Abstract
Purpose
Survival after neuroblastoma relapse is poor. Understanding the relationship between clinical and biologic features and outcome after relapse may help in selection of optimal therapy. Our aim was to determine which factors were significantly predictive of postrelapse overall survival (OS) in patients with recurrent neuroblastoma—particularly whether time from diagnosis to first relapse (TTFR) was a significant predictor of OS.
Patients and Methods
Patients with first relapse/progression were identified in the International Neuroblastoma Risk Group (INRG) database. Time from study enrollment until first event and OS time starting from first event were calculated. Cox regression models were used to calculate the hazard ratio of increased death risk and perform survival tree regression. TTFR was tested in a multivariable Cox model with other factors.
Results
In the INRG database (N = 8,800), 2,266 patients experienced first progression/relapse. Median time to relapse was 13.2 months (range, 1 day to 11.4 years). Five-year OS from time of first event was 20% (SE, ± 1%). TTFR was statistically significantly associated with OS time in a nonlinear relationship; patients with TTFR of 36 months or longer had the lowest risk of death, followed by patients who relapsed in the period of 0 to less than 6 months or 18 to 36 months. Patients who relapsed between 6 and 18 months after diagnosis had the highest risk of death. TTFR, age, International Neuroblastoma Staging System stage, and MYCN copy number status were independently predictive of postrelapse OS in multivariable analysis.
Conclusion
Age, stage, MYCN status, and TTFR are significant prognostic factors for postrelapse survival and may help in the design of clinical trials evaluating novel agents.
INTRODUCTION
Neuroblastoma is a genetically and clinically heterogeneous childhood malignancy arising from the embryonic sympathetic nervous system. Neuroblastoma is metastatic at diagnosis in approximately 50% of patients; more than half of children diagnosed with high-risk neuroblastoma will either not respond to conventional therapies or relapse after treatment, necessitating development of novel treatments. Little is known about how to identify patients who are more likely to respond to therapies and survive longer after primary treatment failure in such a heterogeneous disease. Selection of appropriate therapy is one way in which improved outcome can be achieved, so it is crucial to understand the clinical course of neuroblastoma after failure of conventional therapy.
The length of time from diagnosis to first relapse has been demonstrated to be of prognostic value in multiple types of pediatric tumors.1–5 In one recent single-institution study of high-risk neuroblastoma, time to first relapse was shown to influence length of survival6; however, the relationship between initial tumor biologic and clinical risk factors was not extensively studied with regard to the length of second remission and survival after relapse. To gather additional information about the disease course after relapse, we queried the International Neuroblastoma Risk Group (INRG) database. The INRG project is a collaborative effort among cooperative pediatric oncology groups from Europe, North America, Germany, Australia, New Zealand, and Japan. For the first time, we have a sufficiently large group of annotated relapsed or progressing patients with neuroblastoma to use to identify which clinical and biologic factors are most prognostic of postrelapse survival.7 In this analysis of 2,266 patients with neuroblastoma who suffered relapse or progression, we show that time to first relapse is significantly associated with survival after relapse, in addition to several other biologic and clinical risk factors in multivariable analysis. This information may help guide therapeutic decisions and interpretation of clinical trials of novel agents.
PATIENTS AND METHODS
INRG Database
A total of 8,800 unique patients younger than 21 years of age with pathologically confirmed neuroblastoma who were diagnosed/enrolled between 1990 and 2002 comprise the INRG database.7 An enrollment cutoff of 2002 was chosen to allow for sufficient follow-up time. Patients provided consent and were enrolled shortly after initial diagnosis onto one or more neuroblastoma clinical or biologic trials in Germany, Japan, Italy, Spain, or the United Kingdom or onto a North American Children's Oncology Group study or the European SIOP LNESG1 (International Society of Pediatric Oncology Localized Neuroblastoma European Study) trial. Institutional review board approval and informed patient consent were obtained by each country, cooperative group, and treating institution for their respective studies. In addition to date of diagnosis and follow-up data, information on 35 potential risk factors was included in the INRG database.7 Of the 8,800 patients, the analytic cohort for this report is composed of 2,266 who experienced at least one event (relapse or progression of neuroblastoma or secondary malignancy) and who had available follow-up data. The only other analytic cohort inclusion requirement was that the first event was not death; patients who died at time of relapse/progression were excluded because their OS time could not be calculated. We are interested in patients who require a postrelapse treatment decision.
Statistical Considerations
Time to first event was calculated as time from study enrollment to first occurrence of relapse, progression, or secondary malignancy or time of last patient contact if no event occurred. Overall survival (OS) time was calculated from first event until death or time of last contact if the patient was alive. For ease of discussion, throughout this article, we will refer to these concepts as time to first relapse (TTFR) and OS post relapse, respectively. The method of Kaplan-Meier was used to generate survival curves, and curves were compared using a two-sided log-rank test.8 OS post relapse is presented as the 5-year point estimate (± SE; per method of Peto et al9).
