Abstract
Purpose
To assess the toxicity, pharmacokinetics, and pharmacodynamics of multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in children with relapsed/refractory leukemia.
Patients and Methods
Twelve patients with acute leukemia (11 with acute myeloid leukemia [AML]) received sorafenib on days 1 to 7 and then concurrently with cytarabine (1 g/m2) and clofarabine (stratum one: 40 mg/m2, n = 10; stratum two [recent transplantation or fungal infection]: 20 mg/m2, n = 2) on days 8 to 12. Sorafenib was continued until day 28 if tolerated. Two sorafenib dose levels (200 mg/m2 and 150 mg/m2 twice daily) were planned. Sorafenib pharmacokinetic and pharmacodynamic studies were performed on days 7 and 8.
Results
At sorafenib 200 mg/m2, two of four patients in stratum one and one of two patients in stratum two had grade 3 hand-foot skin reaction and/or rash as dose-limiting toxicities (DLTs). No DLTs were observed in six patients in stratum one at sorafenib 150 mg/m2. Sorafenib inhibited the phosphorylation of AKT, S6 ribosomal protein, and 4E-BP1 in leukemia cells. The rate of sorafenib conversion to its metabolite sorafenib N-oxide was high (mean, 33%; range, 17% to 69%). In vitro, the N-oxide potently inhibited FLT3–internal tandem duplication (ITD; binding constant, 70 nmol/L) and the viability of five AML cell lines. On day 8, sorafenib decreased blast percentages in 10 of 12 patients (median, 66%; range, 9% to 95%). After combination chemotherapy, six patients (three FLT3-ITD and three FLT3 wild-type AML) achieved complete remission, two (both FLT3-ITD AML) had complete remission with incomplete blood count recovery, and one (FLT3 wild-type AML) had partial remission.
Conclusion
Sorafenib in combination with clofarabine and cytarabine is tolerable and shows activity in relapsed/refractory pediatric AML.
INTRODUCTION
Sorafenib is an orally available multikinase inhibitor of C-RAF, B-RAF, c-KIT, FMS-like tyrosine kinase 3 (FLT3), platelet derived growth factor receptors α and β, vascular endothelial growth factor receptors 1, 2, and 3, and multiple intracellular kinases.1,2 Elevated expression or mutations in receptor tyrosine kinases and other kinases that lead to uncontrolled cell growth and survival have been described in acute leukemias.3 In acute myeloid leukemia (AML) cells, mutations in FLT3, such as internal tandem duplications (FLT3-ITD), and in c-KIT are observed in approximately 20% of patients, and aberrant signaling in the phosphoinositide 3-kinase/AKT and RAF/MEK/extracellular signal-regulated protein kinase pathways can be found in the majority of patients.4–6 Constitutive activation of one or more signaling pathways in AML cells is associated with poor prognosis.7 In addition, FLT3 is almost universally expressed in childhood acute lymphoblastic leukemia, especially in patients with MLL gene rearrangement and those with hyperdiploid.8 Hence, signaling inhibitors are an attractive therapeutic approach.
Phase I studies of sorafenib in adults with relapsed/refractory acute leukemias or newly diagnosed AML were recently reported.9–12 When used as a single agent, the maximum tolerated doses (MTDs) were 400 mg twice daily for discontinuous use every 21 to 28 days and 300 mg twice daily with continuous use for 28 days.10–12 Common toxicities were fatigue, nausea/vomiting, diarrhea, dyspnea, and skin rash/hand-foot skin reaction. Here we present the first report to our knowledge of the toxicity profile, dose-limiting toxicities (DLTs), pharmacokinetics, pharmacodynamics, and clinical activity of sorafenib, administered alone and in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia.
PATIENTS AND METHODS
Patient Population
Children, adolescents, and young adults age 21 years or younger with relapsed or refractory leukemia, irrespective of the number of prior salvage regimens, were eligible for the protocol. A Lansky or Karnofsky performance score greater than 50 was required. Patients were required to have shortening fraction of 25% or more by echocardiogram, pulse oxymetry of 93% or more at room air, serum direct bilirubin of 2.0 mg/dL or less, ALT of 4× the upper limit of normal or less, amylase and lipase of 2× the upper limit of normal or less, and an age-adjusted normal serum creatinine or creatinine clearance of 70 mL/min/1.73 m2 or greater.
Exclusion criteria included participants who had relapsed while receiving sorafenib therapy or had a poor response (less than partial remission) to previous clofarabine/cytarabine combination therapy; diastolic blood pressure (BP) greater than the 95th percentile for age and sex, despite optimal medical management; surgery or significant hemorrhage/bleeding event within 4 weeks or history of thrombotic or embolic episodes, angina, or myocardial infarction within 6 months; history of arrhythmia that required treatment; pregnant or breastfeeding women; and clinically significant unrelated systemic illness that would place the participant at undue risk in undergoing treatment. The interval from prior therapy was 14 days for standard chemotherapy and 30 days for investigational agents. This protocol was approved by the St Jude institutional review board and registered at ClinicalTrials.gov. Informed consent was obtained from all patients or their legal guardians.
