Abstract
The filamentous cytoskeletal systems fulfil seemingly incompatible functions by maintaining a stable scaffolding to ensure tissue integrity and simultaneously facilitating rapid adaptation to intracellular processes and environmental stimuli. This paradox is particularly obvious for the abundant keratin intermediate filaments in epithelial tissues. The epidermal keratin cytoskeleton, for example, supports the protective and selective barrier function of the skin while enabling rapid growth and remodelling in response to physical, chemical and microbial challenges. We propose that these dynamic properties are linked to the perpetual re-cycling of keratin intermediate filaments that we observe in cultured cells. This cycle of assembly and disassembly is independent of protein biosynthesis and consists of distinct, temporally and spatially defined steps. In this way, the keratin cytoskeleton remains in constant motion but stays intact and is also able to restructure rapidly in response to specific regulatory cues as is needed, e.g., during division, differentiation and wound healing.
Key words: cytoskeletal filament, cytokeratin, turnover, live cell imaging, microscopy
Introduction
The Greek “πάντα ρεί” (panta rhei), everything is in flow, refers to an ancient concept that can be traced back to the pre-Socratic philosopher Heraklit (∼520-460 BC). Its basic tenet that everything is in motion and subject to continuous change has survived until today. We have chosen this aphorism to characterize properties of a system, which at first sight does not seem to be a good example. Among the cytoplasmic filament systems the intermediate filaments are certainly not known for their rapid changes but rather for their scaffolding function in tissue architecture. Their pronounced stability is reflected by the extremely long half life of their polypeptide components which, in the case of the neuronal cytoskeleton, may be in the range of months or even years.1,2 Multiple diseases further attest to the stabilizing scaffolding functions of these filaments. Thus, a large number of point mutations have been described in the genes encoding epidermal keratins acting in a dominant negative fashion and leading to skin blistering presumably by weakening the epithelial cytoskeleton.3 On the other hand, epithelia cannot be static but must be dynamic to meet the various challenges imposed by their environment. Regeneration is a very efficient mechanism to accomplish this and epithelia are among the fastest dividing tissues. Cell division and subsequent differentiation are accompanied by substantial changes in shape, position and cell architecture (e.g., from the crypt base to the villar tip in the intestine or from the basal to the suprabasal compartment in stratified epithelia). Furthermore, epithelial morphogenetic events are among the most prominent developmental phenomena, for example during gland formation and neural tube formation. The dynamic nature of epithelia becomes even more apparent in various stress situations which require substantial plasticity as is the case, e.g., after wounding. All these properties require a rapidly adaptable and pliable cytoskeleton. In accordance, we have recently observed that the keratin cytoskeleton in cultured cells is not static but subject to constant protein biosynthesis-independent turnover that we refer to as the “keratin cycle” (Fig. 1; details in ref. 4). The cycle supports both continued cytoskeletal filament network integrity and filament network remodeling. We therefore propose that the keratin cycle is tightly linked to epithelial plasticity which is needed for sustaining the complex barrier function that is both resilient and responsive.
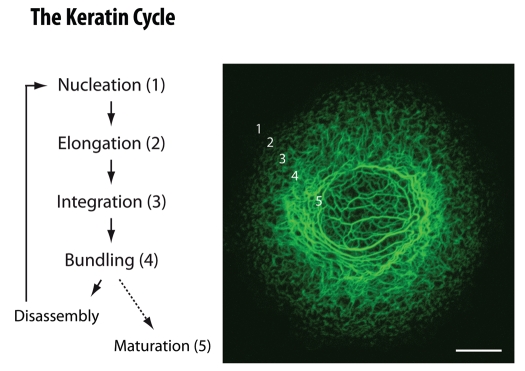
Figure 1.
Schematic representation of the keratin cycle of assembly and disassembly. Steps of the keratin cycle are listed at left which occur in topologically defined regions starting with nucleation in the vicinity of focal adhesions in the cell periphery, followed by particle elongation and particle integration into the peripheral keratin filament network. Filaments within the network further bundle. Inward-transport of keratin particles and keratin filaments relies mainly on actin filaments but also on microtubules. Inward-moving and bundling filaments disassemble into soluble subunits that diffuse throughout the cytoplasm and are re-utilized for another round of assembly. Alternatively, filaments mature and serve as a stable encasement of the nucleus. The fluorescence micrograph at right is taken from a time-lapse recording (see corresponding Video 1) of a vulvar carcinoma-derived A431 subclone producing fluorescent human keratin 13 chimera HK13-EGFP.33 Bar, 10 µm.