The clinical and biologic factors at diagnosis analyzed are listed in Table 1.7,10 For lactate dehydrogenase (LDH) and ferritin, median values from the entire INRG cohort (580 units/L and 96 ng/mL, respectively) were used to dichotomize patients as elevated or not elevated. We then chose to use data-driven splits in the risk to construct a survival tree rather than traditional predefined risk groups.
Table 1.
Clinical and Genetic Characteristics and Postrelapse Survival in the Relapsed INRG Cohort (n = 2,266)
| Factor | Patients |
Postrelapse OS |
5-Year Postrelapse OS |
Postrelapse OS P† | ||
|---|---|---|---|---|---|---|
| No. | % | HR* | 95% CI | OS ± SE (%) | ||
| Histologic classification‡ | 4.5 | 3.6 to 5.8 | < .001 | |||
| Favorable | 246 | 27 | 66 ± 5 | |||
| Unfavorable | 653 | 73 | 11 ± 2 | |||
| INSS stage | 3.5 | 3.1 to 4.0 | < .001 | |||
| 1, 2, 3, 4S | 622 | 28 | 52 ± 3 | |||
| 4 | 1,578 | 72 | 8 ± 1 | |||
| MYCNstatus | 2.7 | 2.4 to 3.1 | < .001 | |||
| Nonamplified | 1,141 | 67 | 32 ± 2 | |||
| Amplified | 562 | 33 | 7 ± 2 | |||
| Initial treatment | 2.6 | 2.3 to 2.9 | < .001 | |||
| Observation, surgery, or standard chemotherapy | 762 | 40 | 41 ± 3 | |||
| Intensive multimodality | 1,143 | 60 | 7 ± 1 | |||
| Age, days | 2.4 | 2.1 to 2.7 | < .001 | |||
| < 547 | 617 | 27 | 47 ± 3 | |||
| ≥ 547 | 1,649 | 73 | 10 ± 1 | |||
| LDH, U/L | 2.4 | 2.1 to 2.8 | < .001 | |||
| < 580 | 466 | 35 | 38 ± 2 | |||
| ≥ 580 | 848 | 65 | 12 ± 2 | |||
| Serum ferritin, ng/mL | 2.1 | 1.8 to 2.4 | < .001 | |||
| < 96 | 321 | 28 | 34 ± 4 | |||
| ≥ 96 | 839 | 72 | 12 ± 2 | |||
| MKI‡ | 1.9 | 1.6 to 2.4 | < .001 | |||
| Low, intermediate | 550 | 74 | 28 ± 3 | |||
| High | 189 | 26 | 12 ± 4 | |||
| 1p | 1.8 | 1.4 to 2.1 | < .001 | |||
| No loss or aberration | 345 | 58 | 32 ± 5 | |||
| LOH, deletion, or imbalance | 252 | 42 | 15 ± 3 | |||
| Ploidy | 1.6 | 1.4 to 2.0 | < .001 | |||
| > 1 (hyperdiploid) | 357 | 57 | 34 ± 4 | |||
| ≤ 1 (diploid, hypodiploid) | 273 | 43 | 16 ± 4 | |||
| Grade of NB differentiation‡ | 1.6 | 1.1 to 2.1 | < .001 | |||
| Differentiating | 72 | 9 | 38 ± 8 | |||
| Undifferentiated | 774 | 91 | 22 ± 3 | |||
| Diagnostic category‡ | ||||||
| 1 (NB, stroma poor) | 992 | 91 | 21 ± 2 | |||
| 2 (GNB, intermixed, stroma rich) | 5 | 0.5 | 0 | |||
| 3 (GNB, well differentiated, stroma rich) | 5 | 0.5 | 0 | |||
| 4 (GNB, nodular [composite]) | 90 | 8 | 10 ± 7 | |||
| 2 and 3 v 1 and 4 | 1.4 | 0.8 to 2.4 | .686 | |||
| 2 and 3 | 10 | 1 | 0 | |||
| 1 and 4 | 1,082 | 99 | 20 ± 2 | |||
| Year of diagnosis | 1.3 | 1.2 to 1.4 | < .001 | |||
| ≥ 1996 | 1,333 | 59 | 25 ± 3 | |||
| < 1996 | 933 | 41 | 17 ± 1 | |||
| 11q | 1.1 | 0.8 to 1.5 | .567 | |||
| Balanced or no aberration | 205 | 65 | 35 ± 6 | |||
| Deletion, imbalance, or unbalanced | 115 | 35 | 20 ± 7 | |||
| 17q | 1.1 | 0.7 to 1.6 | .764 | |||
| No gain | 64 | 41 | 29 ± 8 | |||
| Gain | 91 | 59 | 23 ± 9 | |||
Abbreviations: GNB, ganglioneuroblastoma; HR, hazard ratio; INPC, International Neuroblastoma Pathology Classification; INRG, International Neuroblastoma Risk Group; INSS, International Neuroblastoma Staging System; LDH, lactate dehydrogenase; LOH, loss of heterozygosity; MKI, mitosis karyorrhexis index; NB, neuroblastoma; OS, overall survival.