Treatment Plan
Sorafenib was administered alone on days 1 to 7 and then concurrently with clofarabine (40 mg/m2) and cytarabine (1 g/m2) on days 8 to 12 (stratum one). Single-agent sorafenib was then continued until day 28 if tolerated. Patients who had undergone transplantation within 6 months or experienced fungal infection within 1 month were treated with a lower dose of clofarabine (20 mg/m2; stratum two). Two sorafenib dose levels (200 mg/m2 and 150 mg/m2 twice daily, with maximum doses of 400 mg and 300 mg, respectively) were planned, and interpatient dose de-escalation followed a three-plus-three design in each stratum. Intrapatient dose escalation was not permitted. The starting dose of 200 mg/m2 corresponds to 90% of the adult approved dose based on a body surface area of 1.8. Sorafenib was administered as a combination of capsules (compounded in strengths of 10, 20, 50, and 100 mg) and 200-mg tablets. Supportive measures with moisturizing creams, thick cotton gloves and/or socks, and pain medication were provided for hand-foot skin reaction or skin rash based on 2008 consensus panel recommendations.13 Bone marrow response was evaluated on day 22 and thereafter if hypoplasia was observed. Participants received subsequent courses of sorafenib and clofarabine/cytarabine, maintenance therapy with single-agent sorafenib, or transplantation according to clinical judgment.
Definition of DLT and MTD
This study used the Common Terminology Criteria for Adverse Events version 3.0 for toxicity. DLT was evaluated in the first course and included any grade 3 or 4 nonhematologic toxicities related to therapy except for grade 3 elevation in amylase, lipase, or total bilirubin or grade 3 or 4 elevation of ALT and AST that were asymptomatic and returned to lower than grade 2 elevation within 14 days; grade 3 hypokalemia, hypocalcemia, hypophosphatemia, or hypomagnesemia correctable with oral supplements; and grade 3 infection or fever. Dose-limiting hypertension was defined as confirmed diastolic BP of more than 25 mmHg above the 95th percentile for age and sex or elevated diastolic BP not controlled by a single antihypertensive medication within 14 days. For hand-foot skin reaction, grades and symptoms were based on 2008 consensus panel recommendations.13 Hematologic toxicity was only considered when bone marrow hypocellularity or aplasia (absolute neutrophil count < 300/μL and platelet count < 20,000/μL) for more than 56 days was observed in the absence of leukemia or other causes. The MTD was defined as the dose level at which one or fewer of six patients in a cohort (dose level) experienced DLT.
Pharmacodynamic Studies
Bone marrow was sampled before treatment and on day 8, before administration of clofarabine and cytarabine. Mononuclear cells were labeled with a panel of monoclonal antibodies to identify leukemic blast cells, as described previously.14 Cells were then treated with a membrane-permeabilizing reagent and labeled with rabbit monoclonal antibodies to phospho-AKT (Ser473), phospho–4E-BP1 (Thr37/46), and phospho–S6 ribosomal protein (S6RP; Ser235/236; Cell Signaling Technology, Danvers, MA) conjugated to Alexa-Fluor 488 or 647. The percent inhibition of each phosphoprotein was assessed by comparing geometric mean fluorescence intensity in the pretreatment and day-8 samples.
Pharmacokinetic Studies
Peripheral blood (3 mL) was collected on days 7 and 12 before and 2, 4.5, and 7.5 hours after sorafenib administration. Samples were centrifuged for 10 minutes at 3,000×g, and plasma was stored at −20°C until analysis. The concentration of sorafenib and its metabolite sorafenib N-oxide was measured by using a validated high-performance liquid chromatography–based method with tandem mass spectrometric detection.15 The average steady-state plasma concentration (Css,ave) of each analyte was calculated as the mean concentration of the four samples collected on the specified day. The extent of metabolic conversion was determined as the ratio of sorafenib N-oxide concentration to sorafenib concentration expressed as a percentage.
Evaluation of Sorafenib and Sorafenib N-Oxide in Kinase Assay and AML Cells
Sorafenib and sorafenib N-oxide were evaluated for 442-kinase selectivity profile, FLT3-ITD inhibition, and activity in AML cells, as described in the Appendix (online only).
Response Criteria and Definitions
Bone marrow response to therapy was assessed by morphologic and flow cytometric studies. Remission status was classified with the International Working Group morphologic response criteria.16 Flow cytometry results are reported as percentage of cells expressing leukemia-associated immunophenotype among bone marrow mononucleated cells; a threshold of 0.1% was used to define minimal residual disease (MRD) positivity.14,17
RESULTS
Patient Characteristics
The clinical features of the 12 patients with relapsed/refractory leukemia (11 with AML and one with early T-cell precursor leukemia) treated between September 2009 and October 2010 are summarized in Table 1. All were evaluable for toxicities and responses.
Table 1.
