Abstract
Using twin pairs from the National Survey of Midlife Development in the United States, we estimate that 35 percent of the variance in regular smoking is due to additive genetic influences. When we disaggregate the sample by birth cohort we witness strong genetic influences on smoking for those born in the 1920s, 1930s, and 1950s, but negligible influences for those born in the 1940s and 1960s. We show that the timing of the first Surgeon General’s Report coincides with an increase in the genetic influences on regular smoking, but subsequent legislation prohibiting smoking in public places has significantly reduced these influences. These results are in line with existing gene-environment interaction theory, and we argue that variation in genetic influences across cohorts makes it difficult and potentially misleading to estimate genetic effects on health behaviors from data obtained from a single point in time.
Keywords: genetics, smoking, gene-environment interplay, behavior genetics, heritability
Besides its practical importance as the major source of premature mortality in the United States and many other nations (Jha et al. 2006), smoking has special theoretical importance for the sociology of health: it reflects both social and physiological influences. On one hand, social position (Link 2008), group-based lifestyles (Cockerham 2000), and public policies (Warner, Mendez, and Alshanqeety 2008) greatly influence social patterns and population trends in smoking. Smoking has risen and fallen with social fashion, advertising strategies and restrictions, publicity about its dangers, high taxes, and clean-air laws, and a huge literature has identified the social groups contributing most and least to the trends (see recent volumes of the Surgeon General for comprehensive reviews of social patterns of smoking, U.S. Department of Health and Human Services [USDHHS] 1994, 1998, 2000, 2001). On the other hand, smoking involves physical addiction to nicotine, a stimulating substance that links the behavior to biological or genetic traits. The addictiveness has been well substantiated (USDHHS 1988), and a large literature on biological mechanisms (Bock and Goode 2006; Brunzell 2008), genetic predisposition (Sullivan and Kendler 1999), and related psychological attractions to smoking (Zuckerman 2007) helps explain individual differences.
With some exceptions, the literatures treat the two classes of influence as separate and independent. The social approach implicitly assumes that social patterns of smoking similarly affect persons with varied genetic propensities, and the genetic approach implicitly assumes that individual propensities to addiction remain invariant across social contexts. On the surface, the assumptions justify one literature ignoring the other. After all, largely stable genetic traits cannot explain swings in smoking prevalence, and swings in population smoking prevalence do little to change genetic traits. In other ways, however, assumptions of independence may be flawed. Social conditions may facilitate or inhibit genetic propensities for addiction and smoking, and genetic expression may speed or slow socially generated changes in smoking. If so, understanding patterns of smoking (as well as other aspects of health with biological components) requires combined attention to both social context and genetic propensities.
The importance of social context may show in several ways. When smoking is widely accepted and common, social incentives and motivations to smoke may overwhelm the influence of genetic characteristics. However, when smoking involves controversy over its dangers and the weighing of costs and benefits by individuals, genetic influences may better predict smoking—social smokers with less physical dependence do more to avoid smoking than those with genetic propensities for physical dependence. Social context thus can shape genetic expression, and sociologists can offer much to understanding this expression.
Conversely, genetic propensities can affect the social patterns of smoking. During the initial period of transition from smoking as a normative behavior to one considered controversial, genetic predispositions make avoidance harder for some and slow the pace of change. During later stages of change, however, when institutional policies make smoking difficult for everyone, social constraints on both social and genetic smokers speed abandonment of the practice. Thus, downward trends in cigarette consumption may begin slowly, given the resistance to change of those with genetic propensities, but speed up as disincentives for smoking become stronger.
In this study, we ask, under what social and historical contexts are genetic influences on smoking weakest and strongest? In so doing, we tie together two literatures and draw out the implications of arguments about the combined importance of social and genetic factors for smoking (and, by implication, other health-related behaviors). We argue that genetic influences on smoking are part of a dynamic system that evolves over time rather than remaining independent and random. Going beyond findings that genetic influences on smoking vary across place (Boardman 2009; Boardman et al. 2008), the study links genetic influences on smoking to time, historical events, and social policies that reflect considerable variation in social norms and beliefs about smoking.1 The historically situated arguments give precision and falsifiability to the links between social context and genetic influences.
GENE–ENVIRONMENT INTERPLAY AND SMOKING
From studies that compare the concordance of smoking among identical twin pairs to that of fraternal twin pairs, researchers estimate that roughly 50 to 60 percent of the variance of regular smoking is due to genetic factors (Carmelli et al. 1992; Sullivan and Kendler 1999; Hall, Madden, and Lynskey 2002; Li et al. 2003). However, by comparing reported tobacco use among same-sex twin pairs across three birth cohorts (1910–1924–1925–1939, and 1940–1958), Kendler et al. (2000) demonstrate that heritability estimates are subject to change over time—a result that makes a single figure misleading. Among the first cohort of women they studied, none of the variance in tobacco use came from genetic factors, but by the third cohort, the heritability for regular tobacco use reached nearly 60 percent.
Three complementary mechanisms, two causal and one non-causal, can help explain such changes in the heritability of tobacco use: social control, the social trigger, and a social push gene–environment interaction (G × E). First, a social control model posits that social forces wash out the effects of genetic factors in tobacco use (Shanahan and Hofer 2005:69):
norms and other social forces … “canalize” (i.e., restrict variability in the phenotype of) genetically diverse people. As these canalization forces increase (i.e., norms are more effective and choices are minimal), genetic differences are of diminishing consequence.
For example, the genetic influences on the use of tobacco and alcohol are either muted or nonexistent among those who are raised with a strong religious upbringing with stringent norms against substance use of any kind (Koopmans et al. 1999; Timberlake et al. 2006). Second, according to the social trigger model, genetic factors differentiate between individuals only in the presence of social pressures to consume cigarettes (Perrin and Lee 2007). Therefore, genetic influences on smoking should increase when smoking begins to become widespread, social sanctions against it are removed, and social pressures to smoke emerge. For example, Boardman et al. (2008) show that genetic influences on daily smoking among adolescents are significantly higher for those who attend schools in which the most popular students also smoke the most. In this case, the pro-smoking norms serve as a trigger for genetic influences.
Both of these models attribute a causal influence of the social environment in limiting or exacerbating genetic influences. Shared behavioral expectations and corresponding sanctions cause genes to operate differently by either blocking or enabling their expression. If the social environment makes smoking difficult for everyone, it inhibits the potential for genes to affect smoking; if the social environment presents new choices, it facilitates the potential for genes to affect smoking. But a noncausal model of gene–environment interaction is also possible. The social push model (Raine 2002) posits that changes in social norms regarding smoking can affect the relevance of genetic influences by minimizing or maximizing “noise” that has the potential to overwhelm and hide the influences. On one hand, genetic associations are most clearly observable in benign environments that lack social factors encouraging genetically influenced addictive behaviors. When social noise is minimized, it allows for “biology to shine through” (Raine 2002:14). Conversely, when social factors “push” certain behaviors, then biological factors are harder to identify. As Raine makes clear, the social push perspective does not mean that the environment actually causes genes to operate differently. Rather, by adding or eliminating other sources of variation in behavior, the environment hides or highlights the role of genes to scientific observers.
Applying the social push mechanism to smoking suggests attention to changes in the composition of smoker populations. As large numbers of people (regardless of genetic makeup) begin smoking, there will be a tipping point in the distribution of smoking environments where entrée into smoking becomes a primarily social phenomenon; genetically vulnerable persons are no more likely to begin smoking than genetically resilient persons simply because of the predominant social popularity of smoking. In contrast, if social influences discourage rather than encourage smoking, then genetic influences increase in salience because quitting is physiologically harder for some people than for others. The environment does not cause genetic influences to become more important for smoking but does allow their importance to show.