HRs denote increased risk of event for second row within given category as compared with first row.
Per log-rank test.
Per INPC; per Shimada, if INPC missing.
A Cox proportional hazards regression model was used to calculate the hazard ratio (HR) for increased risk of death (poor outcome category as compared with better outcome category).11 Visual inspection of Kaplan-Meier curves and plots of log(-log(S(t))) versus log(t) were used to identify violations of the proportional hazards assumption.12 Where the proportional hazards assumption was violated, Cox regression modeling was performed using time-dependent covariates to adjust for nonproportionality. Patients were categorized into 6-month cohorts (0 to < 6, 6 to < 12, 12 to < 18, 18 to < 24, 24 to < 30, 30 to < 36, and ≥ 36 months) of TTFR for graphical presentation of the nonlinear relationship between TTFR and OS post relapse. Time-dependent covariates are somewhat analogous to the use of a squared term in an algebraic equation; to approximate the curve in Figure 2, we included a term for TTFR multiplied by OS time and a binary term for TTFR (< 365 v ≥ 365 days) in the Cox model. Inclusion of these terms in the model ensured that patients who progressed within a short time were weighted differently than those who relapsed after therapy.
Fig 2.
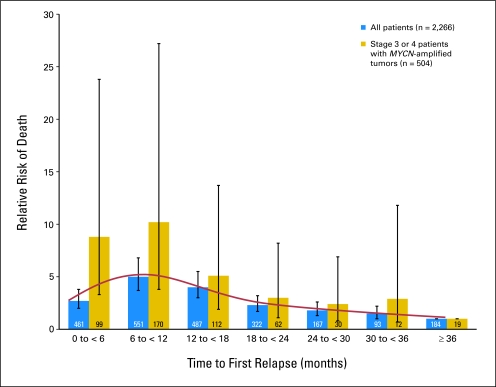
Relative risk (hazard ratio) of death for 6-month cohorts of time to first relapse in comparison with patients whose first relapse occurred more than 36 months from diagnosis. Patients whose first relapse occurred more than 36 months from diagnosis had a relative risk of death equal to 1. Blue bars are all patients (n = 2,266), and gold bars are the subset of stage 3 or 4 patients with MYCN amplified tumors (n = 504). SE bars are shown for relative risk of death for each cohort.
Cox proportional hazards regression models were used to identify the most highly statistically significant variable to create a given split or branch in the survival tree,11–14 and only the Table 1 variables that were statistically significant were tested in the survival tree. To account for the effect of TTFR, each step of the model included TTFR time-dependent covariates; these covariates were retained in all models regardless of their statistical significance. P values less than .05 were considered statistically significant.15
RESULTS
The clinical and biologic characteristics at diagnosis of the 2,266 relapsed/progressing patients are listed in Table 1. Seventy-three percent of patients were 18 months of age or older at diagnosis, 72% had stage 4 tumors, and 33% had MYCN amplified tumors. The median TTFR was 13.2 months (range, 1 day to 11.4 years), and for patients with MYCN amplified tumors (n = 562) or MYCN nonamplified tumors (n = 1,141), it was 11 months (range, 3 days to 7 years) and 14.5 months (range, 7 days to 11 years), respectively (P < .001).
Univariate Survival Analyses
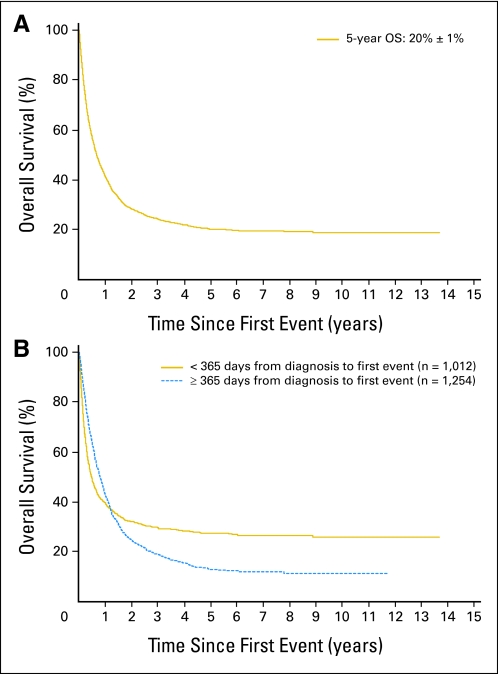
The 5-year OS post relapse was 20% (± SE, 1%; n = 2,266; Fig 1A). The median follow-up time of patients who did not die post relapse was 3.6 years (range, 1 day to 13.7 years). Most factors were statistically significantly associated with OS post relapse (Table 1), including unfavorable histology, stage 4, MYCN amplification, use of intensive multimodality treatment at diagnosis, age 547 days or older, elevated LDH, elevated ferritin, and high mitosis karyorrhexis index (P < .001; HR, ≥ 1.9; Table 1).