The Keratin Cycle Operates in the Absence of Protein Biosynthesis and Allows “Mixing” of Filaments
Biological structures are subject to continuous restructuring thereby allowing architectural and functional adaptation to requirements imposed by the cell itself and its environment. The restructuring can be accomplished by two fundamentally different mechanisms: either by degradation and replacement through de novo synthesis or by disassembly and subsequent reassembly both of which need not be exclusive but may be used in combination. In support of the first mode, keratin ubiquitination has been described presumably leading to subsequent degradation.5–7 This mechanism is upregulated in stress situations, i.e., during cytoskeletal remodeling8,9 and occurs preferentially for mutant, i.e., misfolded keratins.10–12 Furthermore, based on observations on vimentin a model of “dynamic co-translation” was proposed by which de novo filament synthesis is linked to microtubule-dependent integration of newly-formed filaments into the network.13 Observations from our laboratory, however, show that degradation and de novo formation do not account for the full spectrum of keratin filament network plasticity. Complete inhibition of protein biosynthesis does not abrogate filament formation.4 In accordance, using photoactivatable fluorescent keratins it was noted that these keratins “spread” throughout the cell.4 Figure 2A–C′ shows that this is due to co-assembly of the photoactivated keratin with endogenous, non-photoactivated keratins. A similar “mixing” of filaments occurs upon cell fusion. Figure 2D-D″ presents an example of a heterokaryon consisting of a cell producing green keratins and another cell producing red keratins. Increasing amounts of mixed filaments appear over time after cell fusion. Extensive mixing is first observed in the cell periphery and only later in the perinuclear zone. These observations are in full agreement with previous fusion experiments,14 which, in addition, demonstrated isotype-specific differences in mixing kinetics thereby extending in vitro observations on the promiscuity of keratins with pair-specific assembly and stability properties.15,16 Comparable situations occur in vivo and have been well documented. For example, in the epidermis keratins 5/14 mRNAs are predominantly localized in the basal cell compartment. Protein expression, however, persists in the suprabasal cell layers which produce keratins 1/10 mRNAs. Yet, the keratin networks containing the different encoded polypeptides are not separable but completely co-localize.17,18 Thus, switches in keratin network composition occurring during development, differentiation or in various stress paradigms may be accomplished by gradual network exchange through continuous disassembly/reassembly cycles without the need of network disruption or de novo network formation. Consequently, different admixtures of keratins may confer specific intermediate properties on a given epithelial cell thereby fine-tuning context-dependent functions that have been linked to the keratin cytoskeleton such as cell motility, organelle trafficking, translation, signaling, immune response and cell survival.19–26
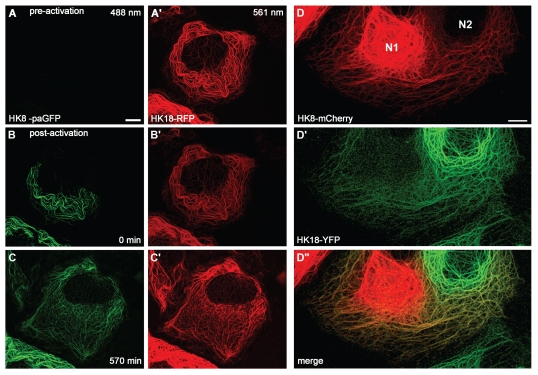
Figure 2.
Filament mixing after photoactivation (A–C′) and cell fusion (D-D″). (A–C′) Hepatocellular carcinoma-derived PLC cells were co-transfected with a construct coding for a photoactivatable GFP fused to human keratin 8 (HK8-paGFP; reviewed in ref. 4) together with a construct coding for a human keratin 18-red fluorescent protein hybrid (HK18-RFP; reviewed in ref. 66). HK8-paGFP was activated by irradiation with UV light (405 nm) at time point 0 min. Fluorescence was recorded at 488 nm and 561 nm by time-lapse fluorescence microscopy. Note that the photoactivated HK8-paGFP is evenly distributed throughout the endogenous network at 570 min. Bar, 10 µm. (D-D″) PLC-derived PK18-5 cells expressing human keratin 18-enhanced yellow fluorescent protein chimera HK18-YFP36 and PK8-7 cells producing human keratin 8-mCherry chimera HK8-mCherry66 were fused by treatment with polyethylene glycol (PEG 1500) after mixing according to published procedures.67 Heterokaryons were fixed with methanol and acetone 3 h after fusion and imaged by confocal laser scanning microscopy. Note mixing of filaments predominantly in the peripheral cytoskeleton. N1, nucleus of HK8-mCherry-producing parent cell; N2, nucleus of HK18-YFP-producing parent cell. Bar, 10 µm.