SMOKING TRENDS AND THE SURGEON GENERAL’S REPORT
Both causal and noncausal models may relate to national trends in cigarette consumption. Cigarette consumption, which reflects both the prevalence and the intensity of smoking, increased more than fivefold from 1920 to 1960, reached a plateau between 1965 and 1975, and has declined consistently since that time. At the peak around 1966, roughly one-half of men and one-third of women in the United States smoked regularly (Forey et al. 2007). Two changes took place during the 1960s and 1970s that had important implications for smoking. These changes first affected the direction of the social push from pro-smoking to antismoking, and later they causally affected the genetic influences on smoking by reinstituting social control. The first event occurred in 1964 when the Surgeon General released the first of a number of reports with clear warnings about the dangers of smoking. This led to the 1965 Federal Cigarette Labeling and Advertising Act, which required that all cigarette packages bear the Surgeon General’s Warning: “Caution: Cigarette Smoking May Be Hazardous to Your Health.” The first report focused on the link between smoking and lung cancer and was followed by a series of reports linking smoking to heart disease (U.S. Department of Health, Education, and Welfare [USDHEW] 1967) and low birth weight (USDHEW 1969), and describing the risks of secondhand smoke for vulnerable populations (USDHEW 1973). These efforts led to the 1971 Public Health Cigarette Smoking Act, which banned the advertising of cigarettes on both television and radio.
The second series of events began in the mid-1970s. In 1973, Arizona passed a comprehensive law that limited smoking in public places, the first effort to formally control public smoking. This was followed by a more restrictive set of laws including the 1975 Minnesota Clean Indoor Air Act, which required restaurants to have nonsmoking sections; another twelve years would pass until Aspen, Colorado, became the first city to formally ban all cigarette smoking in restaurants. The push for bans in all restaurants was bolstered by the nineteenth Surgeon General’s Report (USDHHS 1986), which argued that the “simple separation of smokers and nonsmokers within the same airspace may reduce but cannot eliminate nonsmoker exposure to environmental tobacco smoke” (p. 7).
This historical backdrop provides a unique opportunity to examine both causal and non-causal arguments about gene-by-environment interactions, and the timing of the first Surgeon General’s Report creates a naturally occurring quasi-experimental design to test the value of treating the smoking population as a sociogenetic composition that changes over time. Using the causal/non-causal G × E distinction between the social control, social trigger, and social push arguments in combination with the changing social and institutional forces with respect to smoking, we hypothesize that genetic influences on smoking will change in predictable ways across birth cohorts.
The earliest cohort in our study, those born in the 1920s through the mid-1930s, were in their late teens and young adulthood as smoking emerged from a disreputable activity limited to marginalized groups (lower-class bohemians and upper-class dandies, according to Sobel [1978]) to one accepted in more conventional middle-class groups. Early in the century normative sanctions limited the expression of genetic influences, but the growing acceptance of smoking in the 1920s and 1930s allowed, and possibly encouraged, the expression of genetic influences. Therefore, we believe that genetic influences on smoking will increase and remain quite high for those born during this time. This association is consistent with the social trigger model.2
The next cohort, those born in the middle 1930s to the middle to late 1940s, aged into the risk of smoking at a time when cigarettes were cheap and ubiquitous, with regular images of cultural icons smoking. Because these social mechanisms influenced all individuals regardless of genetic makeup, the genetic influences on smoking will start to decrease for this birth cohort. That is, genetic characteristics are less likely to differentiate smokers from nonsmokers. This decrease is consistent with the social push model.
The third birth cohort, those born in the late 1940s to the mid-1950s, entered young adulthood as the health risks of smoking were becoming clear. The evidence provided by the scientific community created controversy over the dangers of smoking but left individuals to weigh the costs and benefits of starting or continuing the habit. Changes in smoking should be most evident among social smokers and least evident among those for whom smoking is genetically oriented. That is, those for whom quitting is easiest will be the most likely to quit in light of the evidence provided by the Surgeon General, leaving the population of smokers to be composed primarily of genetically vulnerable persons. Thus, the genetic influences on smoking will once again increase. Dominated by a drop in social smoking, this increase is again consistent with the social push model.
The final cohort in our study, those born during the mid- to late 1950s through the 1960s, were socialized about smoking at a time when local, state, and federal lawmakers began to enact and enforce policies aimed to reduce cigarette consumption. When smoking is stigmatized, expensive, and banned in public places, social forces affect smoking among most persons, both with and without genetic tendencies to smoke. These social controls will causally influence the degree to which genetic characteristics differentiate between individuals. Thus, during this period, the genetic influences on smoking will decrease. This change is consistent with the social control model.
In summary, the first period is characterized by the social trigger model in which the rise of smoking is primarily among those with genetic predispositions; the second period of rising social push reflects the rise of smoking among those with social motivations and the declining statistical importance of genetic influences; the third period of declining social push reflects the fall of smoking among those with social motivations and the growing statistical importance of genetic influences; and the fourth period of increasing social control reflects an actual drop in smoking among genetically motivated smokers and the declining importance of genetic influences. These predictions involve something more than claims about steady and small upward or downward trends in heritability. Rather, they involve changes in heritability that match specific events, occur relatively quickly, and involve directional shifts—all making for an original and highly falsifiable test of the value of an integrative gene–environment interaction approach to understanding health behavior.
METHODS
Data
This study uses data from the 1995 National Survey of Midlife Development in the United States (MIDUS) (Brim et al. 1996). MIDUS is a nationally representative survey designed to study the effects of midlife development on the self-reported physical health, psychological well-being, and social consciousness of adults aged 25 to 75. To examine the genetic influences on the social and psychological components in this study, the MIDUS team developed the twin screening project in order to oversample adult twin pairs. The MIDUS twin screening project was conducted by two research organizations, ICR/AUS consultants and Bruskin Associates, who contacted randomly selected households by telephone and asked if there were “any twins in your or your spouse’s immediate family where BOTH of the twins are still living?” During the pre-test period, 14.8 percent of the households said “yes” to this question, with 2.3 percent being members of a twin pair and 12.5 percent of respondents with a twin pair in their family. They were then asked if it would be okay for the Harvard Medical School to contact them and their twin to participate in the study. The sample was limited to twins between the ages of 25 and 74, both with a residential telephone number, living in the United States and English-speaking, and both mentally and physically able to participate in the interview themselves. Roughly one-half of the first contacts were not eligible to participate in the study (mostly because they were too young). Sixty percent of the eligible contacts participated in the study and 21 percent of those who were referred by the first contacts participated, with an overall participation of 26 percent of the identified twin pairs. Overall, a total of 998 adult twin pairs were used in this study. We use only monozygotic (MZ) and same-sex dizygotic (DZ) pairs, dropping pairs with missing information about smoking history or age, and opposite sex DZ pairs. Our final analyses use a total of 340 MZ pairs and 315 same-sex DZ pairs.
Regular smoking was assessed through two questions. Respondents were asked, “Have you ever smoked cigarettes regularly—that is, at least a few cigarettes every day?” Those responding “yes” were then asked, “On average, about how many cigarettes did you smoke per day during the one year in your life when you smoked most heavily?” Respondents indicating that they smoked less than three cigarettes per day during the time of heaviest smoking were considered to have never been regular smokers. Respondents indicating that they had smoked regularly were also asked, “At what age did you begin smoking regularly?” We use lifetime history and the timing of onset to characterize regular smoking. We recognize that this single item does not differentiate between different styles or different amounts of smoking, both of which have genetic and environmental influences. Rather, we emphasize regular smoking because the relatively straightforward description of this variable coupled with the age ranges in this study increase the reliability of smoking recall for this important transition.
Statistical Analysis
To establish the genetic influences on smoking we use a variety of different techniques. All of these methods rely on comparing identical twins and same-sex fraternal twins to examine the genetic influences on regular smoking for the full sample and separately across birth cohorts. The birth cohorts are defined as follows: 1920–1939; 1940–1949; 1950–1959; and 1960–1970.3 Our first demonstration of genetic influences on regular smoking comes from a comparison of concordance for regular smoking among MZ pairs and DZ pairs; evidence of genetic influence is found when the concordance among MZ pairs is significantly higher than the concordance among DZ pairs. These results are presented in Table 1.
Table 1.
Smoking Concordance among Identical and Same-sex Fraternal Twin Pairs Born between 1920 and 1970
| Year of birth | Identical twins (MZ)
|
Same-sex fraternal twins (DZ)
|
MZ-DZ significance test
|
|||
|---|---|---|---|---|---|---|
| N pairs | Concordance | N pairs | Concordance | χ2 | p < | |
| 1920–1939 | 69 | .783 | 69 | .594 | 5.777 | .016 |
| 1940–1949 | 80 | .800 | 85 | .741 | .808 | .369 |
| 1950–1959 | 104 | .865 | 84 | .738 | 4.849 | .028 |
| 1960–1970 | 87 | .793 | 77 | .818 | .164 | .686 |
| Total | 340 | .815 | 315 | .727 | 7.171 | .007 |
Note: Data come from the 1995 National Survey of Midlife Development in the United States (MIDUS) [Brim et al. 1996]. Cell entries represent the number of pairs by zygosity and pairwise concordance rates. Significance test for the change in MZ-DZ differences in concordance is calculated using the following formula from Carey (2003): χ2 = 2[CMZ ln(AMZ) + DMZ ln(1 − AMZ) + CDZ ln(ADZ) + DDZ ln(1 − ADZ) − CTotal ln(ATotal) − DTotal ln(1 − ATotal)], where C is the number of concordant pairs, D is the number of discordant pairs, and C is the concordance rate with df = 1.