Fig 1.
(A) Overall survival post relapse for 2,266 patients with neuroblastoma from the International Neuroblastoma Risk Group database. Median time to first relapse (TTFR) was 13.2 months (range, 1 day to 13.1 years). (B) Overall survival post relapse. TTFR less than 365 days from diagnosis (n = 1,012) versus TTFR 365 days or more from diagnosis (n = 1,254). Log-rank test P value is not valid because of violation of proportional hazards assumption, which can be seen in crossing of two curves.
TTFR was statistically significantly associated with OS time (Table 2), and a nonlinear relationship was identified (Fig 2). Traditional log-rank test comparisons of short versus long TTFR were not supported, because the required proportional hazards assumption was violated (eg, Fig 1B, using 365-day cutoff), regardless of the choice of the cutoff used to categorize patients as short versus long TTFR. TTFR had an effect on OS post relapse, but the strength of the effect varied for different lengths of TTFR (Fig 2). Each of the 6-month TTFR groups had a higher risk of death after relapse than patients with TTFR of 36 months or longer.
Table 2.
Multivariable Analysis of OS After First Event
| Time to First Relapse (months)* | No. of Patients | Risk of Death† |
P | |
|---|---|---|---|---|
| HR | 95% CI | |||
| 0 to < 6 | 461 | 2.7 | 2.0 to 3.8 | < .001 |
| 6 to < 12 | 551 | 5.0 | 3.7 to 6.8 | < .001 |
| 12 to < 18 | 487 | 4.0 | 3.0 to 5.5 | < .001 |
| 18 to < 24 | 322 | 2.3 | 1.7 to 3.2 | < .001 |
| 24 to < 30 | 167 | 1.8 | 1.3 to 2.6 | < .001 |
| 30 to < 36 | 93 | 1.5 | 1.0 to 2.2 | .063 |
| ≥ 36 | 184 | 1.0 | 1.0 to 1.0 | NA |
NOTE. Analysis testing of 6-month categories of time to first relapse compared with risk of death for those relapsing more than 36 months after diagnosis.
Abbreviations: HR, hazard ratio; NA, not applicable; OS, overall survival.
All 6-month categories were simultaneously included in the model, except for ≥ 36, which was the reference level.
In comparison with patients with first relapse more than 36 months after diagnosis.
Patients at the highest risk of death were those who relapsed between 6 and 18 months after diagnosis (peak risk at approximately 12 months). Of patients who relapsed 12 months or longer after diagnosis, 24% underwent stem-cell transplantation during initial therapy, compared with only 10% who relapsed in less than 12 months. The risk of death is approximately the same (approximately 2.5 times higher than in patients with TTFR ≥ 36 months) for patients who relapsed within 6 months as it was for patients who relapsed in 18 to less than 24 months. The group of children who relapsed within 6 months after diagnosis included an unexpectedly high proportion of patients with favorable risk factors. Of 461 children who relapsed within 6 months, 55% (255 of 461 patients) were younger than 18 months old at diagnosis in comparison with 20% (362 of 1,805 patients) of those who relapsed later than 6 months after diagnosis (P < .001; Appendix Table A1, online only). Similarly, 49% (219 of 448 patients) in the early relapse group had INSS stage 1, 2, 3, and 4S tumors, compared with only 23% of those (403 of 1,752 patients) who relapsed later than 6 months (P < .001; Appendix Table A1, online only). Within the subset of relapsed patients who were stage 3 or 4 and MYCN amplified, the nonlinear relationship persisted (Fig 2), with a high risk of death for patients who relapsed within the first 12 months of diagnosis.
Multivariable Survival Analyses
Although many factors were prognostic in a univariate test, few were prognostic in a multivariable Cox model (Table 3). Stage was the most highly predictive of OS post relapse, with increased risk of death for stages 4, 3, and 4S (6.9, 4.3, and 3.5 times higher than stages 1 and 2, respectively), with adjustment for TTFR by simultaneous inclusion in the Cox model.
Table 3.
