Abstract
This research examined whether variations in salivary measures of the hypothalamic-pituitary-adrenal axis (cortisol) and autonomic nervous system (alpha amylase [sAA]) contribute to individual differences in the association between peer victimization and aggression. Children (N = 132; M age = 9.46 years, SD = .33) completed a measure of peer victimization, teachers rated children’s aggression, and children’s saliva was collected prior to, and following, participation in a laboratory-based peer-oriented social challenge task. Children rated their level of frustration at the end of the task. Results revealed that victimization interacted with cortisol and sAA measured in anticipation of the task to predict aggression; the victimization × cortisol contribution to aggression was partly mediated by children’s self-reported frustration level. Victimization also was associated with heightened frustration in girls with high task-related sAA reactivity. Task-related sAA reactivity was associated with heightened aggression, but only for girls. These findings suggest that associations between peer victimization and aggression are moderated by variation in the activity of the major components of the psychobiology of stress; results are discussed in relation to theoretical models of individual differences in biological sensitivity to context.
Keywords: peer victimization, aggression, cortisol, salivary alpha amylase
Peer victimization is a major public health concern. In fact, 10–20% of school children are repeatedly victimized, with many more experiencing periodic victimization (Graham & Juvonen, 1998; Solberg & Olweus, 2003). This victimization can take the form of physical (e.g., hitting), verbal (e.g., name-calling), or psychological (e.g., manipulation) abuse. Unfortunately, this maltreatment can lead to a cycle of violence, wherein victimized youth retaliate against their aggressors (Salmivalli, Karhunen, & Lagerspetz, 1996) or develop more global conduct problems (Sullivan, Farrell, & Kliewer, 2006), yet not all victimized children respond aggressively. Surprisingly little is known about why some victimized youth become aggressors whereas others do not. Understanding the sources of such individual differences can facilitate the early identification of at-risk children, and help focus intervention efforts on interrupting this cycle of violence. In the field of developmental psychopathology, an emerging theme is that individual differences in the psychobiology of the stress response moderate the link between adversity and subsequent adjustment (Scarpa & Ollendick, 2003). Accordingly, the present study used a biobehavioral approach to elucidate why victimization is more strongly associated with aggression in some children than others.
The Biology of Aggression
Much research has examined the biology of aggressive behavior (for reviews, see Scarpa & Raine, 2007; van Goozen, Fairchild, Snoek, & Harold, 2007). This research focuses, in part, on individual differences in arousal and reactivity of the two major psychobiological components of the stress response: the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS). The HPA axis is a slow-acting system; activation of this system triggers a cascade of events that begins with the secretion of corticotropin releasing hormone by the hypothalamus and culminates in the release of cortisol by the adrenal glands into the bloodstream. The ANS is a fast-acting system that is responsible for the classic “fight or flight” response (Chrousos & Gold, 1992), eventuating in the release of catecholamines into the bloodstream.
The HPA axis and ANS are distinct but interrelated systems that provide specialized adaptive biobehavioral responses to environmental change, threat, and challenge (Chrousos & Gold, 1992). Both are reactive to psychological stress, especially subjective interpretations rather than objective features of events (Lundberg & Frankenhaeuser, 1980), and have implications for physical and mental health but there are some key differences in how these systems respond. HPA activation is thought of as a “defeat action,” or a passive response to novel or unpredictable stressors, particularly those perceived as uncontrollable (Lundberg & Frankenhaeuser, 1980). HPA activation typically is linked to negative emotions (Gunnar & Vazquez, 2006). Activation of the sympathetic branch (SNS) of the ANS is thought of as the body’s active, defensive reaction to imposing threats, particularly those perceived as controllable (Henry, 1992), and is believed to reflect generalized alertness, arousal, or vigilance and active engagement with one’s environment (Schachter & Singer, 1962). Given these different but coordinated regulatory roles, Bauer and colleagues (2002) underscore the importance of examining both systems when attempting to link individual variation in the psychobiology of the stress response to children’s externalizing problem behavior.
HPA axis and aggression
Research examining HPA activity and aggression in youth has yielded inconsistent results (for a review, see van Goozen et al., 2007). Whereas some research links aggression with underactivation, as reflected in lower basal levels (Shirtcliff, Granger, Booth, & Johnson, 2005; van Goozen et al., 1998) and dampened reactivity (Gordis et al., 2006; van Goozen et al., 1998), other research links aggression with overactivation, as reflected in higher basal levels (Murray-Close, Han, Cicchetti, Crick, & Rogosch, 2008; van Bokhoven, van Goozen et al., 2005) and heightened reactivity (Lopez-Duran et al., 2009; Scarpa, Fikretoglu, & Luscher, 2000; Susman et al., in press; van Goozen et al., 1998). Many studies also fail to find an association between cortisol and aggression (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001; Scerbo & Kolko, 1994).
ANS and aggression
Research exploring the link between ANS activity and aggression in youth also has yielded equivocal results. Generally, low resting heart rate (reflecting the coordinated effects of input from both the parasympathetic and sympathetic branches of the ANS; El Sheikh et al., 2009) and/or skin conductance (representing SNS activation specifically; El Sheikh et al., 2009) is linked to more aggression (van Bokhoven, Mattys, van Goozen, & van Engeland, 2005; van Goozen et al., 1998; for a review, see Raine 2002). Some research links dampened skin conductance reactivity to aggression (Zahn & Kruesi, 1993) whereas other research links heightened heart rate reactivity to aggression (van Goozen et al., 1998). Meta-analyses reveal that aggressive and antisocial behavior is more consistently associated with lower resting heart rate and skin conductance but increased heart rate reactivity (Lorber, 2004; Ortiz & Raine, 2004).
Recently, developmental scientists have shown a renewed interest in salivary alpha amylase (sAA), an enzyme produced by the salivary glands, as a surrogate marker of SNS activity (Granger et al., 2007). There is debate as to whether sAA is linked specifically to SNS activity or to ANS activation more generally (Nater et al., 2005). Research shows that sAA levels increase in response to physical, nocioceptive, and social-psychological stressors (Nater & Rohleder, 2009) and are positively correlated with norepinephrine (Chatterton et al., 1997; cf. Nater et al., 2006), pre-ejection period (West et al., 2006), skin conductance (El-Sheikh, Buckhalt, Erath, Granger, & Mize, 2008), heart rate, and blood pressure (Stroud et al., 2009). sAA levels also are associated with genetic polymorphisms in the catchol-O-methlytransferase (COMT) enzyme, which plays a crucial role in the central degradation of epinephrine and norepinephrine in postsynaptic neurons (Frigerio et al., 2009). Multiple studies show that individual differences in sAA are associated with children’s behavior and affective states. For example, one study revealed that preschoolers with high basal sAA activation showed more positive affect and approach behavior in a situation that primarily elicited these behaviors, suggesting that sAA activation may reflect sensitivity to context (Fortunato et al., 2008). Another study revealed a curvilinear association between basal sAA and externalizing symptoms, such that both low and high sAA levels predicted more symptoms across two years (Keller & El-Sheikh, 2009). The precise nature of the link between sAA and aggression differs across studies (El-Sheikh et al., 2008; Gordis, Granger, Susman, & Trickett, 2006; Susman et al., in press).