Patient Demographics and Clinical Characteristics
| Patient | Sorafenib Dose Level (mg/m2) | Age (years) | Sex | Disease Status | No. of Previous Regimens | No. of Sorafenib Doses | Response by Morphology | Response by Flow Cytometry (% AML cells) |
HFSR Grade | Skin Rash Grade | Subsequent Therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Day 8 | Day 22 | Before Subsequent Therapy | |||||||||||
| Stratum one, clofarabine 40 mg/m2 | ||||||||||||||
| 1 | 200 | 6 | Female | Relapsed AML | 1 | 26 | Complete | 42 | 13.8 | 0.2 | 0.6 | 2 | 2 | Transplantation |
| 2 | 200 | 14 | Male | Relapsed AML, FLT3-ITD positive (ratio, 0.47) | 2* | 29 | Complete | 80 | 27.4 | 1.2 | 0.3 | 3† | 3† | Sorafenib |
| 3 | 200 | 7 | Male | Refractory AML | 3* | 23 | No response | 45 | 5.3 | 35.6 | Not done | 2 | 2 | Transplantation |
| 4 | 200 | 13 | Male | Refractory AML, FLT3-ITD positive (ratio, 0.59) | 1 | 19 | Complete‡ | 52 | 2.6 | < 1 | < 0.1 | 3† | 3† | Transplantation |
| 5 | 150 | 7 | Male | Relapsed AML with myelodysplasia-related changes | 1 | 34 | No response | 18 | 11.1 | 18.9 | 20.6 | 2 | 2 | Chemotherapy |
| 6 | 150 | 6 | Male | Relapsed AML | 1 | 50 | Complete | 87 | 89 | 5 | < 0.1 | — | 2 | Transplantation |
| 7 | 150 | 9 | Female | Relapsed AML | 1 | 30 | Partial | 86 | 60 | 38.1 | 0.6 | 2 | — | Transplantation |
| 8 | 150 | 17 | Female | Relapsed AML, FLT3-ITD positive (ratio, 0.9) | 1 | 17 | Complete | 70.3 | 30.5 | 1.7 | < 0.1 | — | 1 | Sorafenib |
| 9 | 150 | 6 | Female | Early T-cell precursor leukemia | 4 | 34 | No response | 27 | 31.6 | 85.4 | 82 | — | 1 | Chemotherapy |
| 10 | 150 | 10 | Male | Relapsed AML | 1 | 21 | Complete | 31.2 | 7.0 | < 0.1 | < 0.1 | 2 | 2 | Transplantation |
| Stratum two, clofarabine 20 mg/m2 | ||||||||||||||
| 11 | 200 | 10 | Male | Refractory AML with myelodysplasia-related changes, FLT3-ITD positive (ratio, 0.26) | 5 | 24 | Complete‡ | 79 | 72§ | 31.7 | 10.2 | 3† | — | Sorafenib |
| 12 | 200 | 17 | Female | Relapsed AML, FLT3-ITD positive (ratio, 0.73) | 2* | 29 | Complete | 89.6 | 25.7 | 3.9 | < 0.1 | — | 2 | Sorafenib |
Abbreviations: AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; HFSR, hand-foot skin reaction; ITD, internal tandem duplication.
Includes allogeneic hematopoietic stem-cell transplantation.
Dose-limiting toxicity.
With incomplete blood count recovery.
Decrease in bone marrow cellularity was observed.
Toxicity and MTD
Toxicities attributed to therapy during cycle one are presented in Table 2. Hand-foot skin reaction and/or rash were observed in all patients (Table 1). With sorafenib 200 mg/m2, grade 3 dose-limiting skin toxicities were seen in two of the four patients in stratum one and one of the two patients in stratum two. After reducing the dose of sorafenib to 150 mg/m2 in stratum one, no dose-limiting skin toxicities were seen in the six patients.
Table 2.
Toxicity Profile of Patients
| Toxicity | Toxicity Grade |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratum One |
Stratum Two |
|||||||||||
| 200 mg/m2 (n = 4) |
150 mg/m2 (n = 6) |
200 mg/m2 (n = 2) |
||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Dermatology/skin | ||||||||||||
| Hand-foot skin reaction | 2 | 2* | 3 | 1* | ||||||||
| Rash | 2 | 2* | 2 | 3 | 1 | |||||||
| GI | ||||||||||||
| Abdominal pain | 1 | 2 | ||||||||||
| Nausea | 2 | 3 | ||||||||||
| Vomiting | 1 | 1 | 1 | 4 | ||||||||
| Heartburn/dyspepsia | 1 | |||||||||||
| Diarrhea | 2 | 1 | 1 | |||||||||
| Constipation | 1 | 1 | ||||||||||
| Infection | ||||||||||||
| Febrile neutropenia (infection) | 3 | 4 | 1 | |||||||||
| Grade 1 or 2 ANC | 1 | |||||||||||
| Grade 3 or 4 ANC | 2 | |||||||||||
| Cardiac | ||||||||||||
| Hypotension | 1 | |||||||||||
| Ocular/visual | ||||||||||||
| Conjunctivitis | 1 | 1 | ||||||||||
| Metabolic/laboratory | ||||||||||||
| Increased AST | 1 | 1 | 1 | |||||||||
| Increased ALT | 1 | 2 | 1 | |||||||||
| Increased amylase | 1 | |||||||||||
| Hyperglycemia | 1 | 1 | ||||||||||
| Hyponatremia | 1 | 1 | ||||||||||
| Hypokalemia | 2 | 1 | ||||||||||
| Hypophosphatemia | 1 | |||||||||||
| Hyperuricemia | 1 | 1 | ||||||||||
| Pain | ||||||||||||
| Headache | 1 | 1 | ||||||||||
| Dental | 1 | |||||||||||
| Back | 2 | 1 | ||||||||||
| Limb | 2 | 1 | 2 | |||||||||
| Constitutional symptoms | ||||||||||||
| Fever without neutropenia | 1 | 1 | 1 | |||||||||
Abbreviation: ANC, absolute neutrophil count.