Clinical and Biologic Factors Independently Predictive of OS in Multivariable Analysis (n = 2,266)
| Variable* | Risk of Death |
P | |
|---|---|---|---|
| HR | 95% CI | ||
| Stage 4 | 6.9 | 5.1 to 9.3 | < .001 |
| Stage 3 | 4.3 | 3.1 to 6.1 | < .001 |
| Stage 4S | 3.5 | 2.2 to 5.4 | < .001 |
| MYCN amplification | 2.4 | 2.1 to 2.7 | < .001 |
| Age ≥ 18 months | 1.6 | 1.4 to 1.9 | < .001 |
| TTFR < 12 months | 2.0 | 1.7 to 2.5 | < .001 |
| TTFR × OS time† | 1.0 | 1.0 to 1.0 | < .001 |
Abbreviations: HR, hazard ratio; OS, overall survival; TTFR, time to first relapse.
Variables tested in multivariable model were those significant in survival tree regression: stage, MYCN status, age, ploidy, lactate dehydrogenase, and grade of differentiation.
Interaction term consisting of 12-month TTFR cutoff multiplied by OS time is used to approximate curve shown in Figure 2.
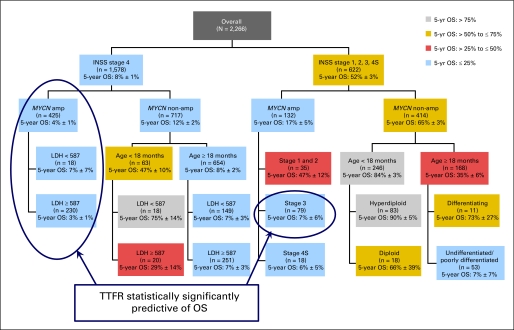
The survival tree regression models identified a small proportion of patients who may have been salvageable after relapse (Fig 3). In the context of relapse therapy administered from 1990 to 2005, the salvageable cohorts seem to be those with stage 1, 2, 3, or 4S disease who had MYCN nonamplified tumors (excluding those with undifferentiated histology); those with stage 4 disease age younger than 18 months who had MYCN nonamplified tumors; and those with stage 1, 2, or 4S disease who had MYCN amplified tumors. Combined, approximately 15% of relapsed/progressing patients seemed to be curable. Within the survival tree subgroups, there were only two subgroups within which TTFR was statistically significantly associated with OS (ie, stage 4 patients with MYCN amplified tumors and stage 3 patients with MYCN amplified tumors).
Fig 3.
Survival tree regression of overall survival post relapse. Modeling was performed with adjustment for time to first relapse. Within each subgroup box, the value of OS post relapse is presented as the 5-year point estimate (± SE). INSS, International Neuroblastoma Staging System; LDH, lactate dehydrogenase; OS, overall survival; TTFR, time to first relapse.
DISCUSSION
TTFR has been demonstrated to be prognostic in different pediatric tumors,1–5 but it has not been thoroughly studied in neuroblastoma. Some studies have shown that a more chronic course of neuroblastoma after failure of primary therapy seems to be associated with longer times of first remission16 or older age at diagnosis.17–19 Cotterill et al20 identified late relapse in a small subset of high-risk patients; however, this study was unable to predict which factors influence long-term survival given the limited follow-up.
In each of the single-institution studies, the number of patients and data collection were limited. In our analysis of 2,266 patients with relapsed neuroblastoma, we have shown that TTFR is an important factor to predict OS after an event. The relationship of TTFR and OS post relapse is complex; the risk of death was highest for patients who relapsed between 6 and 18 months from diagnosis and then decreased steadily, being lowest in patients who relapsed at 36 months or later. The association of TTFR and OS post relapse was the strongest in stage 3 and 4 patients with MYCN amplified tumors. Surprisingly, in the overall cohort, the risk of death for patients who relapsed between 6 and 18 months after diagnosis was higher than for patients who relapsed within 0 to 6 months, or more than 18 months, after diagnosis. A more intuitive relationship would be linear, where those with the shortest TTFR had the highest risk of death, with decreasing risk of death as TTFR increased. A possible explanation for why the patients with the shortest TTFR did not have the highest risk of death may lie in the more favorable clinical characteristics of the patients who relapsed within 6 months; a significantly larger proportion of patients were younger or had stage 1, 2, 3, or 4S disease in comparison with those who relapsed later. Patients who were younger and had a lower stage of disease may have received little or no therapy before relapse and therefore may have responded well to postrelapse treatment. Another possible explanation for the upward slope of the risk-of-death curve with TTFR of less than 12 months and the downward slope of the risk-of-death curve with TTFR of 12 months or longer (Fig 2) is that patients who are able to make it to transplantation (occurring approximately 10 to 12 months post diagnosis) without relapse/progression realize a benefit in terms of prolonged OS.
Accepted prognostic factors for neuroblastoma include age at diagnosis, MYCN gene amplification, and histologic features.21 These factors are standard determinants of initial risk stratification, but their prognostic value after an event had not been studied previously. In our analysis, we identified several factors that were independently prognostic of OS after relapse: stage, MYCN status, age, and TTFR. Many other factors were significantly predictive of poor outcome after relapse in univariate, but not multivariable, analysis. Within the subgroups of patients with MYCN nonamplified tumors or stage 1, 2, or 4S disease, TTFR was not predictive of OS post relapse; it seems that the patients with INSS stage 3 and 4 MYCN amplified tumors were driving the association between TTFR and OS.
