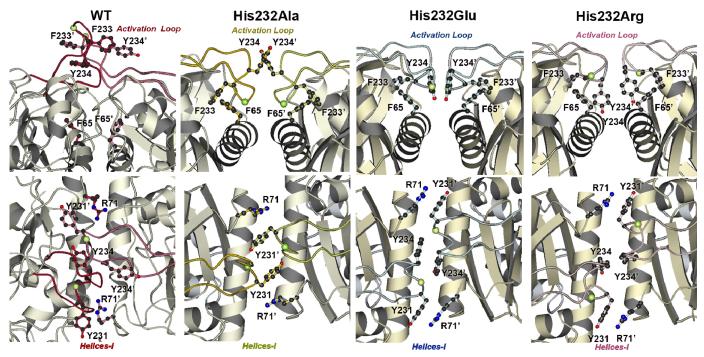
Figure 5. The O-X Dimer Interface Regions of WT, His232Ala, His232Arg, and His232Glu GlpK.
The loop regions of the His232 mutants are similarly folded over domain I but the pairwise interactions across the dimer interface are changed among these structures. The interface perspectives shown in B are rotated approximately 90° from those shown in A. The Cα carbon of residue 232, the site of mutation, is denoted by a yellow-green ball. In the His232Glu, Tyr234 (O) phenolic ring forms face-to-face interactions to Tyr234 (X), with the π–π interactions permitting greater conformational flexibility. In contrast, in addition to changes in the aromatic interactions around Tyr234, an electrostatic network formed by Arg232 further stabilizes the O-X dimer interactions. In the His232Ala structure, additional conformational changes are presented which may also lead to enhanced stabilization of the O-X dimer interactions compared to those found in the His232Glu structure.