A Person x Environment Model
Discrepancies regarding the link between biological activity and aggression have been attributed in part to methodological differences (van Goozen et al., 2007), but also may stem from a failure to consider the context of aggressive behavior (Scarpa & Ollendick, 2003). That is, biological activity may not have a consistent direct effect on aggression but rather may moderate how individuals respond to stressful contexts (El Sheikh et al., 2009; Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010). This contextualized approach may help reconcile differing theoretical perspectives on aggression.
Underarousal theories of aggression (Raine, 2002; van Goozen et al., 2007) suggest that chronic under-activation of the HPA axis and ANS (parasympathetic and sympathetic) drives low levels of fear (fearlessness theory) or efforts to bring individuals toward an optimal level of arousal (sensation-seeking theory); fearlessness and sensation-seeking, in turn, are presumed to trigger proactive aggression, which is a goal-driven behavior that occurs without provocation and is reinforced by reward contingencies (Crick & Dodge, 1996). Overarousal theories of aggression (Keller & El-Sheikh, 2009; Lopez-Duran et al., 2009; Scarpa & Ollendick, 2003; Scarpa & Raine, 1997; van Goozen et al., 1998) suggest that over-reactivity of both systems elicits negative emotionality (e.g., frustration, anger) in response to threat or maladaptive approach-oriented engagement with negative events; negative emotionality and a readiness to fight in response to threat, in turn, are presumed to trigger reactive aggression, which is an impulsive behavior that occurs following provocation or frustration and reflects a hypersensitivity to perceived threat. Physiological over-reactivity (e.g., heightened cortisol reactivity) may be especially likely to promote aggression in the context of an ecological stressor (Scarpa & Ollendick, 2003; Scarpa & Raine, 1997), perhaps due to the heightened vigilance and sensitivity to threat experienced by individual living in stressful contexts.
In support of overarousal theories, high heart rate and cortisol reactivity to provocation, threat, and frustration predict aggression, particularly reactive but not proactive aggression (Hubbard et al., 2002; Lopez-Duran et al., 2009; van Goozen et al., 1998). Few studies directly examine physiological reactivity × social context interactions, but some research in young adults reveals that the combination of heightened cortisol reactivity and exposure to victimization (e.g., physical abuse, community violence) predicts the highest levels of aggression (Scarpa, 1997; Scarpa & Ollendick, 2003). Moreover, opposing action of the sympathetic and parasympathetic nervous systems intensifies the effect of exposure to marital conflict on externalizing symptoms in children (El-Sheikh et al., 2009).
The present study examined the proposal that victimization would be linked to more aggression in children with heightened relative to blunted biological sensitivity (i.e., physiological reactivity to stress). Exposure to peer victimization creates a threatening and unpredictable environment; children with high biological sensitivity may react with aggression aimed at reducing perceived, anticipated, or actual social threats. Moreover, we explored whether perceived frustration mediated victimization × biological sensitivity contributions to aggression. That is, victimization may be more likely to predict frustration in response to social challenge for children with high biological sensitivity; this frustration may, in turn, account for elevated displayed aggression. In contrast, biological sensitivity may be innocuous (unrelated to aggression) or even beneficial (protective against aggression) in the absence of ecological threat (i.e., within low victimization environments) (Boyce & Ellis, 2005).
In particular, this study examined whether cortisol and sAA responses to a laboratory social stressor (a challenging interaction with an unfamiliar peer) moderated the link between peer victimization and the display of aggression against peers in the school context. We examined both anticipatory activation while awaiting a stressor (cortisol and sAA levels prior to the interaction), as well as task-related reactivity (cortisol and sAA changes across the interaction). Most research on biological reactivity explores individual differences in responses to stressors. Yet, recent research suggests that anticipatory activation is a key component of the stress response, especially in natural stressful situations (Afifi, Granger, Aldeis, Joseph, & Denes, 2009; Powers, Laurent, & Granger, 2009; Stroud et al., 2009). A heightened stress response during both the anticipation and the reactivity phases of the stressor may amplify the link between victimization and aggression. Alternatively, children with up-regulated stress-response systems may show peak activation during the anticipation phase, and thus not show reactivity to the challenge itself. In one study, adults showed peak cortisol and sAA responses in anticipation of an upcoming conflict (Powers et al., 2009). In two other studies, children showed high cortisol in anticipation of, but not in reaction to, social stressors (Klimes-Dougan et al., 2001; Stroud et al., 2009). Importantly, anticipatory activation in the context of a threatening peer stressor may generalize to the school setting. That is, victimized children with heightened anticipatory activation may be hyper-vigilant to threat, causing them to feel more frustrated and, consequently, to engage in aggressive behavior in response to even minor perceived incidents of provocation.
Method
Participants
Participants were 132 children (68 girls; 64 boys; M age = 9.46 years, SD = .33; 71.2% White, 28.8% minority) who were recruited from a study of peer victimization. Families were economically diverse as reflected in annual income (50.4% under $60,000, 25.6% $60,000 - 89,999, and 24.0% over $90,000). For the larger study, parent consent forms were given to all eligible 2nd graders across several schools. Of these children, 724 (93%) returned their consents, 576 (86%) of whom received consent and participated. Children provided verbal assent at the time of data collection. Participants and nonparticipants did not significantly differ in sex, χ2(1) = .15, ns, age, t(723) = .63, ns, ethnicity (White vs. minority), χ2(1) = .65, ns, or school lunch status (full pay vs. subsidized), χ2(1) = .35, ns.
Families were invited to participate in a supplemental study involving an interaction with an unfamiliar peer. Parents completed a survey of children’s use of over-the-counter and prescription medications; usage during the past 24 hours was confirmed at the assessment. Of the 318 families contacted, 239 indicated an interest in participating; of those, 132 participated and 107 did not due to scheduling conflicts or exclusion based on medication usage that might influence hormones (Granger, Hibel, Fortunato & Kapelewski, 2009). Both members of the dyad were participants, allowing for a naturally occurring interaction. Participants from different school districts were paired to ensure lack of familiarity between partners; otherwise, children were randomly assigned to same-sex dyads.
Procedure
During the winter of 3rd grade, children completed a measure of peer victimization, and teachers completed a measure of overt aggression. During the following summer and fall, children participated in a 3–4 hour session. Sessions occurred between 1:00 pm and 5:00 pm due to evidence that the HPA axis is more sensitive to stimulation later in the day (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009b). Parents and children provided written consent/assent for the supplemental study. Immediately prior to the social challenge task (Rudolph, Troop-Gordon, & Flynn, 2009), children provided a saliva sample. To ensure a lack of contact, dyadic partners were kept in separate rooms prior to the interaction.