Two previous single-institution studies in neuroblastoma also showed that shorter time to first relapse was a significant adverse factor for survival.6,16 Lau et al16 reviewed 31 patients with neuroblastoma with relapsed disease and found that patients who relapsed less than 12 months from diagnosis had significantly shorter survival time. The only other significant factor in their analysis for survival post relapse was tumor MYCN amplification. Santana et al6 addressed the study of disease-control intervals in 91 high-risk patients with neuroblastoma. The estimated median times to disease recurrence were 18.3, 8.7, and 3.8 months for the first, second, and third recurrences, respectively. Patients with longer initial disease control had a significant postrecurrence survival advantage. This study emphasized the importance of knowing the intervals of disease progression as end points for the design of protocols with new agents.6
Other studies in pediatric malignancies have also shown that earlier relapse portends shorter survival. The Italian Off-Therapy Registry published results on 694 patients, including those with neuroblastoma, CNS tumors, sarcoma, Wilm's tumors, and Hodgkin's lymphoma, who had experienced recurrence. They found significantly different HRs for survival by type of diagnosis and found overall that patients relapsing less than 12 months off therapy had worse survival in univariate, but not in multivariable, analysis.22 Risk factors for survival post relapse in neuroblastoma were not evaluated separately. Interestingly, the patients relapsing in the more recent treatment era had shorter survival, perhaps because the relapse may have occurred after more intensive therapy. A similar trend has been reported in childhood leukemia, in which multiple studies have shown that duration of first remission was the most significant predictor of outcome after relapse of acute lymphoblastic leukemia.4,5
In a retrospective study, Garaventa et al23 described 781 children with neuroblastoma experiencing tumor recurrence. Ten-year OS was 6.8% after progression and 14.4% after relapse. Similar to our series, the factors worsening prognosis in univariate analysis were age older than 18 months, advanced stage, high LDH, MYCN amplification, and abdominal primary (no multivariable analysis). Most relapses occurred early (median interval, 7.8 months), but 86 (24%) occurred late (median, 28 months). Early relapses had a more rapid, unfavorable course, with approximately 80% of deaths occurring within 2 years, whereas survival time was longer for late relapses.
From German protocols NB90, NB97, and NB2004 (n = 493 high-risk patients), Simon et al24 presented data on 254 patients with neuroblastoma who relapsed after autologous bone marrow transplantation as part of initial treatment. MYCN amplification, early recurrence within 18 to 24 months after diagnosis, bone marrow, and lung/pleura metastasis at relapse were independently predictive of poor survival. The 24 patients who underwent a second autologous stem-cell transplantation had better outcome.
Ultimately, understanding the genetic differences in early versus late relapsing patients will facilitate selection of appropriate targeted therapy. Meanwhile, we propose that stratification of relapsed patients according to the timing of first relapse, as well as stage, age, and MYCN status, is critical in certain types of study designs, such as randomized phase II trials, to maintain a balance of less favorable patients between treatment arms. One might also wish to compare two strata for a given treatment to see which has better outcome. TTFR is a significant, readily available prognostic factor for stratification of patients on retrieval trials. In addition, TTFR may be used in therapy selection. Studies of novel agents using time to progression as an end point could be designed to stratify patients based on TTFR so that the effect of the novel agents can be separated from inherent tumor behavior.
We were not able to establish a single clear TTFR cutoff time point for stratification, perhaps because the INRG series is heterogeneous in relation to era of treatment and initial therapy received or because the true relationship between TTFR and OS post relapse is nonlinear. Our results demonstrate that the period from 6 to 18 months from diagnosis to relapse is associated with the shortest survival of relapsing patients, including the subset of patients with stage 3 and 4 MYCN amplified tumors.
In conclusion, time to first relapse is a significant predictor of death after relapse; the risk of death is higher for patients who relapse between 6 and 18 months after diagnosis than it is for patients who relapse more than 18 months from diagnosis. Stratification of relapsed patients with neuroblastoma according to the timing of first relapse, age, stage, and MYCN status is important in retrieval study designs, especially for patients with stage 3 or 4 MYCN amplified tumors.
Acknowledgments
Presented in part at the 14th Advances in Neuroblastoma Research Conference, June 21-24, 2010, Stockholm, Sweden; 46th Annual Meeting of the American Society for Clinical Oncology, June 4-8, 2010, Chicago, IL; and 41st Congress of the International Society of Pediatric Oncology, September 4-9, 2009, Sao Paulo, Brazil.
Appendix
Table A1.
