Abstract
During mitosis, the Golgi complex undergoes a multi-step fragmentation process that is instrumental to its correct partitioning into the daughter cells. To prepare for this segregation, the Golgi ribbon is initially separated into individual stacks during the G2 phase of the cell cycle. Then, at the onset of mitosis, these individual stacks are further disassembled into dispersed fragments. Inhibition of this Golgi fragmentation step results in a block or delay of G2/M transition, depending on the experimental approach. Thus, correct segregation of the Golgi complex appears to be monitored by a ‘Golgi mitotic checkpoint’. Using a microinjection-based approach, we recently identified the first target of the Golgi checkpoint, whereby a block of this Golgi fragmentation impairs recruitment of the mitotic kinase Aurora-A to, and its activation at, the centrosomes. Overexpression of Aurora-A can override this cell cycle block, indicating that Aurora-A is a major effector of the Golgi checkpoint. We have also shown that this block of Aurora-A recruitment to the centrosomes is not mediated by the known mechanisms of regulation of Aurora-A function. Here we discuss our findings in relation to the known functions of Aurora-A.
Key words: mitotic checkpoint, golgi complex, cell cycle, G2, aurora-A, cancer
Introduction
The Golgi complex is the central organelle in the endomembrane system, and it has a well-established role as the processing and sorting station in the transport of protein cargoes to their final destinations. In mammalian cells, the Golgi complex is composed of stacks of cisternae that are interconnected through the tubular non-compact zones, to form a continuous membranous system, known as the Golgi ribbon.1 As with other organelles, during cell division, the Golgi complex must be correctly inherited by the daughter cells.2 G2-specific cleavage of the tubular non-compact zones connecting the stacks (Golgi “unlinking”) is the preparatory step for this segregation3,4 (Fig. 1). Subsequently, at the onset of mitosis, further fragmentation of these separated stacks produces small dispersed fragments from the Golgi, which can then be partitioned between the daughter cells. These Golgi fragments are then reassembled into functional Golgi ribbons in late mitosis.5
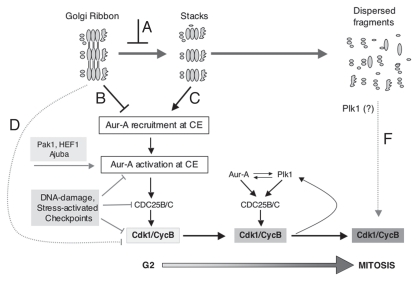
Figure 1.
Unlinking of the Golgi ribbon during G2 inhibits the recruitment and activation of Aur-A at the centrosome trough a novel mechanism. The Golgi complex is organised as a continuous membranous system composed of stacks interconnected by tubules, a structure known as the “Golgi ribbon.” During the G2 phase of the cell cycle, the Golgi ribbon is cleaved into its constituent “stacks.” A block in Golgi fragmentation (A) impairs Aur-A recruitment at the centrosome (CE). This effects on Aur-A can be explained by two models: the triggering of a signalling pathway that causes the release of Aur-A from the centrosomes (B); or the lack of a positive signal that supports the recruitment of Aur-A to the centrosomes (C). The inhibition of Aur-A recruitment and activation at the centrosome in turn impairs the first activation of Cdk1-CycB at the centrosome during early G2 (light grey box). The Golgi-dependent G2 block is not mediated by known Aur-A activators (i.e., Pak1, HEF1, Ajuba), and nor it is indirectly mediated by Plk1 and Cdk1-CycB complex. In addition, the Golgi-dependent G2 block is not regulated by known G2 checkpoint mediators, which act downstream the Golgi checkpoint. At the onset of mitosis the kinases Aur-A, Plk1 and cycB-Cdk1 become functionally connected by a positive feedback loop, leading to irreversible activation of Cdk1-CycB (dark grey box) and progression into mitosis. In the case of a prolonged inhibition of fragmentation of the Golgi ribbon, additional synergistic mechanisms can help to prolong the cell cycle block. For example, a block of Golgi fragmentation could stimulate a transcription-based response that leads to the expression of inhibitors of Cdk1-CycB (D), or it would impede the activation of a Golgi-based Plk1/Cdk1/CycB2 pathway (E).