In the first phase, children were told that whoever constructed a copy of a block model would win a prize. They were given a set of blocks that was sufficient to complete only one model, and were allowed to build for nine minutes. In the second phase, children were informed that they would each receive a prize for their efforts, and were instructed to decide on the distribution of two prizes of noticeably unequal value. This ecologically valid task was intended to elicit a moderate amount of frustration when children realized they were unable to both complete a model and when they had to decide who would receive the better prize. Thus, it was expected that this task would trigger individual differences in children’s anticipation of a social stressor (i.e., an impending interaction with an unfamiliar peer) and reactivity to a social stressor (i.e., potential conflict and perceptions of threat, frustration, and uncontrollability). Children provided two more saliva samples 5 and 20 minutes after the task. Following the task, children reviewed a videotape of the interaction, and provided ratings of their emotion (see Measures). Participants were then debriefed and the one who had received the less valuable prize was given the opportunity to exchange it for a higher valued prize.
Measures
Peer victimization
Children completed a revision of the Social Experiences Questionnaire (Crick & Grotpeter, 1996). The original measure has 5-item subscales that assess overt (e.g., “How often do you get pushed or shoved by another kid?”) and relational (e.g., “How often do other kids leave you out on purpose when it’s time to play or do an activity?”) victimization; 11 items were added to tap other aspects of victimization, yielding a 21-item measure (α = .93). Children checked a box indicating how often they experienced each type of victimization on a 5-point scale. Scores were computed as the mean of the items. Research supports the validity of self-reports of victimization (Bollmer, Harris, & Milich, 2006; Graham & Juvonen, 1998; Ladd & Kochenderfer-Ladd, 2002).
Aggression
Teachers completed the overt aggression subscale of the Children’s Social Behavior Scale (Crick, 1996). This 4-item subscale assesses behaviors intended to harm others through physical damage or the threat of such damage (α = .93; 4 items; e.g., “This child hits or kicks peers.”). Teachers rated each item on a 5-point scale. Scores were computed as the mean of the items. Teacher reports of overt aggression correlate significantly with peer reports (Crick, 1996).
Frustration
Children watched a videotape of the interaction and rated their level of frustration at the end of the task on a 5-point scale. Prior research supports the use of self-reported emotions to assess subjective responses to laboratory social stressors (Stroud et al., 2009). Moreover, in another study using the same task as the present one (Rudolph et al., 2009), self-reported frustration was significantly associated with observer reports of emotion dysregulation. Thus, these self-reports appear to be valid indicators of emotional responses to challenge.
Medication usage
Parents completed a checklist of medication usage. Children were assigned a score of 1 if they had taken medications within the past 24 hours that may interfere with the biological assessments (e.g., steroidal or psychotropic medications) and a score of 0 if they had not. A few children (n = 7) received scores of 1 despite the original exclusionary efforts; these children were included in the study, but all analyses adjusted for medication usage. We also created a more inclusive medication variable reflecting intake of any medications with the past 24 hours. Because cortisol and sAA scores were more strongly correlated with the less inclusive than the more inclusive medication variable, we adjusted for the less inclusive variable in our analyses.
Saliva sample collection and analysis
Saliva samples were collected and handled following Granger and colleagues (2007). All assessments were conducted between 1:00 pm and 5:00 pm to control the influence of the natural diurnal variation in cortisol production. To ensure against contamination from food consumption, children were instructed not to eat for 1 hour prior to their assessment (Gibson et al., 1999). Children provided three saliva samples: immediately prior to the task (pre-task), 5 minutes after the task (to tap sAA reactivity), and 20 minutes after the task (to tap cortisol reactivity). On average, the pre-task sample was collected at 2:19 pm (SD = 33.87 minutes). Children donated whole saliva by passive drool into a 2 mL cryogenic vial. Samples were frozen at −20 to − 80 C until shipped overnight on dry ice to Salimetrics laboratories where they were stored at − 80 C. On the day of assay, samples were brought to room temperature, centrifuged at 3,000 RPM for 15 min, and the clear top-phase of the sample was pipetted into appropriate test tubes. All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay US FDA (510k) cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, PA). The test used 25 μl of saliva, had a lower limit of sensitivity of .007 μg/dl, with a range of sensitivity from .007 to 3.0 μg/dl. Samples were assayed in duplicate; average intra-and inter-assay coefficients of variation were less than 5% and 10%. Averaged duplicate scores were used in all statistical analyses. The assay for sAA employed a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2 min. period) in absorbance at 405 nm. Results are computed in U/mL of sAA using the formula: [Absorbance difference per minute x total assay volume (328 ml) x dilution factor (200)]/[millimolar absorptivity of 2-chloro-p-nitrophenol (12.9) x sample volume (.008 ml) x light path (.97)]. Following Granger and colleagues (2007), all samples were assayed in singlet. When samples and controls are assayed in duplicate using this protocol, intra-and inter-assay coefficients of variation are less than 5% and 10%.
Because pre-task cortisol and sAA distributions were positively skewed, cortisol scores were log-transformed and sAA scores were subjected to a square-root transformation. Statistical outliers (+/− 3 SD) were then recoded to the next highest value in the distribution. To ease interpretation, descriptive statistics (Table 1) are provided on the raw scores. Analyses of anticipatory activation are based on the transformed scores. Given the distinct differences in the kinetic reactivity profiles for the HPA axis and ANS (Granger et al., 2007), cortisol reactivity was computed as the difference between the raw cortisol scores from the third (20 min. post-task) and first (pre-task) assessments, and sAA reactivity was computed as the difference between the raw sAA scores from the second (5 min. post-task) and first (pre-task) assessments (Gordis et al., 2006). Higher scores reflected more reactivity.
Table 1.
Descriptive Statistics and Bivariate Correlations
| M | SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| 1. Pre-task cortisol | .10 | .07 | --- | ||||||||
| 2. Post-task cortisol | .10 | .08 | .57*** | --- | |||||||
| 3. Cortisol reactivity | −.01 | .05 | −.34*** | .52*** | --- | ||||||
| 4. Pre-task sAA | 100.02 | 72.13 | .06 | −.04 | −.09 | --- | |||||
| 5. Post-task sAA | 94.50 | 71.53 | .20* | .08 | −.12 | .78*** | --- | ||||
| 6. sAA reactivity | −5.86 | 53.05 | .18* | .16 | −.02 | −.38*** | .27** | --- | |||
| 7. Peer victimization | 1.92 | .62 | .00 | −.09 | −.14 | −.01 | .04 | .05 | --- | ||
| 8. Frustration | 1.56 | 1.15 | .00 | .02 | .02 | −.18* | −.14 | .04 | .13 | --- | |
| 9. Aggression | 1.40 | .79 | .13 | .06 | −.07 | .04 | .17 | .14 | .26** | .27** | --- |
Note. Means and standard deviations are presented for pre-task and post-task raw scores. Bivariate correlations are based on transformed, windsorized scores.
p < .05.
p < .01.
p < .001.