Postrelapse OS of Patients Who Relapsed Within 6 Months of Diagnosis by Clinical and Biologic Characteristics (n = 461)
| Factor | Patients |
Postrelapse OS |
5-Year Postrelapse OS |
OS P† | ||
|---|---|---|---|---|---|---|
| No. | % | HR* | 95% CI | OS ± SE (%) | ||
| Age, days | 2.4 | 1.9 to 3.1 | < .001 | |||
| < 547 | 255 | 55 | 54 ± 4 | |||
| ≥ 547 | 206 | 45 | 14 ± 3 | |||
| Year of diagnosis | 1.4 | 1.1 to 1.7 | .014 | |||
| ≥ 1996 | 185 | 40 | 42 ± 7 | |||
| < 1996 | 276 | 60 | 32 ± 3 | |||
| Initial treatment | 4.0 | 3.0 to 5.3 | < .001 | |||
| Observation, surgery, or standard chemotherapy | 223 | 64 | 59 ± 4 | |||
| Intensive multimodality | 124 | 36 | 11 ± 4 | |||
| INSS stage | 4.6 | 3.5 to 6.1 | < .001 | |||
| 1, 2, 3, 4S | 219 | 49 | 65 ± 4 | |||
| 4 | 229 | 51 | 11 ± 3 | |||
| Evans stage | 5.1 | 2.9 to 8.9 | < .001 | |||
| I, II, III, IVS | 58 | 53 | 69 ± 8 | |||
| IV | 52 | 47 | 14 ± 5 | |||
| Serum ferritin, ng/mL | 2.9 | 1.8 to 4.6 | < .001 | |||
| < 96 | 56 | 30 | 63 ± 8 | |||
| ≥ 96 | 133 | 70 | 27 ± 5 | |||
| LDH, U/L | 4.1 | 2.7 to 6.2 | < .001 | |||
| < 587 | 90 | 38 | 68 ± 6 | |||
| ≥ 587 | 145 | 62 | 23 ± 4 | |||
| Histologic classification‡ | 5.8 | 3.7 to 9.0 | < .001 | |||
| Favorable | 113 | 47 | 77 ± 5 | |||
| Unfavorable | 127 | 53 | 17 ± 5 | |||
| Diagnostic category‡ | ||||||
| 1 (NB, stroma poor) | 230 | 95 | 39 ± 5 | |||
| 2 (GNB, intermixed, stroma rich) | 2 | 1 | 0 | |||
| 3 (GNB, well differentiated, stroma rich) | 0 | 0 | 0 | |||
| 4 (GNB, nodular [composite]) | 11 | 4 | 0 | |||
| 2 and 3 v 1 and 4 | 1.7 | 0.23 to 11.8 | .617 | |||
| 2 and 3 | 2 | 1 | 0 | |||
| 1 and 4 | 241 | 99 | 38 ± 5 | |||
| Grade of NB differentiation‡ | 1.2 | 0.69 to 2.2 | .459 | |||
| Differentiating | 24 | 11 | 45 ± 12 | |||
| Undifferentiated | 192 | 89 | 41 ± 6 | |||
| MKI‡ | 2.2 | 1.5 to 3.2 | < .001 | |||
| Low, intermediate | 142 | 72 | 46 ± 6 | |||
| High | 54 | 28 | 25 ± 9 | |||
| MYCNstatus | 4.2 | 3.2 to 5.6 | < .001 | |||
| Nonamplified | 235 | 65 | 55 ± 5 | |||
| Amplified | 124 | 35 | 12 ± 5 | |||
| Ploidy | 2.2 | 1.4 to 3.6 | < .001 | |||
| > 1 (hyperdiploid) | 84 | 62 | 55 ± 7 | |||
| ≤ 1 (diploid, hypodiploid) | 52 | 38 | 27 ± 9 | |||
| 11q | 2.2 | 0.91 to 5.3 | .078 | |||
| Balanced or no aberration | 48 | 80 | 64 ± 10 | |||
| Deletion, imbalance, or unbalanced | 12 | 20 | 42 ± 23 | |||
| 1p | 2.3 | 1.4 to 3.6 | < .001 | |||
| No loss or aberration | 73 | 59 | 52 ± 9 | |||
| LOH, deletion, or imbalance | 50 | 41 | 22 ± 7 | |||
| 17q | 1.5 | 0.50 to 4.5 | .472 | |||
| No gain | 19 | 63 | 61 ± 17 | |||
| Gain | 11 | 37 | 46 ± 24 | |||
Abbreviations: GNB, ganglioneuroblastoma; HR, hazard ratio; INPC, International Neuroblastoma Pathology Classification; INSS, International Neuroblastoma Staging System; LDH, lactate dehydrogenase; LOH, loss of heterozygosity; MKI, mitosis karyorrhexis index; NB, neuroblastoma; OS, overall survival.
HRs denote increased risk of event for second row within given category as compared with first row.
Per log-rank test.