Unlinking of the Golgi stacks in G2 is controlled by the fission-inducing protein CtBP1/BARS (BARS) and various kinases (e.g., Raf1, MEK1, ERK2/1c) that can phosphorylate the Golgi-located effectors GRASP65 and GRASP55 (reviewed in ref. 5). Inhibition of this Golgi unlinking process can be achieved through a variety of treatments. Microinjection of blocking antibodies or dominant-negative mutants aimed at a functional block of BARS,6,7 GRASP658 and GRASP55,9 has been shown to result in persistent cell cycle arrest in G2.6,8,10 In addition, inhibition or depletion of the kinases that control this specific Golgi fragmentation step, or expression of point-mutants of GRASP55 or GRASP65, can also result in a significant delay, but not a block, of G2/M transition.5 Collectively, these findings have revealed a novel prerequisite for mitotic entry, suggesting that there is a ‘Golgi checkpoint’ that is dedicated to monitoring the correct mitotic partitioning of the Golgi complex.11 Thus, investigations into the components of this putative checkpoint should lead to the identification of novel proteins and mechanisms involved in cell cycle regulation.
Golgi-Dependent Control of Aurora-A
We have recently addressed the question of how this Golgi fragmentation is functionally connected to cell cycle regulation. Indeed, to block the cell cycle in G2, this inhibition of Golgi fragmentation must affect the activation of cyclin-B-dependent kinase 1 (CycB-Cdk1), which is the key event that initiates mitotic entry.12 As we have shown that G2-specific separation of the Golgi stacks (i.e., the essential step for mitotic entry) is coordinated with the separation of the duplicated centrosomes, which itself coincides with the first activation of CycB-Cdk1,13 we hypothesised that a block of this Golgi-partitioning step would affect the mechanisms governing specific centrosome activation of CycB-Cdk1. A pivotal role in this CycB-Cdk1 activation has been shown for the Ser/Thr kinase Aurora-A (Aur-A; reviewed in ref. 14). Aur-A is recruited to the centrosomes in G2, where it is then activated by phosphorylation on Thr288.15 Once activated, Aur-A phosphorylates Cdc25B phosphatase.16 At the same time, Plk1 is another important regulator of mitotic entry, and it can phosphorylate Cdc25C phosphatase.12 These two Cdc25 phosphatases then together activate the CycB-Cdk1 complex and cooperate in the irreversible entry into mitosis (Fig. 1). Finally, an additional regulation of mitotic entry is operated through DNA-damage-activated or stress-activated checkpoints that can block CycB-Cdk1 activation, which thus occurs should there be incompletely replicated or damaged DNA, or in the presence of other cell stresses.12
To approach our investigations into how the block of Golgi fragmentation affects the centrosome activation of CycB-Cdk1, we decided to use a microinjection-based assay to interfere with the functions of the fission inducing protein BARS3,6 and the Golgi-associated protein GRASP65, the latter of which has an essential role in the maintenance of Golgi structure and function.8 This injection-based approach was aimed at inducing acute inhibition of the Golgi partitioning, and it had already been extensively validated as an effective method for inducing specific, potent and persistent Golgi-dependent G2 block.6,8 In contrast, transfection-based approaches are prone to non-specific alterations to the kinetics of G2/M transition and to the activation of compensatory mechanisms (our unpublished data), and as such they have been shown to induce moderate, rather than persistent, inhibition of G2/M transition. (ref. 4 and Preisinger et al. EMBO J 2005).
We coupled this method to single-cell, immunofluorescence-based investigations of the functional consequences of inhibition of Golgi fragmentation. Although time consuming, this detailed analysis was essential to be able to focus on the processes that are precisely regulated and that occur in limited space and time. Such an analysis would not be feasible by the more ‘usual’ western blotting or FACS-based approaches, and indeed it has been instrumental for revealing the functional consequences of acute inhibition of Golgi fragmentation on the recruitment and activation state of key cell cycle players at the centrosomes during G2.