Results
Overview of Analyses
For descriptive purposes, we examined mean level and individual differences in cortisol and sAA change across the task, as well as bivariate correlations among the variables. We conducted hierarchical multiple regression analyses to examine the independent and interactive contributions of victimization and pre-task cortisol/sAA to frustration and aggression. Because post-task scores were nested within dyad (Kenny, Kashy, & Cook, 2006), we conducted hierarchical linear modeling (HLM; Bryk & Raudenbush, 1992) analyses to examine the independent and interactive contributions of victimization and cortisol/sAA reactivity to frustration and aggression. Intercepts were set as random factors; slopes were set as fixed factors. We also conducted tests of mediated moderation using the approach proposed by Muller and colleagues (Muller, Judd, & Yzerbyt, 2005) to examine whether children’s self-reported frustration mediated the victimization × cortisol/sAA to aggression.
Each analysis adjusted for medication status (0 = no medication; 1 = medication) and time of day. Mean-centered victimization and cortisol/sAA scores and their interaction were included as predictors. Separate analyses were conducted for cortisol and sAA. Significant interactions were decomposed by conducting simple slope analyses for victimization at +1, 0, and −1 SD from the mean of the moderator (Aiken & West, 1991; Bauer & Curran, 2005). To quantify the effects, we examined the SD difference in frustration and aggression for high versus low levels of cortisol/sAA at low and high levels of victimization. Because research suggests possible sex differences in the biological correlates of aggression (Beauchaine, Hong, & Marsh, 2008; Cicchetti & Rogosch, 2001), we included relevant interactions with sex in the analyses. When nonsignificant, results are collapsed across sex. Of the 132 participants, four were missing pre-task cortisol and sAA, and were omitted from the regressions. Because HLM can accommodate missing data, all children were included in those analyses.
Mean Levels and Individual Differences in Reactivity of Cortisol and sAA
Table 1 presents the means and SDs of cortisol and sAA for the two measurements. Paired-samples t-tests revealed no mean change from pre- to post-task for cortisol, t(126) = 1.35, ns, or sAA, t(126) = 1.25, ns. This absence of mean level increases is consistent with research examining cortisol and sAA responses to peer rejection (Stroud et al., 2009) and social-evaluative threat (Yim, Granger, & Quas, in press) in children. Given our focus on individual variation in stress responses, we also examined individual differences in change across the task by computing how many children showed a substantive (at least 10%) change. For cortisol, 42 (32.1%) showed an increase and 57 (43.5%) showed a decrease at 20 minutes post-task. For sAA, 49 (37.4%) children showed an increase and 48 (36.6%) children showed a decrease at 5 minutes post-task. These results are consistent with research suggesting that only a subset of children show reactivity to laboratory tasks (Gunnar, Talge, & Herrera, 2009a). Although the sample as a whole did not show significant reactivity, the fact that a subset of children showed an increase enabled us to examine the effect of individual differences in reactivity.
Bivariate Correlations
Table 1 presents intercorrelations among the variables. Cortisol and sAA levels were highly correlated within biomarker from pre- to post-task. Cortisol and sAA levels, as well as cortisol and sAA reactivity, were not associated across biomarker, except that pre-task cortisol was modestly correlated with post-task sAA and sAA reactivity. These modest associations are consistent with prior research (e.g., Gordis et al., 2006; Fortunato et al., 2008), and support the idea that these two stress-response systems provide primarily non-overlapping information. Given how reactivity scores were calculated, lower pre-task cortisol and sAA levels, and higher post-task cortisol and sAA levels, were associated with more reactivity. Aggression and peer victimization were not significantly associated with any of the cortisol or sAA variables, but were significantly intercorrelated. Frustration was associated with lower pre-task sAA and more aggression.
Anticipatory (Pre-Task) Cortisol and sAA Activation as Moderators of Peer Victimization
Cortisol and frustration
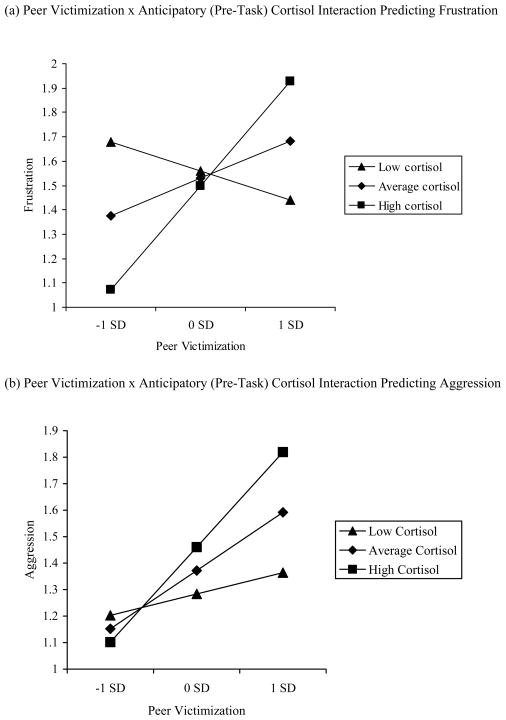
The regression analysis for anticipatory cortisol activation predicting child-reported frustration during the task revealed nonsignificant main effects of victimization and cortisol and a significant Victimization × Cortisol interaction (Table 2). Decomposition of this interaction revealed that victimization was significantly associated with frustration at high, b = .69, t(123) = 3.04, p < .01, but not average, b = .25, t(123) = 1.53, ns, or low, b = −.19, t(123) = −.85, ns, levels of cortisol (Figure 1a). At high levels of victimization, children with heightened cortisol levels had frustration scores .42 SDs greater than children with dampened cortisol levels. At low levels of victimization, children with dampened cortisol levels had frustration scores .53 SDs greater than children with heightened cortisol levels. These effects were not significantly moderated by sex.
Table 2.
Contribution of Peer Victimization, Anticipatory (Pre-Task) Cortisol and sAA Activation, and their Interaction to the Prediction of Frustration and Aggression
| Predictor | Cortisol
|
sAA
|
||
|---|---|---|---|---|
| b | ΔR2 | b | ΔR2 | |
| Frustration
|
||||
| Step 1: | .02 | .02 | ||
| Medication | .66 | .66 | ||
| Time of day | .00 | .00 | ||
| Step 2: | .02 | .05* | ||
| Victimization | .25 | .25 | ||
| Anticipatory Activation | −.04 | −.06* | ||
| Step 3: | .06** | .00 | ||
| Victimization × Anticipatory Activation | .89** | −.02 | ||
|
| ||||
| Aggression
|
||||
| Step 1: | .04† | .04† | ||
| Medication | .70* | .70* | ||
| Time of day | .00 | .00 | ||
| Step 2: | .09** | .07** | ||
| Victimization | .35** | .35** | ||
| Anticipatory Activation | .19 | .01 | ||
| Step 3: | .06* | .07** | ||
| Frustration | .12* | .16** | ||
| Victimization × Anticipatory Activation | .34 | .06* | ||
p < .10.
p < .05.
p < .01.
p < .001.