Dose-limiting toxicity.
Skin toxicities tended to progress rapidly during or after the 5-day course of clofarabine and cytarabine, especially in those in whom sorafenib treatment was continued with early signs of skin toxicities. Therefore, patients were monitored closely and treated with topical emollients, and sorafenib was discontinued at the first sign of skin toxicity. Sorafenib discontinuation first occurred on day 13 (range, days 9-19) and on day 11.5 (range, days 8 to 15) at 200 and 150 mg/m2, respectively. All skin toxicities resolved completely within 1 to 2 weeks after discontinuation of sorafenib with supportive measures.13 No other DLT was observed. A patient (patient one) developed asymptomatic grade 4 elevation of ALT and AST on day 25 of therapy, which reverted to grade 1 on day 36 by withholding sorafenib. All other grade 3 infectious (eight febrile neutropenia, one herpes simplex virus reactivation, and one cellulitis) and metabolic (two hypokalemia and one hypophosphatemia) toxicities improved with medical management. The MTD of sorafenib was 150 mg/m2 (maximum, 300 mg) twice daily for stratum one. Enrollment of patients onto stratum two was discontinued because of slow accrual, and therefore, no dose recommendation was determined.
In 10 patients, sorafenib administration was resumed after resolution of skin toxicity, and all but one (patient 12) tolerated it well; seven patients resumed sorafenib during cycle one and three patients after completion of cycle one, with three of the 10 receiving single-agent sorafenib for up to 5 to 12 months. Patient 12, who had undergone allogeneic hematopoietic stem-cell transplantation 3 months earlier, developed grade 4 hyperbilirubinemia and grade 3 elevated liver enzymes 21 days after sorafenib readministration as a maintenance therapy; these signs improved gradually after discontinuation of sorafenib.
Sorafenib Pharmacodynamics and Pharmacokinetics
We used flow cytometry to simultaneously assess MRD and the status of downstream signaling pathways targeted by sorafenib. Sufficient material was available for these studies in 10 of the 12 patients (Table 3). At baseline, phosphorylation of AKT (three patients), 4E-BP1 (nine patients), and S6RP (eight patients) was detectable in leukemia cells. Independently of FLT3 status, the percentage of positive cells and mean fluorescence intensity of phospho-AKT (all three patients), phospho–4E-BP1 (seven patients), and phospho-S6RP (four patients) declined on day 8 (Table 3).
Table 3.
Changes in Leukemic-Cell Phosphoprotein Expression After Sorafenib Administration
| Patient | Day | Phospho–4E-BP1 (Thr37/46) |
Phospho-AKT (Ser473) |
Phospho–S6 Ribosomal Protein (Ser235/236) |
|||
|---|---|---|---|---|---|---|---|
| Positive Cells (%) | MFI | Positive Cells (%) | MFI | Positive Cells (%) | MFI | ||
| 1 | 0 | 96 | 1,033 | < 1 | 92 | 3,036 | |
| 8 | 59 | 404 | < 1 | 56 | 972 | ||
| 2 | 0 | 52 | 423 | 58 | 372 | 9 | 58 |
| 8 | 15 | 223 | 14 | 252 | 11 | 14 | |
| 5 | 0 | 57 | 550 | 46 | 354 | < 1 | |
| 8 | 6 | 129 | < 1 | Not tested | |||
| 6 | 0 | 59 | 1,373 | < 1 | 10 | 306 | |
| 8 | 54 | 865 | < 1 | 3 | 64 | ||
| 7 | 0 | < 1 | < 1 | < 1 | |||
| 8 | < 1 | < 1 | < 1 | ||||
| 8 | 0 | 40 | 1,362 | < 1 | 62 | 4,004 | |
| 8 | 29 | 399 | < 1 | 83 | 9,838 | ||
| 9 | 0 | 90 | 2,181 | < 1 | 92 | 6,824 | |
| 8 | 68 | 1,268 | < 1 | 95 | 10,906 | ||
| 10 | 0 | 56 | 1,851 | < 1 | 96 | 69,054 | |
| 8 | 91 | 3,332 | < 1 | 97 | 59,235 | ||
| 11 | 0 | 47 | 515 | 35 | 427 | 23 | 393 |
| 8 | 5 | 25 | 14 | 213 | 3 | 39 | |
| 12 | 0 | 67 | 1,067 | < 1 | 24 | 638 | |
| 8 | 85 | 2,261 | < 1 | 54 | 3,325 | ||
Abbreviation: MFI, mean fluorescence intensity of phosphoprotein expression in leukemic-cell population minus mean fluorescence intensity of isotype control.