Having established evidence of genetic influences on regular smoking, we then formally quantify the contribution of genetic factors to regular smoking by using maximum likelihood variance components models (Purcell 2008). This process estimates the best fitting model by comparing the covariance of MZ and DZ pairs, and it provides estimates for two components of genetic influence (additive [A] and dominant [D]) and two components of environmental influence (shared [C] and unshared [E]). We assess the best fitting model by comparing model fit indices (e.g., likelihood ratio tests), and we present the most parsimonious model. The purpose of this model is to establish an average heritability measure for this trait.4 These estimates are presented in Table 2.
Table 2.
Maximum Likelihood Variance Components: Genetic and Environmental Influences on Regular Smoking
| Model | Parameter estimates
|
Model fit for nested model
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A | C | E | −2LL | df | p< | Δ −2LL | Δ df | p < | |
| ACE | .087 | .069 | .092 | .069 | 3 | .995 | |||
| AE | .158 | .087 | 8.668 | 4 | .013 | 8.598 | 1 | .003 | |
| CE | .135 | .113 | 13.236 | 4 | .001 | 13.166 | 1 | .000 | |
| E | .248 | 242.63 | 5 | .000 | 242.560 | 2 | .000 | ||
| ADE | .158 | .000 | .087 | 8.668 | 3 | .034 | |||
| DE | .157 | .087 | 34.474 | 4 | .000 | 25.806 | 1 | .000 | |
| E | .248 | 242.630 | 5 | .000 | 233.961 | 2 | .000 | ||
Note: Data come from the 1995 National Survey of Midlife Development in the United States (MIDUS) [Brim et al. 1996]. The ACE model is the best fitting model, with overall parameter estimates as follows: A = 35%, C = 28%, E = 37%. The following online model fitting routine developed by Purcell (2008) was used to estimate the ACE and ADE parameter estimates: http://statgen.iop.kcl.ac.uk/bgim/twinfit.html.
We then calculate heritability estimates for regular smoking across the four birth cohorts. The goal of this analysis is to examine the anticipated highs and lows described above. If changes in the heritability of smoking are consistent with our expectations, then the highest heritability estimates should be among the first (1920s–1930s) and third (1950s) birth cohorts, and the lowest should be among the second (1940s) and fourth (1960s) cohorts. To demonstrate the reliability of the parameter estimates, we present heritability estimates using four different techniques. The first two methods compare the correlations for regular smoking among MZ and DZ pairs. Heritability is estimated using twice the difference of MZ and DZ correlations (Plomin et al. 2008). The first correlation is simply the bivariate correlation among twin pairs. This estimate is calculated separately for MZ and DZ pairs. We also estimate a pairwise correlation using a second, and more complicated, method that takes into account the age of onset for smoking among sibling pairs as well as censored values for those who may later begin smoking but have not by the time of the survey. This method uses a multivariate survival model with shared frailty among twin pairs by zygosity (Guo and Rodriguez 1992). The frailty variance is similar to a random intercept in a multilevel model and large estimates are indicative of similarity in the timing of smoking among pairs of twins. By comparing the frailty estimates between MZ and DZ twin pairs, we can infer genetic influence on the timing of a particular behavior (Guo and Tong 2006). This model is specified in equation 1.
| (1) |
The values for t are random variables capturing the survival times (the age of onset for regular smoking) for the jth sibling in the ith pair of twins. Thus, the survival function is conditional on this cluster-specific error term wi, and the resulting hazard functions h(tij | wi) are multiplicative frailty models with a baseline hazard λ0(t0).
Our model assumes that wi has a gamma distribution with mean = 1 and a variance of θ. Although θ is a variance component, it has two characteristics that make it a useful statistic for twin analysis. First, 1 + θ can be interpreted as the odds ratio for smoking initiation as a function of the twins smoking status (Clayton 1978). Second, the ratio of θ/(2 + θ) is equivalent to Kendall’s (1962) coefficient of intracluster rank correlation (Oakes 1982; Guo and Rodriguez 1992). Thus, by estimating θ for MZ pairs and DZ pairs separately, we can calculate pairwise correlation coefficients that take into account the duration of exposure and the subsequent onset of regular smoking. We estimate the heritability of regular smoking as twice the difference of the correlations between MZ and DZ pairs. These estimates are shown in Table 3.
Table 3.
Quantitative Genetic Estimates for Regular Smoking across Four Birth Cohorts: A Comparison of Four Different Methods
| Year of birth | MZ pairs
|
DZ pairs
|
Total sample
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frailty estimates
|
Pairwise correlations
|
Frailty estimates
|
Pairwise correlations
|
Heritability estimates
|
||||||||
| Theta | p < | Frailty | Bivariate | Theta | p < | Frailty | Bivariate | Frailty | Bivariate | DF | VC | |
| 1920–1939 | 1.67 | .001 | .46 | .58 | .29 | .096 | .13 | .20 | .66 | .76 | .71 | .58 |
| 1940–1949 | 1.79 | .001 | .47 | .60 | 1.34 | .001 | .40 | .48 | .14 | .24 | .01 | .00 |
| 1950–1959 | 4.89 | .001 | .71 | .72 | .94 | .001 | .32 | .45 | .78 | .54 | .63 | .73 |
| 1960–1970 | 2.44 | .001 | .55 | .56 | 2.45 | .001 | .55 | .60 | .00 | −.08 | −.13 | .00 |
| Total | 2.77 | .001 | .58 | .63 | 1.22 | .001 | .38 | .46 | .40 | .34 | .34 | .35 |
Note: Data come from the 1995 National Survey of Midlife Development in the United States (MIDUS) [Brim et al. 1996]. Columns 1 and 5 describe the shared frailty estimates for MZ and DZ pairs from a survival model predicting the onset of regular smoking. Columns 2 and 6 describe the significance of the frailty estimate for each zygosity-year of birth combination. Columns 3 and 7 describe pairwise smoking correlations using the frailty estimates (r = θ/(θ + 2)). Columns 4 and 8 describe unadjusted bivariate correlations among pairs. The final four columns present the heritability estimates using four different methods. The first two rely on the correlations presented for each pair from columns 3, 4, 7, and 8. The third set of heritability estimates is obtained from a DeFries-Fulker (DF) regression model (DeFries and Fulker 1985), and the final set are the maximum likelihood variance components (VC) estimates (Purcell 2008).
| (2) |
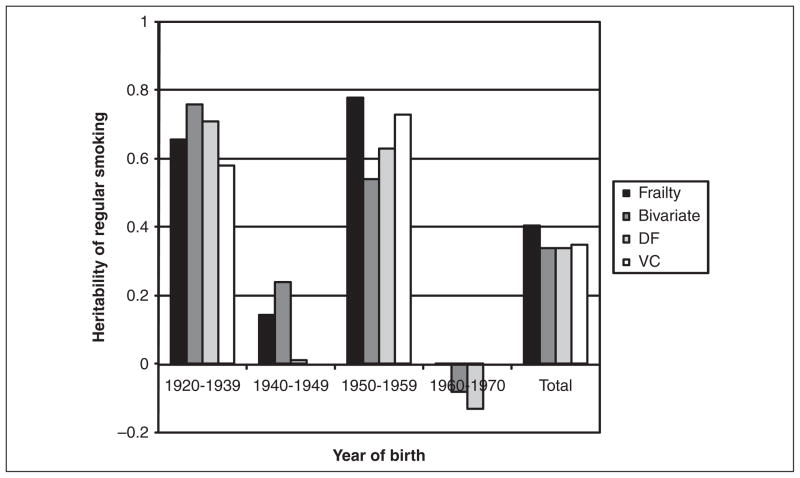
We then use two traditional behavioral genetics techniques to estimate genetic contributions to regular smoking. The first is a DeFries-Fulker model, which is an efficient and robust method to estimate genetic and environmental components (DeFries and Fulker 1985). The DeFries-Fulker model (see equation 2) predicts the outcome of the second sibling of a pair (y2) as a function of the first sibling’s score on the same outcome (y1), a measure of genetic similarity, i.e., proportion of alleles shared identical by descent by the pair—(g = 1 for MZ pairs and g = .5 for DZ pairs), and an interaction between genetic similarity and the sibling’s score (y1g). Two of the parameter estimates obtained from this model (b1 and b3) describe the relative contribution of shared environment (c2) and heritability (h2), respectively, and the remaining proportion is due to nonshared environmental characteristics (e2). Although the most basic DeFries-Fulker model has undergone considerable modifications (Purcell 2002), it is still widely used to assess the genetic contribution to a trait’s overall variation (Cherny, DeFries, and Fulker 1992; Rende 1993; Rodgers and McGue 1994). Although the DeFries-Fulker model is intended for continuously distributed outcomes, the prevalence of smoking in this study is roughly 50 percent, which makes the linear probability model an appropriate extension of this model. In addition to the survival, bivariate correlation, and DeFries-Fulker models, we also calculate cohort-specific heritability estimates using the maximum likelihood variance components technique described above (Purcell 2008). These heritability estimates are presented in Table 3 and summarized in Figure 1.