Thus, we systematically examined whether the Golgi-dependent cell cycle block affected the localization and activation state of a pool of relevant cell cycle regulators. The main outcome of this study was that a block in this Golgi partitioning results in reduced recruitment to and impaired activation at the centrosomes of Aur-A during the G2 phase of the cell cycle17 (Fig. 1). The same treatment did not modify the recruitment or activation of other kinases involved in the activation of Aur-A and CycB-Cdk1, such as Pak and Plk1.17 The localization of Aur-A at the centrosomes during G2 was not influenced by the inhibition of Aur-A itself, or of Plk1 or CycB-Cdk1, or by activation of the DNA-damage checkpoint, which thus shows that this reduced Aur-A recruitment to the centrosomes is not a secondary consequence of a mechanism that affects one of these kinases of G2/M progression. Moreover, we showed that it is only upon entry into mitosis that the further Aur-A activation becomes fully dependent on the activities of Cdk1 and Plk1, in line with other findings18 (Fig. 1). To exclude non-specific effects of this microinjection approach, we showed that the injected blockers of Golgi fragmentation did not affect Aur-A recruitment in cells with a pre-fragmented Golgi ribbon,17 indicating that the injection of blockers of Golgi fragmentation influences Aur-A recruitment only in the presence of an intact Golgi ribbon. An additional indication of the central role of Aur-A in the Golgi checkpoint is that the Golgi-dependent G2 block was overridden by Aur-A overexpression, while it was not affected by expression of the functionally related kinase Plk1. Collectively, these finding revealed that Aur-A is a major effector of the Golgi checkpoint and they suggest that the further definition of these signalling pathways should lead to the identification of novel mechanisms of cell cycle regulation. However, the search for the mechanisms connecting Aur-A recruitment to the status of Golgi (dis) assembly will benefit from the addressing of the next new, and important, questions that we discuss here, including:
Is the Golgi checkpoint a true checkpoint?
Is inhibition of Aur-A recruitment to the centrosomes sufficient to explain the potent and prolonged G2 block?
Future Directions
The first question is whether the Golgi checkpoint encompasses the features of a true checkpoint. Indeed, the effects of the block of Golgi fragmentation on Aur-A can be explained according to two general models: (1) the turning-on of a signalling pathway that causes the release of Aur-A from the centrosomes; or (2) the turning-off of a signalling pathway that stimulates the recruitment of Aur-A to the centrosomes (Fig. 1). This is not a secondary issue, as only the first model is compatible with the correct definition of a checkpoint: a signalling pathway that is turned-on by ‘damage’ to deactivate a key cell cycle element. To demonstrate the existence of such a true checkpoint, we should be able to inhibit an activity to induce the overriding of this G2 block. As we observed that Aur-A recruitment to the centrosomes precedes the initial severing of the Golgi ribbon,17 the most logical interpretation of our data is in line with the first model. Nevertheless, this aspect will be addressable only when more elements of the signalling pathway have been defined. Why mammalian cells have their Golgi in the form of a ribbon and why they have evolved a mechanism to control the correct partitioning of the organelle is an additional important question. A reasonable possibility is that such a complex organization serves specialized functions, such as targeted delivery of cargoes and signalling proteins (for a discussion, reviewed in ref. 19). Moreover, it has been recently shown that the spindle transfers Golgi residents required for post-mitotic reassembly of the Golgi ribbon.20 Such a mechanism would ensure that both daughter cells inherit the ability to form a Golgi ribbon and, thus, to transport proteins in a targeted manner.