All patients completed the pharmacokinetic studies. Individual Css,ave values for sorafenib and sorafenib N-oxide are listed in Table 4; mean steady-state concentrations are shown in Appendix Figure A1 (online only). At 200 mg/m2, mean sorafenib Css,ave was 6.5 mg/L (standard deviation, 3.6 mg/L); at 150 mg/m2, sorafenib Css,ave was 7.0 mg/L (standard deviation, 1.8 mg/L). Sorafenib exposure was similar between the two sorafenib dose levels, a finding that is not unexpected considering the reportedly wide interpatient pharmacokinetic variability of sorafenib.18 No trends were apparent in sorafenib concentrations on day 12 after 5 days of clofarabine/cytarabine. Mean (standard deviation) sorafenib N-oxide Css,ave values were 2.0 (standard deviation, 1.8) and 2.5 (standard deviation, 1.2) mg/L at 200 mg/m2 and 150 mg/m2, respectively. Mean conversion of sorafenib to sorafenib N-oxide was 33% (range, 17% to 69%) and 39% (range, 19% to 50%) on days 7 and 12, respectively.
Table 4.
Sorafenib and Sorafenib N-Oxide Pharmacokinetics
| Patient | Sorafenib Dose (mg/m2)* | Sorafenib Css,ave (mg/L) |
Sorafenib N-Oxide Css,ave (mg/L) |
||
|---|---|---|---|---|---|
| Day 7 | Day 12 | Day 7 | Day 12 | ||
| 1 | 200* | 3.3 | 8.0 | 0.67 | 3.2 |
| 2 | 200* | 10.3 | 9.4 | 1.8 | 4.1 |
| 3 | 200 | 5.9 | 6.7 | 3.1 | 2.9 |
| 4 | 200 | 4.6 | Not done | 0.78 | Not done |
| 11 | 200*† | 11.7 | 5.1‡ | 5.2 | 2.1 |
| 12 | 200† | 3.4 | Not done | 0.69 | Not done |
| Mean | 6.5 | 7.3 | 2.0 | 3.0 | |
| SD | 3.6 | 1.8 | 1.8 | 0.85 | |
| 5 | 150 | 4.9 | 2.6 | 3.4 | 1.3 |
| 6 | 150 | 8.7 | 5.4 | 3.8 | 1.0 |
| 7 | 150 | 8.3 | Not done | 2.4 | Not done |
| 8 | 150 | 8.0§ | Not done | 1.4 | Not done |
| 9 | 150 | 4.5 | Not done | 0.92 | Not done |
| 10 | 150 | 7.9 | Not done | 3.2 | Not done |
| Mean | 7.0 | — | 2.5 | — | |
| SD | 1.8 | — | 1.2 | — | |
Abbreviations: Css,ave, average steady-state plasma concentration; SD, standard deviation.
Sorafenib was administered as compounded capsules (patients 1 and 11) or as combination of compounded capsule and 200-mg tablets (patient 2). All other patients received sorafenib as 200 mg tablets.
Patients treated in stratum two and received lower dose of clofarabine.
Sorafenib schedule was changed from twice to once daily between days 8 and 12 pharmacokinetic assessments.
Value from pretreatment sample on day 7 at steady-state; serial sampling not performed.
Kinase Selectivity Profile and in Vitro Activity of Sorafenib and Sorafenib N-oxide
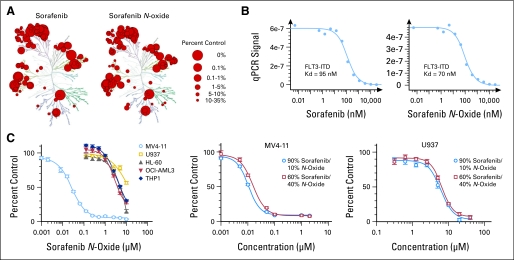
Because conversion of sorafenib to the N-oxide metabolite in our pediatric population was substantially greater than previously reported values of less than 10%,10,19 we evaluated the kinase selectivity of both compounds. Kinase interaction maps are shown in Figure 1A. The percent inhibition of individual kinases is listed in the Data Supplement. Although the kinase interaction maps of sorafenib and sorafenib N-oxide were visually similar, selectivity scores showed the metabolite to be less selective than the parent drug (Appendix Table A1, online only). Sorafenib N-oxide was slightly more potent than sorafenib against FLT3-ITD, with binding constants of 70 and 94 nmol/L, respectively (Fig 1B).
Fig 1.
Activity of sorafenib and sorafenib N-oxide in a human kinase screen and acute myeloid leukemia (AML) cell lines. (A) Kinase interaction maps of sorafenib and sorafenib N-oxide evaluated at 10 μmol/L across panel of 442 kinases by active-site competitive binding assay. Binding inhibition is shown as percentage of kinase that remained bound compared with that in dimethyl sulfoxide (DMSO)–treated control. Larger red circles indicate higher-affinity binding. (B) FMS-like tyrosine kinase 3 (FLT3)–internal tandem duplication (ITD) binding constants of sorafenib and sorafenib N-oxide. Quantity of kinase measured by quantitative real-time polymerase chain reaction (qPCR) signal is plotted against corresponding drug concentration in log10 scale. Binding constants (Kd) were calculated in duplicate experiments by using Hill equation. (C) Activity of sorafenib and sorafenib N-oxide in AML cells in MTT assay. Cells were treated for 72 hours with increasing concentrations of sorafenib N-oxide or indicated mixtures of sorafenib and sorafenib N-oxide. Drug concentration in log scale is plotted against mean percentage of drug-treated cells in relation to DMSO-treated cells. Data points represent two to three independent experiments with six to eight replicates for each drug concentration.