Figure 1.
Changes in the Heritability of Regular Smoking across Four Birth Cohorts in the United States: 1920–1970.
Note: Data come from the 1995 National Survey of Midlife Development in the United States (MIDUS) [Brim et al. 1996]. The values in the figure are obtained from Table 3. The heights of the bars represent the estimated heritability of regular smoking for each of the four birth cohorts.
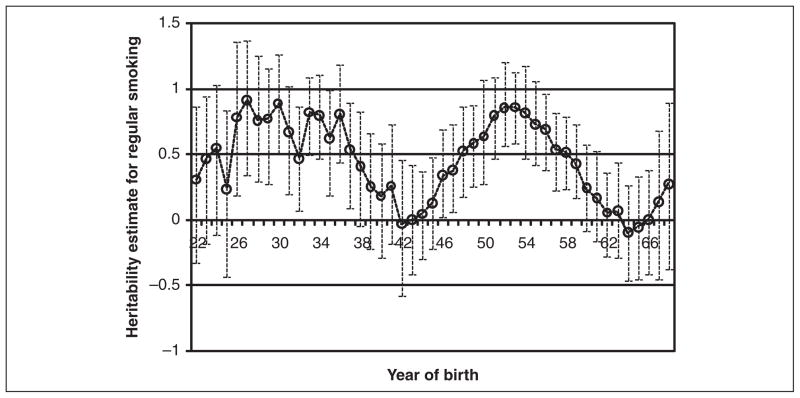
Finally, we examine a more refined series of models by estimating the heritability of regular smoking for each year of birth in our sample. Because of small sample sizes for each birth year in our study, we calculate time-specific estimates for a moving sample with a window of ± four years. For example, an estimate for 1930 includes individuals born between 1926 and 1934. We do this for each year between 1922 and 1968. These models, summarized in Table 4, include controls for the respondent’s age and gender and measures of the equal environments assumption.5 In order to gauge the statistical significance of the estimates, we provide the bootstrapped confidence interval for the yearly estimates. Each year-zygosity model was run 200 times using sample sizes equivalent to the empirical size with replacement. The confidence intervals are, therefore, empirical confidence intervals for the 10th and the 190th ranked value from each distribution. These bootstrapped models were performed using the “coxph” package in the R 2.9.0 statistical program (R Development Core Team 2009). If the confidence interval crosses over zero, then we assume that there are no significant genetic influences on regular smoking for that particular birth year. These estimates are summarized in Figure 2.
Table 4.
Frailty Estimates by Birth Year for the Onset of Regular Smoking by Zygosity
| Birth year | MZ twins
|
Same sex DZ twins
|
Heritability estimates
|
|||||
|---|---|---|---|---|---|---|---|---|
| N | theta | r | N | theta | r | h2 | 95 % C.I. | |
| 22 | 13 | .65 | .24 | 18 | .19 | .09 | .31 | (−.32, .87) |
| 23 | 15 | .62 | .24 | 20 | .00 | .00 | .48 | (−.17, .95) |
| 24 | 18 | .77 | .28 | 21 | .00 | .00 | .56 | (−.10, 1.04) |
| 25 | 18 | .77 | .28 | 27 | .37 | .16 | .24 | (−.43, .84) |
| 26 | 21 | 1.79 | .47 | 30 | .17 | .08 | .79 | (.19, 1.37) |
| 27 | 25 | 1.75 | .47 | 32 | .01 | .01 | .92 | (.35, 1.38) |
| 28 | 26 | 1.25 | .38 | 34 | .00 | .00 | .77 | (.29, 1.26) |
| 29 | 27 | 1.27 | .39 | 32 | .00 | .00 | .78 | (.28, 1.17) |
| 30 | 31 | 1.63 | .45 | 33 | .00 | .00 | .90 | (.51, 1.27) |
| 31 | 36 | 1.15 | .36 | 31 | .05 | .02 | .68 | (.20, 1.03) |
| 32 | 37 | 1.03 | .34 | 33 | .23 | .10 | .47 | (.08, .87) |
| 33 | 45 | 1.45 | .42 | 35 | .01 | .01 | .83 | (.50, 1.10) |
| 34 | 47 | 1.39 | .41 | 34 | .02 | .01 | .80 | (.47, 1.11) |
| 35 | 47 | 1.38 | .41 | 37 | .21 | .09 | .63 | (.19, 1.00) |
| 36 | 46 | 1.42 | .42 | 36 | .02 | .01 | .81 | (.44, 1.19) |
| 37 | 45 | 1.37 | .41 | 43 | .31 | .14 | .54 | (.09, .90) |
| 38 | 47 | 1.37 | .41 | 47 | .50 | .20 | .42 | (−.04, .84) |
| 39 | 49 | 1.16 | .37 | 56 | .62 | .24 | .26 | (−.21, .67) |
| 40 | 47 | 1.05 | .34 | 60 | .67 | .25 | .19 | (−.29, .58) |
| 41 | 50 | 1.38 | .41 | 67 | .76 | .28 | .26 | (−.18, .74) |
| 42 | 48 | .86 | .30 | 74 | .93 | .32 | −.03 | (−.57, .47) |
| 43 | 57 | 1.23 | .38 | 73 | 1.22 | .38 | .00 | (−.41, .43) |
| 44 | 68 | 1.47 | .42 | 73 | 1.32 | .40 | .05 | (−.29, .38) |
| 45 | 77 | 1.87 | .48 | 81 | 1.44 | .42 | .13 | (−.21, .48) |
| 46 | 86 | 2.10 | .51 | 80 | 1.03 | .34 | .34 | (.03, .70) |
| 47 | 84 | 2.49 | .55 | 76 | 1.13 | .36 | .38 | (.07, .73) |
| 48 | 85 | 3.10 | .61 | 70 | 1.03 | .34 | .53 | (.18, .87) |
| 49 | 89 | 4.28 | .68 | 71 | 1.26 | .39 | .59 | (.26, .88) |
| 50 | 96 | 3.52 | .64 | 71 | .93 | .32 | .64 | (.27, 1.08) |
| 51 | 95 | 3.99 | .67 | 74 | .72 | .27 | .80 | (.48, 1.09) |
| 52 | 101 | 4.48 | .69 | 76 | .70 | .26 | .86 | (.57, 1.21) |
| 53 | 93 | 5.16 | .72 | 77 | .81 | .29 | .87 | (.59, 1.13) |
| 54 | 91 | 4.61 | .70 | 72 | .80 | .29 | .82 | (.47, 1.18) |
| 55 | 91 | 5.17 | .72 | 74 | 1.10 | .35 | .73 | (.43, 1.07) |
| 56 | 99 | 4.90 | .71 | 81 | 1.13 | .36 | .70 | (.38, .97) |
| 57 | 97 | 4.51 | .69 | 85 | 1.46 | .42 | .54 | (.23, .82) |
| 58 | 95 | 3.77 | .65 | 92 | 1.29 | .39 | .52 | (.24, .80) |
| 59 | 94 | 4.51 | .69 | 88 | 1.82 | .48 | .43 | (.17, .73) |
| 60 | 91 | 3.59 | .64 | 81 | 2.16 | .52 | .25 | (−.08, .58) |
| 61 | 79 | 2.98 | .60 | 78 | 2.11 | .51 | .17 | (−.16, .53) |
| 62 | 81 | 2.74 | .58 | 75 | 2.44 | .55 | .06 | (−.28, .37) |
| 63 | 79 | 2.84 | .59 | 73 | 2.44 | .55 | .07 | (−.29, .45) |
| 64 | 75 | 2.15 | .52 | 70 | 2.60 | .57 | −.09 | (−.46, .27) |
| 65 | 73 | 1.98 | .50 | 63 | 2.19 | .52 | −.05 | (−.45, .34) |
| 66 | 70 | 2.13 | .52 | 58 | 2.10 | .51 | .01 | (−.41, .39) |
| 67 | 60 | 2.22 | .53 | 43 | 1.68 | .46 | .14 | (−.44, .68) |
| 68 | 48 | 1.81 | .48 | 36 | 1.01 | .33 | .28 | (−.37, .90) |
Note: Data come from the 1995 National Survey of Midlife Development in the United States (MIDUS) [Brim et al. 1996]. Cell entries represent parameter estimates obtained from separate survival models (for MZ and DZ twins separately) by year of birth with shared frailty estimates capturing similarity in the onset of regular smoking among twin pairs. Heritability estimates are obtained by comparing the correlations of MZ and DZ pairs (h2 = 2 × (rMZ–rDZ).