The second question relates to whether the potent and prolonged G2 block that results from a block in Golgi fragmentation can be explained entirely by the inhibition of Aur-A recruitment to the centrosomes. A complication in addressing this issue is that although the essential role of Aur-A in the control of G2/M progression has been clearly established,21,22 the mechanism of its activation is still an ill-defined event in the cell cycle. Aur-A expression increases as cells pass through S phase; then during G2, Aur-A is recruited to the centrosomes, where it is activated by either autophosphorylation or transphosphorylation on Thr288. Recent studies have demonstrated that activation of Aur-A is required for centrosome maturation and separation, and for G2/M transition.22,23 Moreover, although there is a certain degree of overlap in the cellular functions that are regulated by Plk1 and Aur-A in mammalian cells, Aur-A has recently been shown to be an upstream regulatory kinase of Plk1 during G2 (Fig. 1), further confirming the key role of Aur-A in G2/M transition.22 Nevertheless, small-molecule chemical inhibitors of Aur-A have shown variable levels of effects on cell cycle progression, and generally they result in a delay in G2/M transition and a more prominent block in prometaphase.24 How are these findings related to the role of Aur-A in the Golgi checkpoint?
A first consideration here is that the block of Golgi fragmentation does not simply result in inhibition of Aur-A activation; it also inhibits Aur-A recruitment to (or causes Aur-A release from) the centrosomes. As discussed above, Aur-A recruitment to the centrosomes during G2 is independent of its kinase activity.17 Therefore, the effects on Aur-A recruitment that are triggered by blocking Golgi fragmentation are inherently different from the effects of Aur-A kinase inhibitors, and this is indicative of a more complex mechanism that will also involve other proteins. Small-interfering (si)RNA-mediated depletion of Aur-A represents a similar approach that has indeed been shown to result in a more pronounced reduction of cell entry into mitosis, with its potency strictly related to the degree of reduction in total Aur-A levels.
Moreover, although our findings have shown that Aur-A is the key element in this G2 arrest that is induced by a block in Golgi fragmentation, as this G2 arrest can be overridden by overexpression of Aur-A, but not of Plk1,17 these data do not exclude other additional synergistic mechanisms that can help to prolong the cell cycle block. One interesting possibility has been suggested by a series of reports that have shown that small-molecule chemical inhibitors of Plk1 or Aur-A can promote persistent G2 block when this treatment is concomitant with cell stress, such as that which occurs after DNA damage.24 Thus, we can hypothesise that inhibition of Golgi fragmentation has two main outcomes: (1) the activation of signalling pathways that cause Aur-A release from the centrosomes; and (2) the activation of an unknown cell stress, which would render the cells fully dependent on Aur-A for entry into mitosis.
A further possibility is based on an analogy with the organisation of other cell cycle checkpoints. These essential systems include the G1/S, G2/M and spindle-assembly checkpoints, and they are designed to monitor various types of DNA damage and inappropriate microtubule attachment to the kinetochores. In response to such damage, the first intervention is through a fast-acting and kinase-based response and the delaying of cell cycle progression.12 In the case of more intense damage, the additional intervention of a transcriptionally regulated response results in increased levels of Cdk inhibitors, which makes the cell cycle block more persistent12 (Fig. 1). As a consequence, the cells are arrested at a specific stage of the cell cycle for repair, or should the errors or damage be too severe to be resolved, they are committed to programmed cell death.12
Similarly, the intensity and duration of the Golgi checkpoint might be correlated to the severity of the ‘damage’ [in relation to the experimental procedures and to the protein(s) targeted], and to the possibility to activate compensatory mechanisms to achieve correct segregation of the Golgi membranes. Thus, the Golgi checkpoint is potentially composed of two modules: a fast response that triggers the immediate release of Aur-A from the centrosomes, and a transcription-based response that contributes to the prolonged block of G2/M progression by altering the levels/activities of cell cycle regulators. The development of new assays that can induce the Golgi checkpoint will contribute to the addressing of this question.
