesis was 7.6, corresponding to a negative free energy change of 1,230 cal per mole.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOJANOWSKI R., GAUDY E., VALENTINE R. C., WOLFE R. S. OXAMIC TRANSCARBAMYLASE OF STREPTOCOCCUS ALLANTOICUS. J Bacteriol. 1964 Jan;87:75–80. doi: 10.1128/jb.87.1.75-80.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMNAS A. Amide metabolism in yeasts. II. The uptake of amide and amide like compounds by yeast. J Biochem. 1962 Sep;52:149–154. [PubMed] [Google Scholar]
- Gaudy E. T., Bojanowski R., Valentine R. C., Wolfe R. S. Ureidoglycolate synthetase of Streptococcus allantoicus. I. Measurement of glyoxylate and enzyme purification. J Bacteriol. 1965 Dec;90(6):1525–1530. doi: 10.1128/jb.90.6.1525-1530.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES M. E., ANDERSON A. D., ANDERSON C., HODES S. Citrulline synthesis in rat tissues. Arch Biochem Biophys. 1961 Dec;95:499–507. doi: 10.1016/0003-9861(61)90182-5. [DOI] [PubMed] [Google Scholar]
- SISTER M R SOHLER, SISTER M A SEIBERT, KREKE C. W., COOK E. S. Depression of catalase activity by organic mercurial compounds. J Biol Chem. 1952 Sep;198(1):281–291. [PubMed] [Google Scholar]
- SMITH R. A., GUNSALUS I. C. Isocitritase; enzyme properties and reaction equilibrium. J Biol Chem. 1957 Nov;229(1):305–319. [PubMed] [Google Scholar]
- VALENTINE R. C., BOJANOWSKI R., GAUDY E., WOLFE R. S. Mechanism of the allantoin fermentation. J Biol Chem. 1962 Jul;237:2271–2277. [PubMed] [Google Scholar]