Keratin Cycling is Spatially Organized to Support Polarized Cell Shape Change and Motility
A distinguishing feature of intermediate filaments in comparison to the other cytoskeletal filaments is their lack of polarity because of the full symmetry of their tetrameric assembly units.27 It was therefore suggested that intermediate filament plasticity is governed by lateral exchange of subunits throughout the entire network (reviewed in ref. 28–30). The heterogeneity in filament diameter further supports this notion (for keratins see ref. 31). Our own observations in living cells, however, show that other modes exist and may even be prevalent.4,32–34 Video 1 presents a typical example of a time lapse-recording of fluorescently-labeled keratins in a cultured epithelial cell. Perpetual inward motion of fluorescent material is readily apparent starting with small particles that appear in the cell periphery (“nucleation”), enlarge (“elongation”), and are subsequently incorporated into the peripheral network (“integration”). Filaments move further toward the nucleus while they fuse laterally (“bundling”). Movement of growing and bundling filaments is orchestrated primarily by actin filaments but also relies on microtubules.34,35 Some filaments dissolve into soluble subunits (“disassembly”) while others are stabilized, notably those that are anchored to the nucleus and to desmosomes (“maturation”). Disassembled subunits diffuse rapidly throughout the cytoplasm and are re-utilized for another round of assembly. Details of this process have been worked out for cultured cells4,32–36 and are summarized in Figure 1. The point that we would like to make here is that this mode of keratin cycling is, in contrast to the previously proposed non-selective lateral exchange mechanism, compartmentalized. Thus, specialized regions exist within cells that favor/allow keratin filament assembly whereas other regions favor/allow keratin disassembly. In this way, the intrinsically non-polar network becomes differentially organized, which is needed in polarized cells, most notably in migrating cells. In this instance, the network needs to assemble/ grow toward the leading edge while filaments in the trailing edge need to be disassembled/retracted. This is indeed what is observed in migrating cells.35,37
Keratin Cycling is Linked to the Environment through Cell Adhesions
Intricately involved in cell migration are focal adhesions, which are hotspots of mechanical coupling and signaling between cells and their surrounding extracellular matrix. Focal adhesions coordinate cytoskeletal organization by anchorage of actin stress fibers (e.g., reviewed in ref. 38–40) and by cross talk with microtubules.41,42 We have further shown that focal adhesions serve as major determinants of the keratin cycle.43 The exact signals that foster keratin filament formation, however, are still unknown. Yet, some candidate proteins have been described that may be involved in the physical linkage of focal adhesions and keratins. Among them, plectin is certainly a prime candidate, because certain isoforms have been localized to focal adhesions and because of its cytoskeletal cross-linking function.44,45 A recent study46 provides evidence for such a link in the case of vimentin intermediate filaments. Further potential mediators of this association are integrins, vinculin/metavinculin, talin and zyxin.47–51
Keratin intermediate filaments are anchored to the extracellular matrix by hemidesmosomes and to adjacent cells by desmosomes. These adhesion sites are established upon stable tissue formation to support the tight mechanical coupling of cells to their neighbors and the underlying connective tissue necessitating a switch from a dynamic to a more static cytoskeleton. And this is indeed what preliminary experiments seem to suggest: desmosome-and hemidesmosome-anchored keratin filaments exit the keratin cycle of assembly and disassembly and become much more stable (see ref. 4; unpublished observations). The increased stability of these mature filaments is reflected by an increased resilience to disruption as observed in cells treated with the tyrosine phosphatase inhibitor vanadate.36 The elucidation of the underlying molecular mechanisms will touch on basic questions of eptihelial cell biology, especially the understanding of the balance between the migratory/invasive and sessile/tissue-promoting phenotypes during normal developmental processes and malignant transformation.