Finally, another unexplored and speculative possibility arises as several key regulatory components of G2/M transition have also been found on the Golgi complex, including the Cdc25C phosphatases, Myt1 and CycB2, or have been shown to interact with Golgi-associated proteins during mitosis, as is the case for the Plk1 association with GRASP65.5,25 Although it has been suggested that the roles of these proteins are to regulate post-G2 fragmentation of the Golgi stacks,5 an intriguing possibility is that they can cooperate, in turn, to stimulate a Golgi-based pathway of CycB2-Cdk1 activation.
Indeed, mammals express two B-type cyclins, CycB1 and CycB2. CycB1 is located at the centrosomes, spindle and nucleus, while CycB2 is located at the Golgi complex and ER.26 However, following knock-out experiments in mice, it was reported that CycB1 is an essential gene for cell viability, while nullizygous CycB2 mice develop normally and did not show any obvious abnormalities.27 This led to the conclusion that CycB2 is not essential for cell function. Nevertheless, a recent siRNA-based analysis revealed a more complex scenario, as extensive depletion of CycB1 in HeLa cells led to only minor effects on G2/M progression.28 It was only the co-depletion of CycB1 with CycB2 that induced a profound arrest in G2.28 These data indicate that CycB1 and CycB2 can cooperate to promote stable mitotic entry, and they offer another example of how complex cell cycle-regulatory circuits can give rise to compensatory mechanisms. Thus, we can speculate that the consequences of blocking Golgi fragmentation could be two-fold: from one side, it would result in the release of Aur-A from the centrosomes, thus turning off the centrosome-based activation of Cdk1/CycB1; and from the other side, it would impede the activation of a Golgi-based Plk1/Cdk1/CycB2 pathway (Fig. 1). Only the contemporary inhibition of these two pathways would trigger prolonged G2 block.
In conclusion, our studies have revealed that a block in Golgi partitioning induces a strong impairment of the recruitment to and activation at the centrosomes of the mitotic kinase Aur-A through a novel mechanism. These findings have important physiological and pharmacological consequences. Indeed, inhibition of Aur-A activity is considered a clinically sound anticancer therapy for tumours that are characterised by high levels of Aur-A overexpression, as is the case in a variety of human cancers, including breast, colorectal, bladder, pancreatic, gastric and ovarian cancers.24 For this reason, several Aur-A inhibitors have been developed as antimitotic drugs. These are now undergoing clinical trials for anticancer treatments, although they show complex phenotypes and can show side effects.24 Thus, the definition of the molecular mechanisms that link Golgi fragmentation and Aur-A regulation of G2/M should lead to the identification of novel regulatory elements of the role of Aur-A, and thus to the identification of additional targets for cancer therapies.
Acknowledgements
The authors would like to thank C.P. Berrie for critical reading of the manuscript. A.C. and D.C. acknowledge the Italian Association for Cancer Research (AIRC, Milan, Italy; IG 6074 and IG 4664) and Telethon (Italy) for financial support. R.I.C. is recipient of a “Loredana Gualandi Sabotti” Fellowship awarded by the Italian Association for Cancer Research (AIRC, Milan, Italy); M.L.B. is recipient of a Fellowships awarded by the Italian Foundation for Cancer Research (FIRC, Milan, Italy).