Figure 2.
Yearly Heritability Estimates for Regular Smoking in the United States: 1922–1968.
Note: Data come from the 1995 National Survey of Midlife Development in the United States (MIDUS) [Brim et al. 1996]. Estimates are obtained from a series of multivariate Cox regression models with shared frailty among twin pairs, and they describe the heritability of regular smoking for each year of birth. The bars around each point estimate describe the 95% confidence intervals for each year. These confidence intervals were bootstrapped from 200 runs for each zygosity using the coxph package for R 2.7.1 for all years between 1922 and 1968. Each birth year contains the four years before and after and thus describes a nine-year window for the estimated genetic influences on smoking at that time.
RESULTS
Table 1 presents concordance rates for regular smoking among the twin pairs in this study. Of the 340 MZ twin pairs, 81.5 percent were concordant for regular smoking status compared to 72.7 percent of same-sex DZ twin pairs. This difference is statistically significant (p < .01), providing evidence for genetic influences on regular smoking for the full sample. The estimates provided in Table 2 quantify the genetic contributions to regular smoking more formally. According to these results, the ACE (additive genetic [A], shared environment [C], and unshared environment [E]) model is the best fitting of the seven models.
These numbers indicate that total variance in regular smoking (σ2 = .248) is composed of 35 percent (A = .087/.248) additive genetic influences, 28 percent (C = .069/.248) shared environmental influences, and 37 percent (E = .092/.248) unique environmental influences. In other words, roughly one-third of the reason that individuals smoke regularly is due to genetic factors, and the remaining two-thirds is due to their social environments.
Returning to Table 1, it is important to note that of the four birth cohorts examined MZ pairs have a significantly higher concordance rate, compared to DZ twins, for regular smoking in only two cohorts. These findings are in line with the anticipated changes in heritability described above. In other words, these findings suggest that for those born in the 1940s and 1960s, genetic factors do not significantly contribute to the risk of regular smoking. The same results are evident in Table 3, which summarizes the outcomes from the four methods used to characterize the heritability of regular smoking. The first row of Table 3 presents estimates for those born between 1920 and 1939. Among MZ pairs, the frailty estimate (θ = 1.67) translates into a pairwise .46 correlation. As described earlier, these models control for respondent’s age, gender, and a measure for the equal environments assumption among twin pairs. The bivariate correlation, based on the frailty estimates, for this birth cohort is .58. For DZ pairs, the frailty estimate (θ = .29) is considerably smaller, and it translates into a pairwise .13 correlation. Using twice the difference of the MZ-DZ correlations as a rough heritability estimate, we calculate the heritability of regular smoking to be in the range of .66 to .76 for this birth cohort. These estimates are in line with the other two methods used to estimate heritability (hDF2 = .71; hVC2 = .58). The heritability estimates for the four birth cohorts are presented for each method in Figure 1. Although there are slight differences in these estimates using the four different methods, it is quite clear from Figure 1 that there are systematic differences in the heritability of regular smoking across the four birth cohorts. As Table 1 shows, only the first and third of these cohorts demonstrate a sizable genetic influence on regular smoking.
To provide more precise yearly estimates and to document the trend in the heritability of regular smoking, the survival models presented in Table 3 were repeated 47 times for the birth years 1922 to 1968.6 The yearly heritability estimates and the bootstrapped 95 percent confidence intervals are presented in Table 4 and Figure 2. These results correspond with the results presented in Table 3. However, they also provide a more detailed picture regarding temporal changes in the genetic influences on smoking. That is, the averages described in Table 3 are still evident, but important changes occur within each birth cohort, and these changes are theoretically linked to the causal and noncausal forms of G × E described above. The genetic influences on regular smoking appear to be the most pronounced for those born in the early 1930s and the mid-1950s. Sharp changes in the sociogenetic composition of the smoking population occur for those born after 1936 and again for those born after 1954. The first minimum in this figure occurs in 1942, which corresponds with the Surgeon General’s Report; those born during the early to mid-1940s were at their prime smoking ages (in their early 20s) when the report was released. However, following this first transition there is a persistent and steady increase in the genetic influences on regular smoking until a maximum is reached for those born in 1954. We argue that this increase captures a noncausal form of gene–environment interactions where the sociogenetic composition of smokers is changing over time; those for whom quitting smoking is relatively easy may be the first to quit in light of the evidence about the health risks. Smoking desistance is the most highly heritable smoking phenotype (Vink, Willemsen, and Boomsma 2005). Therefore, those who have the hardest time quitting may also be those who have a stronger physiologic dependence on nicotine. As nondependent individuals are removed from the smoking population, genetic factors responsible for nicotine dependence become relatively more important.
The first legislative efforts to limit or ban smoking in public places occurred during the early and mid-1970s. According to our hypotheses, the genetic contributions to regular smoking will decrease under noncausal changes due to the social composition or if there are causal social forces (normative, institutional, or both) that act to control the behaviors of individuals. For those born after the mid-1950s, entry into regular smoking took part under increasingly strict and formal social control. These controls, we argue, are responsible for the measured decline in the heritability of regular smoking that is seen in Figure 2. This period extends until the mid-1990s and is characterized by an increasing number of federal, state, and local laws that controlled the advertisement, sale, distribution, and smoking of tobacco. In other words, changes in the social orientation of smoking did not causally influence genetic factors related to smoking onset or persistence until laws were developed and enforced that placed physical limits on this behavior. These legislative efforts reflect the forces described by Shanahan and Hofer (2005) that restrict the variation of genetic factors, and the steep drop in the genetic influences on regular smoking fits the social control perspective on gene–environment interactions.
DISCUSSION
Both environmental and genetic factors are implicated in the onset of regular smoking among U.S. adults. We show that, on average, over one-third of the variance in regular smoking is due to additive genetic influences. However, we also show variation in heritability estimates for regular smoking for different birth cohorts. Specifically, we show that the genetic influences are significantly higher among those born between 1925 and 1935 and those born between 1951 and 1956, and are largely unimportant for those born in the early 1940s and those born in the mid-1960s. The minimum heritability corresponds with a birth cohort that was in its early twenties at the time of the 1964 Surgeon General’s report on the dangers of cigarette smoking. The increasing genetic influence among the earliest cohort supports the causal social trigger model, and the declining genetic influence for those born after the mid-1950s supports the causal social control model. We argue that the smoking behavior of the birth cohorts between 1935 and 1954 is best characterized by the noncausal social push perspective.