Note. Coefficients are unstandardized bs. Measurement units: ug/dL (cortisol) and U/mL (sAA).
Figure 1.
sAA and frustration
The regression analysis for anticipatory sAA activation predicting child-reported frustration during the task revealed a significant main effect of sAA, but nonsignificant effects of victimization and the Victimization × sAA interaction (Table 2). Heightened sAA levels were associated with less frustration. These effects were not significantly moderated by sex.
Cortisol and aggression
The regression analysis for anticipatory cortisol activation predicting teacher-reported aggression revealed significant main effects of victimization and frustration. Consistent with the premise that frustration mediates the joint influence of victimization and cortisol activation on aggression, the Victimization × Cortisol interaction was significant when frustration was not included in the regression, b = .45, t(124) = 2.12, p < .05, ΔR2 = .03, but was no longer significant with frustration included (Table 3). Decomposition of the original interaction revealed that victimization was significantly associated with aggression at high, b = .58, t(124) = 3.80, p < .001, and average, b = .35, t(124) = 3.22, p < .01, but not low, b = .13, t(124) = .13, ns, levels of cortisol (Figure 1b). At high levels of victimization, children with heightened cortisol levels had aggression scores .58 SDs greater than children with dampened cortisol levels. There was only a modest difference (.13 SDs) in aggression scores at low levels of victimization.
Table 3.
Contribution of Peer Victimization, Task-Related Cortisol and sAA Reactivity, and their Interaction to the Prediction of Frustration and Aggression
| Predictor | Cortisol | sAA |
|---|---|---|
| Frustration
|
||
| Medication | .58 | .31 |
| Time of day | .00 | .00 |
| Victimization | .24 | −.05 |
| Task-Related Reactivity | .79 | .00 |
| Victimization × Reactivity | −2.59 | .00a |
|
| ||
| Aggression
|
||
| Medication | .48 | .43 |
| Time of day | .00 | .00 |
| Victimization | .29** | .48** |
| Task-Related Reactivity | −.91 | .00a |
| Frustration | .15† | .14† |
| Victimization × Reactivity | −2.21 | .00 |
p < .10.
p < .05.
p < .01.
p < .001.
Note. Measurement units: ug/dL (cortisol) and U/mL (sAA).
Significant interaction with sex (see text).
These findings meet the conditions of mediated moderation (Muller et al., 2005): (a) a significant interaction between the main effect (victimization) and the moderator (cortisol) in the prediction of the outcome (aggression), (b) a significant interaction between the main effect and the moderator in the prediction of the mediator (frustration), and (c) the main effect of the mediator in the prediction of the outcome remains significant when controlling for the interaction between the main effect and the moderator, and the interaction between the main effect and the moderator is reduced. Providing further support of mediation, a Sobel (1982) test revealed a significant indirect effect of victimization on aggression through frustration at high levels of cortisol, Z = 1.67, p < .05, one-tailed. These findings suggest that victimization predicted more frustration in response to social challenge in children with heightened anticipatory cortisol levels, and frustration predicted more aggression.
sAA and aggression
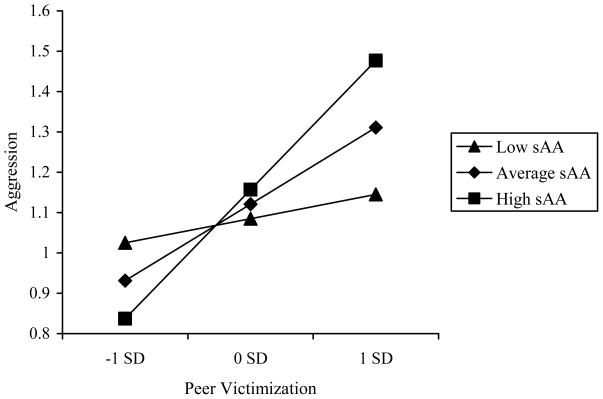
The regression analysis for anticipatory sAA activation predicting teacher-reported aggression revealed significant main effects of victimization and frustration. The main effect of victimization was qualified by a significant Victimization × sAA interaction (Figure 2). Decomposition of this interaction revealed that victimization was significantly associated with aggression at high, b = .51, t(123) = 3.36, p < .001, and average, b =.31, t(123) = 2.91, p < .01, but not low, b = .10, t(123) = .61, ns, levels of sAA. At high levels of victimization, children with heightened sAA levels had aggression scores .42 SDs greater than children with dampened sAA levels. At low levels of victimization, children with dampened sAA levels had aggression scores .24 SDs greater than children with heightened sAA levels. These effects were not significantly moderated by sex.
Figure 2.
Peer Victimization x Anticipatory (Pre-Task) sAA Interaction Predicting Aggression
Task-Related Cortisol and sAA Reactivity as Moderators of Peer Victimization
Cortisol and frustration
The HLM analysis for task-related cortisol reactivity predicting child-reported frustration during the task revealed no significant main or interactive effects of victimization or cortisol (Table 3); moreover, there were no significant interactions with sex.
sAA and frustration
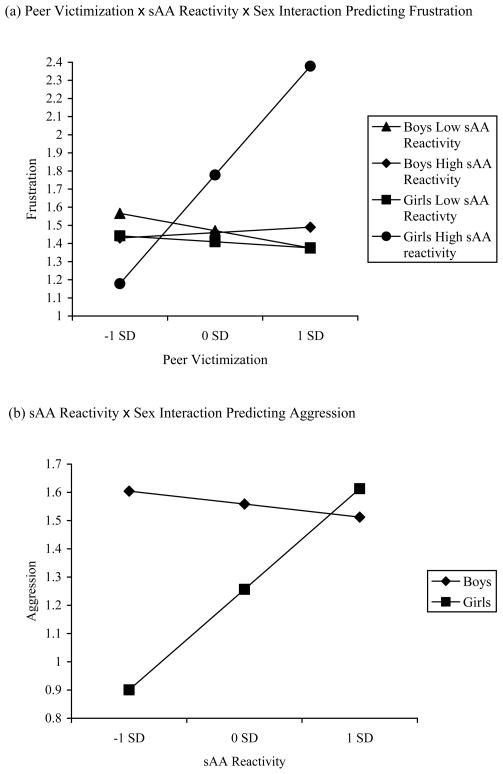
The HLM analysis for task-related sAA reactivity predicting child-reported frustration during the task revealed no significant main or interactive effects of victimization or sAA (Table 3). However, there was a significant Victimization × sAA × Sex interaction, coefficient = .01, t(117) = 1.99, p < .05. Decomposition of this interaction revealed that victimization was significantly associated with frustration for girls with high sAA reactivity, b = .96, t(123) = 2.60, p < .05, but not for girls with low sAA reactivity, b = −.05, t(123) = −.20, ns, boys with high sAA reactivity, b = .05, t(123) = .14, ns, or boys with low sAA reactivity, b = −.15, t(123) = −.69, ns (Figure 3a).