The activities of sorafenib and its metabolite were examined in AML cell lines. MV4-11 cells with FLT3-ITD were more sensitive to sorafenib N-oxide (IC50, 25.8 nmol/L) compared with other AML cell lines, which had wild-type FLT3 (IC50, 3.9 to 13.3 μmol/L; Fig 1C). However, MV4-11 cells were even more sensitive to mixtures of N-oxide and sorafenib (60% sorafenib: IC50, 16.4 nmol/L; 90% sorafenib: IC50, 11.0 nmol/L). A similar pattern was observed in U937 cells (IC50 with 0%, 60% and 90% sorafenib were 13.3, 6.4, and 5.5 μmol/L, respectively). These data suggest that sorafenib is more active than sorafenib N-oxide against AML cells.
Leukemia Response
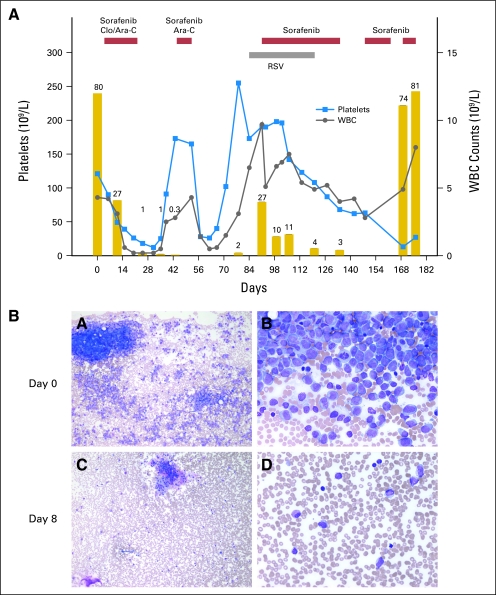
After 7 days of single-agent sorafenib therapy, 10 patients had a median decrease in bone marrow blast percentage from baseline by 66% (range, 9% to 95%), whereas two had an increase from baseline of 2% and 17%, respectively (Table 1). Four of five patients with FLT3-ITD AML (patients 2, 4, 8, and 12) and three of six with wild-type FLT3 AML (patients 1, 3, and 10) had a blast reduction that exceeded 50%. Patient 11 (FLT3-ITD AML) had a remarkable decrease in bone marrow cellularity with persistence of blasts. After receiving sorafenib in combination with clofarabine and cytarabine, six patients with AML achieved complete remission (four MRD negative), two with complete remission with incomplete blood count recovery (one MRD negative), and one patient had a partial remission. Responses were observed not only in all five patients with FLT3-ITD AML but also in four of the six with wild-type FLT3 AML. Six patients proceeded to allogeneic hematopoietic stem-cell transplantation. Three other patients with FLT3-ITD AML were offered transplantation, but patients 2 and 11 developed respiratory syncytial virus infection, and patient 8 declined this option. Patients 8 and 11 have been receiving single-agent sorafenib for 5 and 12 months, respectively. Patient 2 became refractory at 6 months; his clinical course is shown in Figure 2.
Fig 2.
Clinical course and bone marrow response in patient 2. (A) Patient achieved complete remission with persistence of minimal residual disease after receiving sorafenib with clofarabine (Clo) and cytarabine (Ara-C); acute myeloid leukemia recurred during respiratory syncytial virus (RSV) infection. Single-agent sorafenib induced third complete remission, and RSV infection cleared. Five weeks later, after sorafenib was interrupted because of thrombocytopenia, blast cells increased to 74%; there was no response to readministration of sorafenib. Gold bars represent percentage of bone marrow blast cells. (B) Bone marrow response to initial 7-day treatment with single-agent sorafenib. Magnification ×10 (panels A, C); ×40 (panels B, D).
DISCUSSION
In this study, we evaluated the safety, pharmacokinetics, pharmacodynamics, and clinical activity of sorafenib alone and in combination with clofarabine and cytarabine in children and adolescents with relapsed/refractory leukemia. Hand-foot skin reaction and/or skin rash were DLTs at 200 mg/m2/dose twice daily, and the MTD was defined as 150 mg/m2/dose twice daily.
Skin toxicities were observed in all our patients; however, adults treated with single-agent sorafenib had a lower overall incidence of hand-foot skin reaction (9% to 62%) and skin rash (19% to 66%).13 In adult phase I studies, the incidence and severity of skin toxicity increased with sorafenib dose and plasma exposure.13 Because sorafenib plasma concentrations were similar between our pediatric patient population and adults,18 high sorafenib concentrations alone do not likely explain the 100% incidence of skin toxicity. The rate of conversion of sorafenib to the active metabolite, sorafenib N-oxide, was three to four times that reported in healthy adults and adults with AML.10,19 Therefore, higher exposure to the metabolite may have contributed to the increased skin toxicity. Because both clofarabine and cytarabine are associated with skin toxicities, their concurrent administration with sorafenib also may have contributed to the severity of skin toxicities. In addition to supportive measures, close monitoring and discontinuation of sorafenib at the first sign of hand-foot skin reaction or rash are essential. After resolution of skin toxicities, sorafenib can be resumed in most patients. Because of the wide interpatient variability of sorafenib pharmacokinetics, monitoring of sorafenib and sorafenib N-oxide levels may help dose adjustments, but the optimal schedule of administration of sorafenib with cytarabine-based regimens (eg, simultaneous v sequential) for AML with FLT3-ITD and wild-type FLT3 remains to be determined.