Keratin Cycling is Modified in Various Ways to Allow Graded Structural and Functional Responses
Regulation of the keratin cycle may act either globally or at individual stages and/or may induce alternative routes. When considering any kind of regulation one should keep in mind that self-assembly of keratins into 10 nm filaments and further self-organization into bundles and networks are intrinsic properties of this unusual filament system.27,52 The prime modifier of filament dynamics is certainly phosphorylation, since keratins are highly phosphorylated.53 The complex patterns of phosphorylation make this modification suitable to confer graded effects on keratin cycling. Direct evidence for such a mechanism, however, is lacking and requires experimental testing. Yet, it has been shown that keratin cycling can be completely and reversibly stopped by inhibition of p38 MAPK which has been shown to target keratins.54–56
Selective accumulation of cycle intermediates has been observed in various situations. Thus, the soluble keratin pool is increased upon phosphorylation occurring during mitosis and upon increased stress.57,58 Elevated bundle formation and loss of soluble keratins upon shear stress was also reported and was interpreted as a functional adaptation of the cytoskeleton to withstand the shear forces more effectively.59
A disruption of the cycle occurs in disease. When mutant keratins that cause the blistering disease epidermolysis bullosa simplex are expressed in cultured cells, often only a residual filamentous keratin network is found. Instead, granular aggregates are seen in the cell periphery. Live cell imaging shows that these keratin assemblies are still generated in the vicinity of focal adhesions and are transported towards the cell interior much like the typical nascent keratin filament precursors. But these particles do not elongate, they are rather short-lived and rarely give rise to filamentous structures.43,60 The keratin granules that are formed disassemble rapidly into subunits and enter another abortive cycle of assembly. Thus, keratin cycling still occurs but is abbreviated resulting in a dysfunctional cytoskeleton.
Conclusions and Outlook
A tempting, yet testable, hypothesis is that keratin cycling is a crucial prerequisite of epithelial function and that (minor) disturbances are sufficient to interfere with the stress-protective properties of the keratin cytoskeleton.57 This may be of particular relevance for keratin mutations that do not directly perturb network organization and do not directly induce disease but are considered to be disease risk factors.61 It will therefore be interesting to examine, how keratin cycling is affected in the different transgenic mouse models carrying mutant human keratins that have been shown to be associated with altered susceptibility to liver disease.22,62,63 Recent observations link sumoylation to keratin cycling in these situations64 and observations in the model organism C. elegans further support this notion, since reduced intermediate filament turnover because of altered sumoylation results in embryonic developmental defects.65
The keratin cycle provides a conceptual framework that should help to understand the cross talk between the keratin cytoskeleton and epithelial plasticity as a prime prerequisite for proper tissue function. Cycling may range from barely detectable in fully differentiated cells to very rapid in motile cells and thus cover the full dynamic spectrum occurring at different temporal and spatial scales and thereby providing copious evidence for the pre-Socratic idea of “panta rhei.” In contrast to the old philosophers' view, however, this phenomenon is not only a pervasive property of all matter but is part of the “cycle of life,” which is economical and makes use of available material to continuously probe the environment in the pursuit of functional and structural optimization.
Acknowledgements
We thank Ursula Wilhelm for expert technical assistance and Dr. George Patterson (NIH, Bethesda USA) for the plasmid encoding paGFP. We are also grateful for the many stimulating discussions on the topic with Drs. Thomas Magin and Michael Beil. The work was supported by the German Research Council (LE 566/10, WI 731/6-1).