Figure 3.
Cortisol and aggression
The HLM analysis for task-related cortisol reactivity predicting teacher-reported aggression revealed a significant main effect of victimization and a marginal main effect of frustration but nonsignificant effects of cortisol and the Victimization × Cortisol interaction (Table 3). These effects were not significantly moderated by sex.
sAA and aggression
The HLM analysis for task-related sAA reactivity predicting teacher-reported aggression revealed a significant main effect of victimization and a marginal main effect of frustration but nonsignificant effects of sAA and the Victimization × sAA interaction (Table 3). However, there was a significant sAA × Sex interaction, coefficient = .01, t(116) = 2.85, p < .01. Decomposition of this interaction revealed that sAA reactivity was significantly associated with aggression in girls, b = .01, t(116) = 3.30, p < .01, but not boys, b = .00, t(116) = −.42, ns (Figure 3b).
Discussion
Understanding why some victimized children are aggressive yet others are not is critical for preventing an emerging cycle of violence. This study supported the idea that variations in biological sensitivity to social stress contribute to individual differences in aggressive behavior in the context of peer victimization. Specifically, victimization was associated with aggression in children who showed high but not low pre-task levels of cortisol and sAA while awaiting a social challenge. In contrast, this biological sensitivity had either innocuous or even some beneficial effects (i.e., protecting children against feeling frustrated and behaving aggressively) when children were exposed to low levels of peer victimization. Victimization also was associated with heightened frustration in response to social stress in girls with high sAA reactivity over the course of a stressor.
Moderational Role of Variations in Anticipatory (Pre-Task) Cortisol and sAA Activation
As expected, victimization was associated with more aggression in children with heightened but not dampened anticipatory cortisol and sAA activation. Victimized children are likely to show vigilance and cognitive sensitivity to social threat (Hazler, Carney, & Granger, 2006; Lochman & Dodge, 1998). If these children have highly reactive biological stress-response systems, they may be prone to frustration and maladaptive approach-oriented behavior (e.g., a readiness to fight) in response to threat, resulting in aggression (Keller & El-Sheikh, 2009; Scarpa & Raine, 1997; van Goozen et al., 1998). Indeed, children’s self-reported frustration partially mediated the victimization × cortisol contribution to aggression. These results are consistent with research suggesting that cortisol reactivity is associated with more negative mood states (e.g., frustration, anger) following uncontrollable stress (Scarpa, Friedman, Smalley, & Luscher, 1997), and, more specifically, high levels of cortisol predict more anger about bullying experiences (Hamilton, Newman, Delville, & Delville, 2008).
We examined anticipatory cortisol and sAA activation by informing children that they would be interacting with an unfamiliar peer. In victimized children, high levels of activation in this context may reflect a hyper-alertness to social threat. In fact, it has been suggested that children with up-regulated stress-response systems are more vigilant and have a lower threshold for anticipating threat in ambiguous or unfamiliar situations (Boyce & Ellis, 2005). This hyper-alertness may prompt maladaptive approach-oriented efforts to cope with social stressors. If victimized children show this anticipatory activation in daily social situations, they may react to perceived or actual threat by striking out aggressively against peers. This interpretation is consistent with overarousal theories of aggression, which suggest that activation of psychobiological stress responses promotes aggression when faced threatening or provoking contexts (Scarpa & Raine, 1997; van Goozen et al., 1998). Our findings also are consistent with research linking aggression to HPA and ANS activation in the context of stress, including provocation and frustration in the laboratory (Lopez-Duran et al., 2009; van Goozen et al., 1998) and naturally occurring ecological stressors such as victimization (Scarpa & Ollendick, 2003).
Although heightened cortisol and sAA played a similar role (amplifying aggression) at high levels of victimization, findings diverged somewhat across these two indexes at low levels of victimization. For sAA but not cortisol, heightened anticipatory activation seemed to be somewhat protective against aggressive behavior. It has been suggested that activation of the ANS reflects active engagement, either positive or negative, with one’s social context (Schachter & Singer, 1962). For low-victimized children, activation may foster a positive engagement with the environment, thereby suppressing aggressive behavior. Notably, this bivalent, context-dependent effect of high activation may help to reconcile discrepant findings in past research concerning the link between ANS activation and aggression. Heightened anticipatory activation of the HPA axis also seemed to be protective against self-reported frustration at low levels of victimization. Collectively, these results suggest that the meaning of heightened HPA and ANS activation depends on children’s contexts.
Moderational Role of Variations in Cortisol and sAA Task-Related Reactivity
Contrary to expectations, task-related cortisol reactivity did not moderate the association between victimization and frustration or aggression. Our goal was to examine activation in the context of an ecologically meaningful stressor. Although the interaction task was designed to be somewhat stressful, there was likely individual variation in the actual nature of the interaction. Thus, children’s anticipation of the interaction may have triggered more of a sense of threat than the interaction itself. Indeed, other research suggests that measuring anticipatory activation may be essential for understanding psychobiological responses to naturalistic stressors (Afifi et al., 2009; Klimes-Dougan et al., 2001; Powers et al., 2009; Stroud et al., 2009).
Task-related sAA reactivity did moderate the link between victimization and frustration in girls; heightened sAA reactivity also was significantly associated with aggression in girls. Inspection of the graphs revealed that dampened sAA reactivity protected girls from experiencing frustration (at high levels of victimization) and from engaging in aggression, yet heightened sAA reactivity was somewhat protective against frustration at low levels of victimization. It has been suggested that stressors involving social threat typically induce a “tend and befriend” response in females (Taylor et al., 2000) that involves downregulation of psychobiological stress responses and an effort to promote social relationships. Thus, it may be that girls who can effectively manage interpersonal challenges (such as a challenging interaction with an unfamiliar peer), as reflected in dampened sAA reactivity, represent a gender-normative group that displays fairly low levels of aggression, whereas girls who mount a strong sAA response to interpersonal challenge reflect a gender-atypical group of children who are less adept at coping with social stress, particularly if they have been previously victimized, and consequently feel more frustrated and resort to aggression. More research is clearly needed to better understand sex differences in the link between autonomic responses to challenge and aggression but this study provides preliminary data suggesting that a heightened sAA response to social stress represents a risk factor for girls, perhaps especially those with a history of victimization.