In seven of 12 patients (seven [63%] of 11 patients with AML; 95% CI, 30% to 90%), the percentage of bone marrow blasts was reduced by more than 50% from baseline levels regardless of FLT3 status after 7 days of treatment with sorafenib as a single agent. Furthermore, maintenance treatment with single-agent sorafenib controlled leukemia activity for a prolonged period in three patients with FLT3-ITD. Thus, sorafenib clearly has significant antileukemic activity in relapsed or refractory pediatric AML. Mean sorafenib steady-state concentrations in our pediatric population (6.5 and 7.0 mg/L at 200 mg/m2 and 150 mg/m2, respectively) were consistent with the range reported in adult phase I studies of sorafenib 400 mg twice daily (4.0 to 6.4 mg/L).18 The promising activity of sorafenib in pediatric AML could be the result of the much higher rate of conversion of sorafenib to the active metabolite sorafenib N-oxide observed in our patients (mean, 33%) as compared with that reported in adult patients (mean, < 10%).10 We were also able to document inhibition of phospho-AKT, phospho-S6RP, and phospho–4E-BP1 in leukemia cells in vivo. Although its precise mechanism is unknown, sorafenib may produce antileukemic effects through inhibition of protein translation mediated by S6RP and 4E-BP120 and through removal of the negative regulatory effect of AKT on Bad and induction of apoptosis.21
Zhang et al22 showed synergistic effects in FLT3 wild-type OCI-AML3 cells simultaneously exposed to sorafenib and cytarabine. Similarly, we previously demonstrated synergism or additive effects of simultaneous treatment with sorafenib and cytarabine in seven AML cell lines (six with wild-type FLT3) and primary pediatric AML cells.23 We also reported that sorafenib administered simultaneously with cytarabine prolonged survival in an AML xenograft model with wild-type FLT3.23 In vitro, sorafenib upregulates proapoptotic Bim, Bad, Bax, and Bak proteins and downregulates Mcl-1, X-linked inhibitor of apoptosis, and survivin, which leads to activation of the intrinsic apoptotic pathway and may sensitize AML cells to cytarabine-based chemotherapy.22,24 Treatment with sorafenib in combination with clofarabine and cytarabine achieved an overall response in nine of 12 patients (nine [82%] of 11 patients with AML; 95% CI, 48% to 98%); four of the six patients with FLT3 wild-type AML responded, in addition to all five patients with FLT3-ITD AML.
In conclusion, sorafenib 150 mg/m2/dose twice daily is the recommended dose when administered in combination with 5 days of clofarabine (40 mg/m2/d) and cytarabine (1,000 mg/m2/d) in children with relapsed/refractory leukemia who have not undergone a recent transplantation or fungal infection. Hand-foot skin reaction and/or skin rash are DLTs and require close monitoring. The regimen described here seems to be promising for the treatment of high-risk pediatric AML. These results justify the incorporation of sorafenib into future pediatric AML trials.
Supplementary Material
Acknowledgment
We thank Shelley Orwick for assistance with in vitro experiments, Chris Clark for technical assistance in phosphoprotein studies, and our research and pharmacokinetic nurses.
Appendix
Kinase Selectivity Profile
The kinome-wide selectivity profiles of sorafenib (Chemie Tek, Indianapolis, IN) and sorafenib N-oxide (Toronto Research Chemicals, North York, Ontario, Canada) were determined at a concentration of 10 μmol/L using a 442-kinase active-site competitive binding assay (Ambit Biosciences, San Diego, CA; Fabian MA, Biggs WH 3rd, Treiber DK, et al: Nat Biotechnol 23:329-336, 2005). Binding inhibition was expressed as the percentage of the kinase that remained bound after drug treatment versus dimethyl sulfoxide–treated control. A lower-percentage control is associated with greater inhibition. A kinase interaction map was constructed using the visualization software TREEspot (www.kinomescan.com). A selectivity score was determined as previously described (Karaman MW, Herrgard S, Treiber DK, et al: Nat Biotechnol 26:127-132, 2008).
FMS-Like Tyrosine Kinase 3–Internal Tandem Duplication Inhibition Assay
Inhibition of FMS-like tyrosine kinase 3–internal tandem duplication activity by sorafenib and sorafenib N-oxide was determined by an active-site competitive binding assay (Ambit Biosciences; Fabian MA, Biggs WH 3rd, Treiber DK, et al: Nat Biotechnol 23:329-336, 2005). Both compounds were tested over a concentration range of 0.508 to 30,000 nmol/L. The binding constant was calculated by using the Hill equation; the Levenberg-Marquardt algorithm was used to generate a nonlinear least-squares fit (Hill A: J Physiol 40:4-7, 1910; Levenberg K: Quart Appl Math 2:164-168, 1944).