References
- 1.Nixon RA, Logvinenko KB. Multiple fates of newly synthesized neurofilament proteins: evidence for a stationary neurofilament network distributed non-uniformly along axons of retinal ganglion cell neurons. J Cell Biol. 1986;102:647–659. doi: 10.1083/jcb.102.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millecamps S, Gowing G, Corti O, Mallet J, Julien JP. Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J Neurosci. 2007;27:4947–4956. doi: 10.1523/JNEUROSCI.5299-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolsch A, Windoffer R, Wurflinger T, Aach T, Leube RE. The keratin-filament cycle of assembly and disassembly. J Cell Sci. 2010;123:2266–2272. doi: 10.1242/jcs.068080. [DOI] [PubMed] [Google Scholar]
- 5.Ku NO, Omary MB. Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J Cell Biol. 2000;149:547–552. doi: 10.1083/jcb.149.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogel MR, Jaitovich A, Ridge KM. The role of the ubiquitin proteasome pathway in keratin intermediate filament protein degradation. Proc Am Thorac Soc. 2010;7:71–76. doi: 10.1513/pats.200908-089JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikanth B, Vaidya MM, Kalraiya RD. O-GlcNAcylation determines the solubility, filament organization and stability of keratins 8 and 18. J Biol Chem. 2010;285:34062–34071. doi: 10.1074/jbc.M109.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaitovich A, Mehta S, Na N, Ciechanover A, Goldman RD, Ridge KM. Ubiquitin-proteasome-mediated degradation of keratin intermediate filaments in mechanically stimulated A549 cells. J Biol Chem. 2008;283:25348–25355. doi: 10.1074/jbc.M801635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na N, Chandel NS, Litvan J, Ridge KM. Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. FASEB J. 2010;24:799–809. doi: 10.1096/fj.08-128967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loffek S, Woll S, Hohfeld J, Leube RE, Has C, Bruckner-Tuderman L, et al. The ubiquitin ligase CHIP/STUB1 targets mutant keratins for degradation. Hum Mutat. 2010;31:466–476. doi: 10.1002/humu.21222. [DOI] [PubMed] [Google Scholar]
- 11.Lee D, Santos D, Al-Rawi H, McNeill AM, Rugg EL. The chemical chaperone trimethylamine N-oxide ameliorates the effects of mutant keratins in cultured cells. Br J Dermatol. 2008;159:252–255. doi: 10.1111/j.1365-2133.2008.08596.x. [DOI] [PubMed] [Google Scholar]
- 12.Chamcheu JC, Virtanen M, Navsaria H, Bowden PE, Vahlquist A, Torma H. Epidermolysis bullosa simplex due to KRT5 mutations: mutation-related differences in cellular fragility and the protective effects of trimethylamine N-oxide in cultured primary keratinocytes. Br J Dermatol. 2010;162:980–989. doi: 10.1111/j.1365-2133.2009.09615.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Shav-Tal Y, Trcek T, Singer RH, Goldman RD. Assembling an intermediate filament network by dynamic cotranslation. J Cell Biol. 2006;172:747–758. doi: 10.1083/jcb.200511033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paramio JM, Casanova ML, Alonso A, Jorcano JL. Keratin intermediate filament dynamics in cell heterokaryons reveals diverse behaviour of different keratins. J Cell Sci. 1997;110:1099–1111. doi: 10.1242/jcs.110.9.1099. [DOI] [PubMed] [Google Scholar]
- 15.Hatzfeld M, Franke WW. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985;101:1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann I, Franke WW. Heterotypic interactions and filament assembly of type I and type II cytokeratins in vitro: viscometry and determinations of relative affinities. Eur J Cell Biol. 1997;72:122–132. [PubMed] [Google Scholar]
- 17.Lersch R, Fuchs E. Sequence and expression of a type II keratin, K5, in human epidermal cells. Mol Cell Biol. 1988;8:486–493. doi: 10.1128/mcb.8.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichel J, Bussow H, Grund C, Magin TM. Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol Biol Cell. 2001;12:1557–1568. doi: 10.1091/mbc.12.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depianto D, Kerns ML, Dlugosz AA, Coulombe PA. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet. 2010;42:910–914. doi: 10.1038/ng.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Kellner J, Lee CH, Coulombe PA. Interaction between the keratin cytoskeleton and eEF1Bgamma affects protein synthesis in epithelial cells. Nat Struct Mol Biol. 2007;14:982–983. doi: 10.1038/nsmb1301. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 22.Ku NO, Toivola DM, Strnad P, Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol. 2010;12:876–885. doi: 10.1038/ncb2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long HA, Boczonadi V, McInroy L, Goldberg M, Maatta A. Periplakin-dependent re-organisation of keratin cytoskeleton and loss of collective migration in keratin-8-downregulated epithelial sheets. J Cell Sci. 2006;119:5147–5159. doi: 10.1242/jcs.03304. [DOI] [PubMed] [Google Scholar]