Implications for Theories of Biological Sensitivity to Stress
Broadly, this research contributes to theories of individual differences in reactivity to the environment. The findings are consistent with two different models (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007): (a) a dual risk model, and (b) a differential susceptibility model. According to a dual risk model, compared to less vulnerable individuals, more vulnerable individuals (e.g., those with particular genetic, biological, or temperamental profiles) show more maladaptive outcomes in the context of high stress but no differences in the context of low stress. According to a differential susceptibility model, compared to less susceptible individuals, more susceptible individuals show more maladaptive outcomes in the context of high stress but more positive outcomes in the context of low stress. For example, the biological sensitivity to context (BSC) theory (Boyce & Ellis, 2005; Obradovic et al., 2010) proposes that individuals with up-regulated stress-response systems show health risks in risky environments but health benefits in supportive environments, such that individuals with high BSC are more attuned to both positive and negative aspects of their social contexts.
Dual risk and differential susceptibility models are similar in their prediction that victimization will be associated with aggression in children with more versus less sensitive stress-response systems, but differ in their prediction about aggression at low levels of victimization. Whereas a dual risk model holds that non-victimized children with more versus less sensitive stress-response systems will show similar (low) levels of aggression, a differential susceptibility model holds that non-victimized children with more sensitive stress-response systems will show particularly low levels of aggression.
Consistent with a dual risk model, children with more versus less sensitive HPA systems differed in their expression of aggression at high levels of victimization but were similar at low levels of victimization. Yet, consistent with a differential susceptibility model, children with more sensitive HPA systems showed less frustration at low levels of victimization. Also consistent with a differential susceptibility model, sAA activation conferred risk (augmenting aggression) within a stressful environment (high victimization) but benefits (dampening aggression) within a low-stress environment (low victimization). This pattern tentatively supports the idea that sAA activation reflects a generalized, context-sensitive form of arousal (Fortunato et al., 2008). Because few studies directly examine how biological sensitivity moderates environmental contributions to frustration and aggression, more research is needed to determine the best-fitting models for these two stress-response systems.
Limitations and Future Directions
Consistent with a contemporary focus on studying biological stress responses within naturalistic contexts (Stroud et al., 2009), this study used an ecologically valid task designed to tap several relevant characteristics of stress, including unpredictability, uncontrollability, and social-evaluative threat. However, one consequence of this naturalistic assessment is that the task may not have been uniformly stressful, as reflected in the absence of mean level cortisol and sAA increases across the task. It also is possible that mean level change was tempered in this age group given that biological stress responses are larger in adolescents and adults than preadolescents (Stroud et al., 2009; Yim et al., in press). Alternatively, consistent with other research on naturalistic interpersonal stressors (Afifi et al., 2009; Powers et al., 2009; Stroud et al., 2009), the most salient stressor may have been anticipation of the upcoming interaction. It will be helpful for future research to clarify the differential roles of anticipatory versus reactivity phases of the stress response.
Because HPA and ANS activation varies across stressors (Stroud et al., 2009), research also would benefit from examining the specificity of these effects with regard to both the context of assessment (e.g., interpersonal vs. performance challenge) and the ecological context (e.g., peer victimization vs. other stressors). Specifically, it will be intriguing to explore whether dual vulnerability and differential susceptibility models hold more strongly if there is a match between the context of assessment and the ecological context of interest or whether these effects generalize across contexts.
Our research also was limited by the use of a global measure of aggression. We speculated that aggressive behavior in the context of peer victimization may reflect a reactive form of aggression displayed in response to threat or provocation (e.g., an impulsive effort to retaliate against aggressors) rather than a proactive form of aggression that is reinforced by rewards. However, more explicit measurement of reactive versus proactive aggression would provide a stronger basis for concluding that heightened activation is linked specifically to reactive aggression. We did find that victimization interacted with pre-task cortisol activation to predict task-specific frustration, and frustration partly accounted for the victimization × cortisol contribution to aggression, suggesting that heightened arousal may be linked to frustration-based aggression. Future research should expand on this finding to investigate whether broader ecological contexts (e.g., exposure to victimization) also interact with biological stress responses to predict task-specific aggressive behavior.
Finally, in line with a recent emphasis on multi-system measurement of stress responses (Bauer et al., 2002; Granger et al., 2006; Stroud et al., 2009), we examined both cortisol and sAA activity and found somewhat different patterns of results. Beyond examining differential effects across the two systems, however, recent research suggests that it may be important to consider their interactive contributions to emotion and behavior. For example, one study revealed that elevated baseline cortisol was associated with internalizing and externalizing symptoms in youth with high but not low sAA (El-Sheikh et al., 2008). Another study found that high sAA protected youth against aggression in the context of cortisol hyporeactivity (Gordis et al., 2006). Our relatively small sample size precluded our ability to adequately examine multiple three-way (victimization × sAA × cortisol) or four-way (victimization × sAA × cortisol × sex) interactions; thus, we focused on the independent role of these two systems. It will be important for future research to test more complex models that consider how the joint activity of these systems moderates the link between social context and behavior.
Conclusion
This research provides insight into individual variation in aggressive behavior in the context of peer victimization, suggesting that exposure to victimization is most likely to be associated with aggression in children who show heightened cortisol and sAA activation in anticipation of social stress and in girls who show heightened sAA reactivity to social stress. More broadly, this study elucidates how biological sensitivity moderates the contribution of high-stress versus low-stress environments to children’s health, suggesting that this sensitivity is harmful within threatening contexts but innocuous or perhaps even beneficial within non-threatening contexts.
Acknowledgments
We would like to thank the families and schools who participated in this study. We are grateful to Jamie Abaied, Monica Agoston, Hannah Banagale, Molly Bartlett, Sarah Kang, Megan Flynn, Nicole Llewelyn, and Niwako Sugimura for their assistance in data collection and management. This research was funded by a University of Illinois Arnold O. Beckman Award and National Institute of Mental Health Grant MH68444 awarded to Karen D. Rudolph.
Footnotes
In the interest of full disclosure, Douglas A. Granger is the founder and president of Salimetrics LLC (State College, PA).