The findings presented in this paper speak to a large body of work that quantifies genetic and environmental contributions to smoking in the population, with the goal of anticipating dispersion in genetic influences across different social settings (Shanahan and Hofer 2005). The gene–environment interaction perspective anticipates that social environments may either enhance or suppress latent genetic tendencies in a causal manner or they may simply obscure or clarify the influence of genetic factors in a noncausal manner. The context for this dispersion may be discrete social settings like schools (Rowe et al. 1999; Boardman et al. 2008) or neighborhoods (Cleveland 2003); but, as we show here, it may also be a social historical trend or birth cohort. This point is made nicely by Rutter (2006, p. 60):
There is not, and cannot be, any absolute value for the strength of genetic influences on a trait, no matter how accurately the trait is measured or how carefully the genetic effect is assessed. As behavioral geneticists have long recognized, and emphasized, heritability figures are necessarily specific to populations and to time periods. (emphasis added)
Despite the general acceptance of this perspective, we know of only one other study to consider the influence of birth cohort on the genetic influences on smoking. That study, which used data from twin pairs in Sweden, examined birth cohorts from 1910 to 1958 (Kendler et al. 2000), and it showed an increasing heritability of smoking for women across the cohorts but a relatively stable heritability among men. Kendler and colleagues’ analyses considered those born between 1940 and 1958 as one birth cohort, so it is difficult to compare their results to ours. That is, it is possible that heritability peaked before the end of this cohort and then declined as in our results. But there are also clear differences in the trends in regular tobacco use between Western European nations (Pampel 2003) and the United States (Forey et al. 2002). The United States began a shift to regular smoking earlier than Sweden and peaked much earlier. Importantly, these differences set up an opportunity for future researchers to elaborate upon the temporal variation in genetic factors linked to smoking by comparing these associations across multiple settings and birth cohorts.
Social scientists have been slow to incorporate the work of genetic epidemiologists and quantitative geneticists into their work, and genetic researchers have been slow to incorporate the work of sociologists. If anything is clear from this study, it is that each discipline needs to consider the large body of findings from the other. For example, these findings and the trend-based perspective on genetic influences are highly relevant to genetic epidemiologists in the recent push to identify single nucleotide polymorphisms (SNPs) that predict smoking (Li 2008). Estimates from genome-wide association studies (Lange et al. 2003) may be subject to periodic highs and lows in the genetic influences on a particular trait; if a genome-wide study on regular smoking were conducted on a national cohort of U.S. adults born in 1942, the researchers would have a very difficult time identifying SNPs that differentiate smokers from non-smokers. The current methods certainly consider this factor (e.g., the population prevalence is a key component of the estimation techniques), but they do not necessarily consider that any sample is drawn from a specific historical moment in a larger cycle with predictable ebbs and flows.
Similarly, although sociologists have examined smoking trends at great length, very few efforts have been made to synthesize this work with findings from behavioral genetic research. For example, Pampel (2005) examines social trends in smoking for U.S. adults born between the turn of the century and the mid-1970s by comparing the correlation between years of education and smoking across birth cohorts. He shows a steady increase in the association between education and smoking for white men born between 1931 and 1944. This correlation drops for those born between 1945 and 1949 and returns to a high level for those born after 1950. The steady rise in the correlation between education and smoking corresponds with our findings regarding the steady decline in the genetic influences on smoking. Likewise, his finding that the educational correlation peaks for those born in the early 1940s corresponds with our finding that genetic factors bottom out at this time. In other words, what may at first appear to be a random change in the data due to unique sampling characteristics takes on a different meaning in light of the findings presented in this article. The same might well prove true for other environmental moderators, such as schools, neighborhoods, or peer groups.
Limitations
There are several important limitations that should be considered in interpreting these results. First, although our multivariate models control for the equal environments assumption with a series of questions regarding the similarity of twins’ environments, it is difficult to know whether this control fully captures these effects. Several studies have tested this assumption, and the results are somewhat mixed (Kessler et al. 2004; Kendler et al. 1993). Further, as the survival models used in this study have been used in only a few behavioral genetic studies, there is no agreed-upon method to reliably adjust parameter estimates for differential environments for MZ and same-sex DZ twins. In ancillary analyses (results not shown), we examined MZ-DZ differences in the equal environment measure and found a monotonic decline over time. That is, differences in MZ and DZ environments were much higher among the earliest birth cohorts and smaller among the most recent cohorts. Therefore, heritability estimates may be exaggerated for the earlier cohorts of the study. However, as this change is consistently decreasing over time, it cannot explain the increasing–decreasing changes that we observed in our study across birth cohorts.
Second, there is only one published study that has used the shared frailty technique to examine genetic influences on behaviors (Guo and Tong 2006). Importantly, that article examined the age of first intercourse, and whereas nearly all individuals will have had intercourse in their lifetimes, the proportion of adults who become regular smokers is much smaller and the normative contexts of the two behaviors are very different from one another. Although we believe that the timing of smoking onset better characterizes the genetics of smoking compared to a binary question about lifetime use, and that our heritability estimates are in line with the others, it is important to bear in mind that the extension of this model to smoking onset is relatively novel.
Third, we do not differentiate between non-smokers who have never tried a cigarette and those who have tried cigarettes but never progressed to regular smoking. This distinction is important because different genes are believed to underlie smoking initiation and regular smoking (Madden et al. 1999). The initiation of smoking is influenced by impulsivity, whereas regular tobacco use is linked more strongly to individual differences in nicotine metabolism (Lerman et al. 1999; Pianezza, Sellers, and Tyndale 1998; Gu et al. 2000). The genetic factors responsible for these more proximate phenotypes are quite different from one another and therefore may respond very differently to the same environmental forces. In the same manner, the social moderation of genetic factors does not mean that the same genes are being turned off or turned on across time. Although examining these molecular processes would clarify this complex picture, it is not possible to perform this kind of analysis with these data. We chose to focus on regular smoking primarily because we felt that it might have greater reliability for the older respondents in our survey. That is, we believed that answers from former (or current) regular smokers about when they began regular smoking would be more reliable than answers from people who never became regular smokers about when they first tried cigarettes. Further research is needed to differentiate between the different genetic processes that may be influencing the results presented here. Finally, although this is a national study of twins, it is possible that these findings are unique to this sample. Although we show convergence in these findings using multiple methods, replication of these findings using data obtained from different samples across similar cohorts is necessary to fully validate our results.
CONCLUSION
In sum, sociological analysis of genetic phenomena represents an exciting and important opportunity. Sociologists are uniquely positioned to comment on social and institutional forces that structure behavioral trends. While they may appear to be unlikely compatriots with geneticists, their expertise in social contexts, such as schools, neighborhoods, workplaces, and families, makes them central to gene–environment interaction studies. The fact that genetic influences on behaviors such as smoking vary not only across social spaces (Boardman 2009; Boardman et al. 2008) but also across historical periods ensures that sociology will have an important voice in the science of genetic association studies. Recently, a number of genetic epidemiologists have called for more thorough accounts of the biology of gene–environment interactions (Rutter 2008). However, it is also clear that testable hypotheses about the biological pathways through which genes operate require a corresponding set of testable hypotheses regarding the social-environmental factors that enable or restrict the influence of genes. This is precisely the type of work that sociologists have engaged in over the past two centuries. If, indeed, the social environment is a fundamental cause of health (Link and Phelan 1995), then it is likely a fundamental cause of genotype–phenotype associations. The proximate associations (e.g., the influence of specific genotypes or haplotypes on a particular phenotype) may change over time, but the normative and institutional context in which behaviors reside remains fundamental to these more proximate causes.
Acknowledgments
FUNDING
Funding for this research was provided in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant R21 HD 051146 to the University of Colorado Population Center, an NICHD-funded early career award (K01 HD 50336), and the MIDUS Pilot Grant Competition.
Biographies
Jason D. Boardman is associate professor of sociology and research associate of the Population Program in the Institute of Behavioral Science at the University of Colorado, Boulder. His primary research is in the field of social epidemiology, with an emphasis on gene–environment interplay related to health and health behaviors.
Casey L. Blalock is a graduate student in the Department of Sociology and a research assistant with the Population Program in the Institute of Behavioral Science at the University of Colorado, Boulder. His research focuses on labor migration and migrant networks.
Fred C. Pampel is professor of sociology and research associate of the Population Program in the Institute of Behavioral Science at the University of Colorado, Boulder. His research focuses on trends in smoking disparities across socioeconomic status groups, the effects of tobacco use on changes in the sex differential in mortality, and global patterns of cigarette adoption and diffusion. His recent article in the Journal of Health and Social Behavior examines life course changes in smoking among whites and blacks.