MTT Cell Viability Assay
The activity of sorafenib and sorafenib N-oxide in five acute myeloid leukemia cell lines (MV4-11, HL-60, U937, THP-1, and OCI-AML3) was compared using a 72-hour drug incubation and the MTT Cell Proliferation Kit I (Roche, Indianapolis, IN), as previously described (Hu S, Niu H, Minkin P, et al: Mol Cancer Ther 7:1110-1120, 2008). MV4-11 and U937 cells were also treated with a 90% sorafenib plus 10% N-oxide mixture or a 60% sorafenib plus 40% N-oxide mixture. Results were measured as a mean percentage of dimethyl sulfoxide–treated control cells at each concentration. Two to three independent experiments with six to eight replicates each were performed for each compound and cell line.
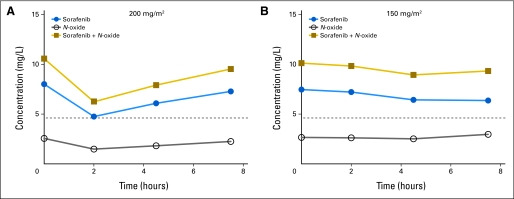
Fig A1.
Mean steady-state sorafenib and sorafenib N-oxide plasma concentrations. Sorafenib (A) 200 mg/m2 or (B) 150 mg/m2 was administered twice daily, and serial pharmacokinetic sampling was performed on day 7. Dashed line indicates sorafenib concentration of 10 μmol/L (4.65 mg/L).
Table A1.
Selectivity Scores of 10 μmol/L Sorafenib and Sorafenib N-Oxide in Screen of 442 Kinases
| Compound | Selectivity Score Type | No. of Hits | No. of Nonmutant Kinases | Selectivity Score |
|---|---|---|---|---|
| Sorafenib | S (35) | 98 | 386 | 0.254 |
| S (10) | 63 | 386 | 0.163 | |
| S (1) | 30 | 386 | 0.078 | |
| Sorafenib N-oxide | S (35) | 121 | 386 | 0.313 |
| S (10) | 79 | 386 | 0.205 | |
| S (1) | 36 | 386 | 0.093 |
Footnotes
Supported in part by National Institutes of Health Grant No. R01 CA138744 (S.D.B) and Cancer Center Support Grant No. P30 CA021765; by the American Lebanese Syrian Associated Charities; and by Bayer/Onyx Pharmaceuticals (H.I.). C.-H.P. is an American Cancer Society professor.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, December 4-7, 2010, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00908167.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Hiroto Inaba, Bayer Pharmaceuticals, Onyx Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hiroto Inaba, Jeffrey E. Rubnitz, Robbin Christensen, Stanley B. Pounds, Ching-Hon Pui, Raul C. Ribeiro, Dario Campana, Sharyn D. Baker
Financial support: Hiroto Inaba, Ching-Hon Pui, Sharyn D. Baker
Administrative support: Hiroto Inaba, Robbin Christensen, Ching-Hon Pui
Provision of study materials or patients: Hiroto Inaba, Jeffrey E. Rubnitz, Elaine Coustan-Smith, Kenneth M. Heym, Mihaela Onciu, Raul C. Ribeiro, Dario Campana
Collection and assembly of data: Hiroto Inaba, Jeffrey E. Rubnitz, Elaine Coustan-Smith, Brian D. Furmanski, Gerard P. Mascara, Kenneth M. Heym, Robbin Christensen, Mihaela Onciu, Sheila A. Shurtleff, Sharyn D. Baker
Data analysis and interpretation: Hiroto Inaba, Elaine Coustan-Smith, Lie Li, Brian D. Furmanski, Gerard P. Mascara, Mihaela Onciu, Sheila A. Shurtleff, Stanley B. Pounds, Ching-Hon Pui, Dario Campana, Sharyn D. Baker
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fabian MA, Biggs WH, 3rd, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: A direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 3.Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 4.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–4269. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholl C, Gilliland DG, Fröhling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol. 2008;35:336–345. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Masson K, Rönnstrand L. Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell Signal. 2009;21:1717–1726. doi: 10.1016/j.cellsig.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Kornblau SM, Womble M, Qiu YH, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown P, Levis M, Shurtleff S, et al. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005;105:812–820. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- 9.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratz KW, Cho E, Levis MJ, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24:1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump M, Hedley D, Kamel-Reid S, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: A NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma. 2010;51:252–260. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 12.Borthakur G, Kantarjian H, Ravandi F, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2010;96:62–68. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 14.Coustan-Smith E, Ribeiro RC, Rubnitz JE, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zhao M, Navid F, et al. Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3033–3038. doi: 10.1016/j.jchromb.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: A review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 19.Lathia C, Lettieri J, Cihon F, et al. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57:685–692. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Chapuis N, Tamburini J, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010;95:819–828. doi: 10.3324/haematol.2009.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori S, Cortes J, Kantarjian H, et al. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49:2246–2255. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Niu H, Inaba H, et al. Enhanced activity of the multikinase inhibitor sorafenib in combination with cytarabine in acute myeloid leukemia. J Natl Cancer Inst. 2011;103:893–905. doi: 10.1093/jnci/djr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci MS, Kim SH, Ogi K, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.