References
- Afifi TD, Granger DA, Aldeis D, Joseph A, Denes A. The influence of divorce and parents’ communication skills on adolescents’ and young adults’ stress reactivity and recovery. 2009. Manuscript submitted for publication. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Bollmer JM, Harris MJ, Milich R. Reactions to bullying and peer victimization: Narratives, physiological arousal, and personality. Journal of Research in Personality. 2006;40:803–828. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 1992. [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Hudgens GA. Hormonal responses to psychological stress in men preparing for skydiving. Journal of Clinical Endocrinology and Metabolism. 1997;82:2503–2509. doi: 10.1210/jcem.82.8.4133. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Crick NR. The role of overt aggression, relational aggression, and prosocial behavior in the prediction of children’s future social adjustment. Child Development. 1996;67:2317–2327. [PubMed] [Google Scholar]
- Crick NR, Dodge KA. Social information-processing mechanisms in reactive and proactive aggression. Child Development. 1996;67:993–1002. [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Children’s treatment by peers: Victims of relational and overt aggression. Development and Psychopathology. 1996;8:367–380. [Google Scholar]
- El-Sheikh M, Buckhalt JA, Erath SA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L, Beauchaine T, Moore GA. Marital conflict and children’s externalizing behavior: Interactions between sympathetic and parasympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74:1–55. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato CK, Dribin AE, Granger DA, Buss KA. Salivary alpha-amylase and cortisol in toddlers: Differential relations with affective behavior. Developmental Psychobiology. 2008;50:807–818. doi: 10.1002/dev.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio A, Ceppi E, Rusconi M, Giorda R, Raggi ME, Fearon PJ. The role played by the interaction between genetic factors and attachment in the stress response in infancy. Journal of Child of Psychology and Psychiatry. 2009;50:1513–1522. doi: 10.1111/j.1469-7610.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosomatic Medicine. 1999;61:214–24. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Graham S, Juvonen J. Self-blame and peer victimization in middle school: An attributional analysis. Developmental Psychology. 1998;34:538–587. doi: 10.1037//0012-1649.34.3.587. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on saliva cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Buckhalt JA, Stroud LR, Handwerger K, Schwartz EB. Integrating the measurement of salivary α-amylase into studies of child health, development, and social relationships. Journal of Personal and Social Relationships Special Issue: Physiology and Human Relationships. 2006;23:267–290. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis E, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009a;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in HPA activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009b;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LD, Newman ML, Delville CL, Delville Y. Physiological stress response of young adults exposed to bullying during adolescence. Physiology and Behavior. 2008;95:617–624. doi: 10.1016/j.physbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hazler RJ, Carney JV, Granger DA. Integrating biological measures into the study of bullying. Journal of Counseling and Development. 2006;84:298–307. [Google Scholar]
- Henry JP. Biological basis of the stress response. Integrative Physiological and Behavioral Science. 1992;27:66–83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing KF, et al. Observational, physiological, and self-report measures of children’s anger: Relations to reactive versus proactive aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M. Salivary alpha-amylase as a longitudinal predictor of children’s externalizing symptoms: Respiratory sinus arrhythmia as a moderator of effects. Psychoneuroendocrinology. 2009;34:633–643. doi: 10.1016/j.psyneuen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York: Guilford; 2006. [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Ladd GW, Kochenderfer-Ladd B. Identifying victims of peer aggression from early to middle childhood: Analysis of cross-informant data for concordance, estimation of relational adjustment, prevalence of victimization, and characteristics of identified victims. Psychological Assessment. 2002;14:74–96. doi: 10.1037//1040-3590.14.1.74. [DOI] [PubMed] [Google Scholar]
- Lochman JE, Dodge KA. Distorted perceptions in dyadic interactions of aggressive and nonaggressive boys: Effects of prior expectations, context, and boys’ age. Development and Psychopathology. 1998;10:495–512. doi: 10.1017/s0954579498001710. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. Journal of Abnormal Child Psychology. 2009;37:169–182. doi: 10.1007/s10802-008-9263-3. [DOI] [PubMed] [Google Scholar]
- Lorber M. The psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. Journal of Psychosomatic Research. 1980;24:125–130. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89:852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Han G, Cicchetti D, Crick NR, Rogosch FA. Neuroendocrine regulation and physical and relational aggression: The moderating roles of child maltreatment and gender. Development and Psychopathology. 2008;44:1160–1176. doi: 10.1037/a0012564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, et al. Stress-induced changes in human salivary alpha-amylase activity–Associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. International Journal of Psychophysiology. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Powers SI, Laurent HK, Granger DA. Individual coordination and dyadic attunement of HPA and SNS reactions to relationship conflict in dating couples. 2009. Manuscript submitted for publication. [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Flynn M. Relational victimization predicts children’s social-cognitive and self-regulatory responses in a challenging peer context. Developmental Psychology. 2009;45:1444–1454. doi: 10.1037/a0014858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmivalli C, Karhunen J, Lagerspetz KMJ. How do the victims respond to bullying? Aggressive Behavior. 1996;22:99–109. [Google Scholar]
- Scarpa A. Aggression in physically abused children: The interactive role of emotion regulation. In: Raine A, Brennan PA, Farrington DP, Mednick SA, editors. Biosocial bases of violence. New York: Plenum; 1997. pp. 341–344. [Google Scholar]
- Scarpa A, Fikretoglu D, Luscher K. Community violence exposure in a young adult sample: II. Psychophysiology and aggressive behavior. Journal of Community Psychology. 2000;28:417–426. [Google Scholar]
- Scarpa A, Friedman BH, Smalley K, Luscher KA. Physiological reactivity moderates stress-induced mood [Abstract] Psychophysiology. 1997;34(Suppl):78. [Google Scholar]
- Scarpa A, Raine A. Biosocial bases of violence. In: Flannery DJ, Vazsonyi AT, Waldman ID, editors. The Cambridge handbook of violent behavior and aggression. NY: Cambridge University Press; 2007. pp. 151–169. [Google Scholar]
- Scarpa A, Ollendick TH. Community violence exposure in a young adult sample: III. Psychophysiology and victimization interact to affect risk for aggression. Journal of Community Psychology. 2003;31:321–338. [Google Scholar]
- Scarpa A, Raine A. Psychophysiology of anger and violent behavior. Psychiatric Clinics of North America. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer J. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;171:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic intervals for indirect effects in structural equations models. In: Leinhart S, editor. Sociological methodology 1982. San Francisco, CA: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- Solberg ME, Olweus D. Prevalence estimation of school bullying with the Olweus Bully/Victim Questionnaire. Aggressive Behavior. 2003;29:239–268. [Google Scholar]
- Stroud LR, Foster E, Papandonatos G, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus social rejection stress. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TN, Farrell AD, Kliewer W. Peer victimization in early adolescence: Association between physical and relational victimization and drug use, aggression, and delinquent behaviors among urban middle school students. Development and Psychopathology. 2006;18:119–137. doi: 10.1017/S095457940606007X. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo JA, Dorn LD. Cortisiol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behavior in young adolescents. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2009.09.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, Matthys W, Van Goozen SHM, Van Engeland H. Prediction of adolescent outcome in children with disruptive behaviour disorders: A study of neurobiological, psychological and family factors. European Child and Adolescent Psychiatry. 2005;14:153–163. doi: 10.1007/s00787-005-0455-x. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, van Goozen SHM, van Engeland H, Schaal B, Arseneault L, Se’guin JR, et al. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. Journal of Neural Transmission. 2005;112:1083–1096. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, Van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Yim I, Granger DA, Quas JA. Children’s and adults’ salivary alpha-amylase responses to a laboratory stressor and to verbal recall of the stressor. Developmental Psychobiology. doi: 10.1002/dev.20453. (in press) [DOI] [PubMed] [Google Scholar]
- Zahn TP, Kruesi MJP. Autonomic activity in boys with disruptive behavior disorders. Psychophysiology. 1993;30:605–614. doi: 10.1111/j.1469-8986.1993.tb02086.x. [DOI] [PubMed] [Google Scholar]