Footnotes
The social environment for tobacco use today differs so starkly from the 1950s, when advertising, films, and even some medical professionals encouraged smoking, that comparisons over time maximize our ability to observe the influence of social context. Comparisons across places in today’s largely anti-smoking social environment lack the same contrast in social context.
The truncated historical range of our data does not allow for a full test of the change, but the analysis can examine whether genetic influences at the end of the period are high, as predicted by the hypothesis.
The hypothesized changes in the genetic influences on regular smoking correspond with midpoints of these birth cohorts. We chose to present data by these time frames because they correspond with natural breaks in decades. We also chose to combine the 1920s and the 1930s because of small sample sizes in the 1920s.
Heritability estimates describe the proportion of phenotypic variance that is due to genetic influences. In the case of this article, the primary estimate describes the proportion of regular smoking that is due to additive genetic causes.
Violations of the equal environments assumption, resulting from MZ twins being treated more similarly than DZ twins, can increase concordance among MZ twins and overestimate heritability. The MIDUS twin data set includes data from three questions assessing how often twins were dressed alike, were placed in the same classrooms, and had the same playmates. These measures have been used to gauge and correct for violations of the equal environments assumption (Kessler et al. 2004). We create a composite EEA score using a polychoric principal components analysis of the pair’s mean response on the three items, and we include this estimate as a control in all models. We do not expect twin pairs who are treated more similarly to one another to be more likely to smoke; rather, they will simply be more like one another. Thus, if MZ pairs are like one another because they are more likely to share environments, then this control should reduce the frailty variance estimate for MZ pairs more than for DZ pairs.
It is important to consider mortality selection among smokers and nonsmokers that may complicate comparisons across cohorts. The selection effects are potentially more influential among the older cohorts in our study. To examine the influence of selection, we weighted each case by age-specific relative risks of death for former and current smokers compared to nonsmokers. This technique has been used in previous research (Pampel 2005) and is based on life expectancies tabulated by Rogers and Powell-Griner (1991) from the National Health Interview and National Mortality Followback Surveys. For each age–gender combination, cumulative probabilities of dying among current and former smokers are divided by the cumulative probability of dying among nonsmokers. Nonsmokers receive a weight of 1, and then the final weights are divided by the sum of weights so that the average weight is 1.
References
- Boardman Jason D. State-Level Moderation of Genetic Tendencies to Smoke. American Journal of Public Health. 2009;99:480–86. doi: 10.2105/AJPH.2008.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman Jason D, Saint Onge Jarron M, Haberstick Brett C, Timberlake David S, Hewitt John K. Do Schools Moderate the Genetic Determinants of Smoking? Behavior Genetics. 2008;38(3):234–46. doi: 10.1007/s10519-008-9197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock Gregory, Goode Jamie., editors. Understanding Nicotine and Tobacco Addiction. Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- Brim Orville Gilbert, Baltes Paul B, Bumpass Larry L, Cleary Paul D, Featherman David L, Hazzard William R, Kessler Ronald C, Lachman Margie E, Markus Hazel Rose, Marmot Michael G, Rossi Alice S, Ryff Carol D, Shweder Richard A. National Survey of Midlife Development in the United States (MIDUS), 1995–1996 [MRDF], 2nd ICPSR version. Ann Arbor, MI: DataStat, Inc; Boston, MA: Harvard Medical School, Department of Health Care Policy [producers]; Ann Arbor, MI: Inter-University Consortium for Political and Social Research [distributor]; 1996. [Google Scholar]
- Brunzell Darlene. Neurochemistry of Nicotine Dependence. In: Karch Steven B., editor. Neurochemistry of Abused Drugs. Boca Raton, FL: CRC Press; 2008. pp. 23–28. [Google Scholar]
- Carey Gregory. Human Genetics for the Social Sciences. Thousand Oaks, CA: Sage; 2003. Retrieved December 20, 2008 ( http://psych.colorado.edu/~carey/hgss/hgssadvanced/TwinConcordance.pdf) [Google Scholar]
- Carmelli Dorit, Swan Gary E, Robinette Dennis, Fabsitz Richard R. Genetic Influence on Smoking: A Study of Male Twins. New England Journal of Medicine. 1992;327(12):829–33. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- Cherny Stacey S, DeFries John C, Fulker David W. Multiple-Regression Analysis of Twin Data: A Model-Fitting Approach. Behavior Genetics. 1992;22:489–97. doi: 10.1007/BF01066617. [DOI] [PubMed] [Google Scholar]
- Clayton DG. Model for Association in Bivariate Life Tables and its Application in Epidemiological Studies of Familial Tendency in Chronic Disease Incidence. Biometrika. 1978;65:141–51. [Google Scholar]
- Cleveland H Harrington. Disadvantaged Neighborhoods and Adolescent Aggression: Behavioral and Genetic Evidence of Contextual Effects. Journal of Research on Adolescence. 2003;13:211–38. [Google Scholar]
- Cockerham William C. The Sociology of Health Behavior and Health Lifestyles. In: Bird Chloe E, Conrad Peter, Fremont Allen M., editors. Handbook of Medical Sociology. Upper Saddle River, NJ: Prentice Hall; 2000. pp. 159–72. [Google Scholar]
- DeFries John C, Fulker David W. Multiple-Regression Analysis of Twin Data. Behavior Genetics. 1985;15:467–73. doi: 10.1007/BF01066239. [DOI] [PubMed] [Google Scholar]
- Forey Barbara, Hamling Jan, Lee Peter, Wald Nicholas. International Smoking Statistics: WEB Edition. P. N. Lee Statistics and Computing Ltd; 2007. Retrieved April 8, 2008 ( http://www.pnlee.co.uk/ISS.htm) [DOI] [PubMed] [Google Scholar]
- Gu Dongfeng, Hinks Leslie J, Morton Newton E, Day Ian NM. The Use of Long PCR to Confirm Three Common Alleles at the CYP2A6 Locus and the Relationship between Genotype and Smoking Habit. Annals of Human Genetics. 2000;64:383–90. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- Guo Guang, Rodriguez German. Estimating a Multivariate Proportional Hazard Model for Clustered Data Using the EM Algorithm, with an Application to Child Survival in Guatemala. Journal of the American Statistical Association. 1992;87(420):969–76. [Google Scholar]
- Guo Guang, Tong Yuying. Age at First Sexual Intercourse, Genes, and Social Context: Evidence from Twins and the Dopamine d4 Receptor Gene. Demography. 2006;43(4):747–69. doi: 10.1353/dem.2006.0029. [DOI] [PubMed] [Google Scholar]
- Hall Wayne, Madden Pamela, Lynskey Michael T. The Genetics of Tobacco Use: Methods, Findings and Policy Implications. Tobacco Control. 2002;11(2):119–24. doi: 10.1136/tc.11.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha Prabhat, Peto Richard, Zatonski Witold, Boreham Jillian, Jarvis Martin J, Lopez Alan D. Social Inequalities in Male Mortality, and in Male Mortality from Smoking: Indirect Estimation from National Death Rates in England and Wales, Poland, and North America. The Lancet. 2006;368:367–70. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- Kendall Maurice G. Rank Correlation Methods. London: Griffin; 1962. [Google Scholar]
- Kendler Kenneth S, Neale Michael C, Kessler Ronald C, Heath Andrew C, Eaves Lindon J. A Test of the Equal Environment Assumption in Twin Studies of Psychiatric Illness. Behavior Genetics. 1993;23(1):21–27. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- Kendler Kenneth S, Thornton Laura M, Pedersen Nancy L. Tobacco Consumption in Swedish Twins Reared Apart and Reared Together. Archives of General Psychiatry. 2000;579:886–92. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- Kessler Ronald C, Gilman Stephen E, Thornton Laura M, Kendler Kenneth S. Health, Well-Being, and Social Responsibility in the MIDUS Twin and Sibling Subsamples. In: Brim OG, Ryff CD, Kessler RC, editors. How Healthy Are We? A National Study of Well-Being at Midlife. Chicago, IL: University of Chicago Press; 2004. pp. 124–52. [Google Scholar]