Abbreviations
- BARS
brefeldin A ADP-ribosylated substrate
- GRASP
golgi reassembly stacking protein
- Plk1
polo-like kinase1
- ERK1
extracellular signal-regulated kinase 1
- MEK1
MAPK/ERK kinase 1
References
- 1.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 2.Lowe M, Barr FA. Inheritance and biogenesis of organelles in the secretory pathway. Nat Rev Mol Cell Biol. 2007;8:429–439. doi: 10.1038/nrm2179. [DOI] [PubMed] [Google Scholar]
- 3.Colanzi A, Hidalgo Carcedo C, Persico A, Cericola C, Turacchio G, Bonazzi M, et al. The Golgi mitotic checkpoint is controlled by BARS-dependent fission of the Golgi ribbon into separate stacks in G2. EMBO J. 2007;26:2465–2476. doi: 10.1038/sj.emboj.7601686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein TN, Linstedt AD. Mitogen-activated protein kinase kinase 1-dependent Golgi unlinking occurs in G2 phase and promotes the G2/M cell cycle transition. Mol Biol Cell. 2007;18:594–604. doi: 10.1091/mbc.E06-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei JH, Seemann J. Mitotic division of the mammalian Golgi apparatus. Semin Cell Dev Biol. 2009;20:810–816. doi: 10.1016/j.semcdb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo Carcedo C, Bonazzi M, Spano S, Turacchio G, Colanzi A, Luini A, et al. Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science. 2004;305:93–96. doi: 10.1126/science.1097775. [DOI] [PubMed] [Google Scholar]
- 7.Corda D, Colanzi A, Luini A. The multiple activities of CtBP/BARS proteins: the Golgi view. Trends Cell Biol. 2006;16:167–173. doi: 10.1016/j.tcb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Sutterlin C, Hsu P, Mallabiabarrena A, Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. doi: 10.1016/s0092-8674(02)00720-1. [DOI] [PubMed] [Google Scholar]
- 9.Duran JM, Kinseth M, Bossard C, Rose DW, Polishchuk R, Wu CC, et al. The role of GRASP55 in Golgi fragmentation and entry of cells into mitosis. Mol Biol Cell. 2008;19:2579–2587. doi: 10.1091/mbc.E07-10-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura S, Yoshioka K, Barr FA, Lowe M, Nakayama K, Ohkuma S, et al. Convergence of cell cycle regulation and growth factor signals on GRASP65. J Biol Chem. 2005;280:23048–23056. doi: 10.1074/jbc.M502442200. [DOI] [PubMed] [Google Scholar]
- 11.Rabouille C, Kondylis V. Golgi ribbon unlinking: an organelle-based G2/M checkpoint. Cell Cycle. 2007;6:2723–2729. doi: 10.4161/cc.6.22.4896. [DOI] [PubMed] [Google Scholar]
- 12.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 13.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 14.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 15.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Cazales M, Schmitt E, Montembault E, Dozier C, Prigent C, Ducommun B. CDC25B phosphorylation by Aurora-A occurs at the G2/M transition and is inhibited by DNA damage. Cell Cycle. 2005;4:1233–1238. doi: 10.4161/cc.4.9.1964. [DOI] [PubMed] [Google Scholar]
- 17.Persico A, Cervigni RI, Barretta ML, Corda D, Colanzi A. Golgi partitioning controls mitotic entry through Aurora-A kinase. Mol Biol Cell. 2010;21:3708–3721. doi: 10.1091/mbc.E10-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Horn RD, Chu S, Fan L, Yin T, Du J, Beckmann R, et al. Cdk1 activity is required for mitotic activation of aurora A during G2/M transition of human cells. J Biol Chem. 2010;285:21849–21857. doi: 10.1074/jbc.M110.141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol. 2010;188:621–628. doi: 10.1083/jcb.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei JH, Seemann J. The mitotic spindle mediates inheritance of the Golgi ribbon structure. J Cell Biol. 2009;184:391–397. doi: 10.1083/jcb.200809090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 22.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 24.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta D, Linstedt AD. Mitotic inhibition of GRASP65 organelle tethering involves Polo-like kinase 1 (PLK1) phosphorylation proximate to an internal PDZ ligand. J Biol Chem. 2010;285:39994–40003. doi: 10.1074/jbc.M110.189449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackman M, Firth M, Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 1995;14:1646–1654. doi: 10.1002/j.1460-2075.1995.tb07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandeis M, Rosewell I, Carrington M, Crompton T, Jacobs MA, Kirk J, et al. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci USA. 1998;95:4344–4349. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soni DV, Sramkoski RM, Lam M, Stefan T, Jacobberger JW. Cyclin B1 is rate limiting but not essential for mitotic entry and progression in mammalian somatic cells. Cell Cycle. 2008;7:1285–1300. doi: 10.4161/cc.7.9.5711. [DOI] [PubMed] [Google Scholar]