Per INPC; per Shimada, if INPC missing.
Footnotes
Supported in part by the Little Heroes Pediatric Cancer Foundation and Forbeck Foundation (W.B.L.); by Grants No. FIS 09/02323 and GVBE10/08 from Generalitat Valenciana (V.C.); and by National Cancer Institute Grant No. PO1 81403, the Dougherty Foundation, the Conner Fund, and the Alex Lemonade Foundation (K.K.M.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Wendy B. London, Victoria Castel, Andrew D.J Pearson, Akira Nakagawara, Katherine K. Matthay
Financial support: Wendy B. London
Administrative support: Wendy B. London, Victoria Castel
Provision of study materials or patients: Victoria Castel, Peter F. Ambros, Frank Berthold, Akira Nakagawara, Ruth L. Ladenstein, Tomoko Iehara
Collection and assembly of data: Wendy B. London, Victoria Castel, Tom Monclair, Peter F. Ambros, Susan L. Cohn, Frank Berthold, Ruth L. Ladenstein, Tomoko Iehara
Data analysis and interpretation: Wendy B. London, Victoria Castel, Andrew D.J Pearson, Katherine K. Matthay
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Barker LM, Pendergrass TW, Sanders JE, et al. Survival after recurrence of Ewing's sarcoma family of tumors. J Clin Oncol. 2005;23:4354–4362. doi: 10.1200/JCO.2005.05.105. [DOI] [PubMed] [Google Scholar]
- 2.Leavey P, Mascarenhas L, Marina N, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51:334–338. doi: 10.1002/pbc.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantonello T, Int-Veen C, Winkler P, et al. Initial patient characteristics can predict pattern and risk of relapse in localized rhabdomyosarcoma. J Clin Oncol. 2008;26:406–413. doi: 10.1200/JCO.2007.12.2382. [DOI] [PubMed] [Google Scholar]
- 4.Malempati S, Gaynon P, Sather H, et al. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800–5807. doi: 10.1200/JCO.2007.10.7508. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen K, Devidas M, Cheng S, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santana V, Furman W, McGregor L, et al. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112:2796–2801. doi: 10.1002/cncr.23507. [DOI] [PubMed] [Google Scholar]
- 7.Cohn S, Pearson A, London W, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Peto R, Pike M, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 12.Segal MR. Regression trees for censored data. Biometrics. 1988;44:35–47. [Google Scholar]
- 13.Davis RB, Anderson JR. Exponential survival trees. Stat Med. 1989;8:947–961. doi: 10.1002/sim.4780080806. [DOI] [PubMed] [Google Scholar]
- 14.Leblanc M, Crowley J. Survival trees by goodness of split. J Am Stat Assoc. 1993;88:457–467. [Google Scholar]
- 15.Fleming TR, Harrington DP. New York, NY: John Wiley & Sons; 1991. Counting Processes and Survival Analysis. [Google Scholar]
- 16.Lau L, Tai D, Weitzman S, et al. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol. 2004;26:227–232. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Kushner BH, Kramer K, Cheung NK. Chronic neuroblastoma. Cancer. 2002;95:1366–1375. doi: 10.1002/cncr.10800. [DOI] [PubMed] [Google Scholar]
- 18.Franks LM, Bollen A, Seeger RC, et al. Neuroblastoma in adults and adolescents: An indolent course with poor survival. Cancer. 1997;79:2028–2035. doi: 10.1002/(sici)1097-0142(19970515)79:10<2028::aid-cncr26>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Conte M, Parodi S, De Bernardi B, et al. Neuroblastoma in adolescents: The Italian experience. Cancer. 106:1409–2006. doi: 10.1002/cncr.21751. [DOI] [PubMed] [Google Scholar]
- 20.Cotterill SJ, Pearson AD, Pritchard J, et al. Late relapse and prognosis for neuroblastoma patients surviving 5 years or more: A report from the European Neuroblastoma Study Group “Survey.”. Med Pediatr Oncol. 2001;36:235–238. doi: 10.1002/1096-911X(20010101)36:1<235::AID-MPO1057>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.George RE, Variend S, Cullinane C, et al. Relationship between histopathological features, MYCN amplification, and prognosis: A UKCCSG study United Kingdom Children Cancer Study Group. Med Pediatr Oncol. 2001;36:169–176. doi: 10.1002/1096-911X(20010101)36:1<169::AID-MPO1041>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Ceschel S, Casotto V, Valsecchi M, et al. Survival after relapse in children with solid tumors: A follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer. 2006;47:560–566. doi: 10.1002/pbc.20726. [DOI] [PubMed] [Google Scholar]
- 23.Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse: A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45:2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Simon T, Berthold F, Klingenbiel T, et al. Do relapsed high risk neuroblastoma patients have a second chance. Pediatr Blood Cancer. 2009;53(5):736. [Google Scholar]