- Koopmans Judith R, Slutske Wendy S, Heath Andrew C, Neale Michael C, Boomsma Dorret I. The Genetics of Smoking Initiation and Quantity Smoked in Dutch Adolescent and Young Adult Twins. Behavior Genetics. 1999;29:383–93. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Lange Christoph, Silverman Edwin K, Xu X, Weiss Scott T, Laird Nan M. A Multivariate Family-Based Association Test Using Generalized Estimating Equations: FBAT-GEE. Biostatistics. 2003;4:195–306. doi: 10.1093/biostatistics/4.2.195. [DOI] [PubMed] [Google Scholar]
- Lerman Caryn, Caporaso Neil E, Audrain Janet, Main David, Bowman Elise D, Lockshin Benjamin, Boyd Neil R, Shields Peter G. Evidence Suggesting the Role of Specific Genetic Factors in Cigarette Smoking. Health Psychology. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- Li Ming D. Identifying Susceptibility Loci for Nicotine Dependence: 2008 Update Based on Recent Genome-Wide Linkage Analyses. Journal of Human Genetics. 2008;123(2):119–31. doi: 10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- Li Ming D, Cheng Rong, Ma Jennie Z, Swan Gary E. A Meta-Analysis of Estimated Genetic and Environmental Effects on Smoking Behavior in Male and Female Adult Twins. Addiction. 2003;98(1):23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Link Bruce G. Epidemiological Sociology and the Social Shaping of Population Health. Journal of Health and Social Behavior. 2008;49(4):367–84. doi: 10.1177/002214650804900401. [DOI] [PubMed] [Google Scholar]
- Link Bruce G, Phelan Jo. Social Conditions as Fundamental Causes of Disease. Journal of Health and Social Behavior. 1995;(extra issue):80–94. [PubMed] [Google Scholar]
- Madden Pamela AF, Heath Andrew C, Pedersen Nancy L, Kaprio Jaako, Koskenvuo Markku J, Martin Nicholas G. The Genetics of Smoking Persistence in Men and Women: A Multicultural Study. Behavior Genetics. 1999;29(6):423–31. doi: 10.1023/a:1021674804714. [DOI] [PubMed] [Google Scholar]
- Oakes David. A Model for Association in Bivariate Survival Data. Journal of the Royal Statistical Society, Series B. 1982;51:127–38. [Google Scholar]
- Pampel Fred C. Age and Education Patterns of Smoking among Women in High-Income Nations. Social Science & Medicine. 2003;57(8):1505–14. doi: 10.1016/s0277-9536(02)00543-9. [DOI] [PubMed] [Google Scholar]
- Pampel Fred C. Diffusion, Cohort Change, and Social Patterns of Smoking. Social Science Research. 2005;34(1):117–39. doi: 10.1016/j.ssresearch.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin Andrew J, Lee Hedwig. The Under-theorized Environment: Sociological Theory and the Ontology of Behavioral Genetics. Sociological Perspectives. 2007;50(2):303–22. [Google Scholar]
- Pianezza Micahel L, Sellers Edward M, Tyndale Rachel F. Nicotine Metabolism Defect Reduces Smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- Plomin Robert, DeFries John, McClearn Gerald, McGuffin Peter. Behavioral Genetics. 5. New York: Worth Publishers; 2008. [Google Scholar]
- Purcell Shaun. Variance Components Models for Gene-Environment Interaction in Twin Analysis. Twin Research. 2002;5:554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Purcell Shaun. Statistical Methods in Behavioral Genetics. In: Robert Plomin, DeFries John, McClearn Gerald, McGuffin Peter., editors. Appendix in Behavioral Genetics. 5. New York: Worth Publishers; 2008. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- Raine Adrian. Biosocial Studies of Antisocial and Violent Behavior in Children and Adults: A Review. Journal of Abnormal Child Psychology. 2002;30(4):311–26. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rende Richard. Genes, Environment, and Addictive Behavior: Etiology of Individual Differences and Extreme Cases. Addiction. 1993;88:1183–88. doi: 10.1111/j.1360-0443.1993.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Rodgers Joseph Lee, McGue Matt. A Simple Algebraic Demonstration of the Validity of DeFries-Fulker Analysis in Unselected Samples with Multiple Kinship Levels. Behavior Genetics. 1994;24:259–62. doi: 10.1007/BF01067192. [DOI] [PubMed] [Google Scholar]
- Rogers Richard G, Powell-Griner Eve. Life Expectancies of Cigarette Smokers and Nonsmokers in the United States. Social Science & Medicine. 1991;32:1151–59. doi: 10.1016/0277-9536(91)90092-q. [DOI] [PubMed] [Google Scholar]
- Rowe David C, Almeida David M, Jacobson Kristen C. School Context and Genetic Influences on Aggression in Adolescence. Psychological Science. 1999;10:277–80. [Google Scholar]
- Rutter Michael. Genes and Behavior: Nature-Nurture Interplay Explained. London: Blackwell; 2006. [Google Scholar]
- Rutter Michael. Biological Implications of Gene-Environment Interaction. Journal of Abnormal Child Psychology. 2008;36:969–75. doi: 10.1007/s10802-008-9256-2. [DOI] [PubMed] [Google Scholar]
- Shanahan Michael J, Hofer Scott M. Social Context in Gene-Environment Interactions: Retrospect and Prospect. Journals of Gerontology: Series B. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Sobel Robert. They Satisfy: The Cigarette in American Life. New York: Anchor Press/Doubleday; 1978. [Google Scholar]
- Sullivan Patrick F, Kendler Kenneth S. The Genetic Epidemiology of Smoking. Nicotine & Tobacco Research. 1999;1:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Timberlake David S, Rhee Soo Hyun, Haberstick Brett C, Hopfer Christian, Ehringer Marissa, Lessem Jeffrey M, Smolen Andrew, Hewitt John K. The Moderating Effects of Religiosity on the Genetic and Environmental Determinants of Smoking Initiation. Nicotine & Tobacco Research. 2006;8(1):123–33. doi: 10.1080/14622200500432054. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services [USD-HHS] The Health Consequences of Involuntary Smoking: A Report of the Surgeon General. 1986 Retrieved April 8, 2008 ( http://profiles.nlm.nih.gov/NN/B/C/P/M/_/nnbcpm.pdf)
- U.S. Department of Health and Human Services [USD-HHS] The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General. Rockville, MD: Public Health Service, Office of the Surgeon General; 1988. [Google Scholar]
- U.S. Department of Health and Human Services [USD-HHS] Youth and Tobacco: Preventing Tobacco Use among Young People: A Report of the Surgeon General. Rockville, MD: Public Health Service, Office on Smoking and Health; 1994. [Google Scholar]
- U.S. Department of Health and Human Services [USD-HHS] Tobacco Use among U.S. Racial/Ethnic Minority Groups: A Report of the Surgeon General. Rockville, MD: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998. [Google Scholar]
- U.S. Department of Health and Human Services [USD-HHS] Reducing Tobacco Use: A Report of the Surgeon General. Washington, DC: Department of Health and Human Services, U.S. Public Health Service; 2000. [Google Scholar]
- U.S. Department of Health and Human Services [USD-HHS] Women and Smoking: A Report of the Surgeon General. Washington, DC: Department of Health and Human Services, U.S. Public Health Service; 2001. [Google Scholar]
- U.S. Department of Health, Education, and Welfare [USDHEW] The Health Consequences of Smoking: A Public Health Service Review. 1967 Retrieved April 8, 2008 ( http://profiles.nlm.nih.gov/NN/B/B/K/M/_/nnbbkm.pdf)
- U.S. Department of Health, Education, and Welfare [USDHEW] The Health Consequences of Smoking: 1969 Supplement to the 1967 Public Health Service Review. 1969 Retrieved April 8, 2008 ( http://profiles.nlm.nih.gov/NN/B/B/L/H/_/nnbblh.pdf)
- U.S. Department of Health, Education, and Welfare [USDHEW] The Health Consequences of Smoking. 1973 Retrieved April 8, 2008 ( http://profiles.nlm.nih.gov/NN/B/B/P/M/_/nnbbpm.pdf)
- Vink Jacqueline M, Willemsen Gonneke, Boomsma Dorret I. Heritability of Smoking Initiation and Nicotine Dependence. Behavior Genetics. 2005;35(4):397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Warner Kenneth E, Mendez David, Alshanqeety Omar. Tobacco Control Success versus Demographic Destiny: Examining the Causes of the Low Smoking Prevalence in California. American Journal of Public Health. 2008;98:268–69. doi: 10.2105/AJPH.2007.112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman Marvin. Sensation Seeking and Risky Behavior. Washington, DC: American Psychological Association; 2007. [Google Scholar]