- 24.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005;15:608–617. doi: 10.1016/j.tcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Vijayaraj P, Kroger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol. 2009;187:175–184. doi: 10.1083/jcb.200906094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 28.Ngai J, Coleman TR, Lazarides E. Localization of newly synthesized vimentin subunits reveals a novel mechanism of intermediate filament assembly. Cell. 1990;60:415–427. doi: 10.1016/0092-8674(90)90593-4. [DOI] [PubMed] [Google Scholar]
- 29.Miller RK, Vikstrom K, Goldman RD. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol. 1991;113:843–855. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon KH, Yoon M, Moir RD, Khuon S, Flitney FW, Goldman RD. Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J Cell Biol. 2001;153:503–516. doi: 10.1083/jcb.153.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel A, Eichner R, Aebi U. Polymorphism of reconstituted human epidermal keratin filaments: determination of their mass-per-length and width by scanning transmission electron microscopy (STEM) J Ultrastruct Res. 1985;90:323–335. doi: 10.1016/s0022-5320(85)80010-1. [DOI] [PubMed] [Google Scholar]
- 32.Windoffer R, Leube RE. Imaging of keratin dynamics during the cell cycle and in response to phosphatase inhibition. Methods Cell Biol. 2004;78:321–352. doi: 10.1016/s0091-679x(04)78012-7. [DOI] [PubMed] [Google Scholar]
- 33.Windoffer R, Leube RE. Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J Cell Sci. 1999;112:4521–4534. doi: 10.1242/jcs.112.24.4521. [DOI] [PubMed] [Google Scholar]
- 34.Woll S, Windoffer R, Leube RE. Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur J Cell Biol. 2005;84:311–328. doi: 10.1016/j.ejcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Kolsch A, Windoffer R, Leube RE. Actin-dependent dynamics of keratin filament precursors. Cell Motil Cytoskeleton. 2009;66:976–985. doi: 10.1002/cm.20395. [DOI] [PubMed] [Google Scholar]
- 36.Strnad P, Windoffer R, Leube RE. Induction of rapid and reversible cytokeratin filament network remodeling by inhibition of tyrosine phosphatases. J Cell Sci. 2002;115:4133–4148. doi: 10.1242/jcs.00096. [DOI] [PubMed] [Google Scholar]
- 37.Rolli CG, Seufferlein T, Kemkemer R, Spatz JP. Impact of tumor cell cytoskeleton organization on invasiveness and migration: a microchannel-based approach. PLoS One. 2010;5:8726. doi: 10.1371/journal.pone.0008726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 39.Patla I, Volberg T, Elad N, Hirschfeld-Warneken V, Grashoff C, Fassler R, et al. Dissecting the molecular architecture of integrin adhesion sites by cryoelectron tomography. Nat Cell Biol. 2010;12:909–915. doi: 10.1038/ncb2095. [DOI] [PubMed] [Google Scholar]
- 40.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efimov A, Kaverina I. Significance of microtubule catastrophes at focal adhesion sites. Cell Adh Migr. 2009;3:285–287. doi: 10.4161/cam.3.3.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krylyshkina O, Anderson KI, Kaverina I, Upmann I, Manstein DJ, Small JV, et al. Nanometer targeting of microtubules to focal adhesions. J Cell Biol. 2003;161:853–859. doi: 10.1083/jcb.200301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Windoffer R, Kolsch A, Woll S, Leube RE. Focal adhesions are hotspots for keratin filament precursor formation. J Cell Biol. 2006;173:341–348. doi: 10.1083/jcb.200511124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezniczek GA, Abrahamsberg C, Fuchs P, Spazierer D, Wiche G. Plectin 5′-transcript diversity: short alternative sequences determine stability of gene products, initiation of translation and subcellular localization of isoforms. Hum Mol Genet. 2003;12:3181–3194. doi: 10.1093/hmg/ddg345. [DOI] [PubMed] [Google Scholar]
- 45.Rezniczek GA, Janda L, Wiche G. Plectin. Methods Cell Biol. 2004;78:721–755. [PubMed] [Google Scholar]
- 46.Burgstaller G, Gregor M, Winter L, Wiche G. Keeping the vimentin network under control: cell-matrix adhesion-associated plectin 1f affects cell shape and polarity of fibroblasts. Mol Biol Cell. 2010;21:3362–3375. doi: 10.1091/mbc.E10-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 48.Kreis S, Schonfeld HJ, Melchior C, Steiner B, Kieffer N. The intermediate filament protein vimentin binds specifically to a recombinant integrin alpha2/beta1 cytoplasmic tail complex and co-localizes with native alpha2/beta1 in endothelial cell focal adhesions. Exp Cell Res. 2005;305:110–121. doi: 10.1016/j.yexcr.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 49.Sun N, Critchley DR, Paulin D, Li Z, Robson RM. Identification of a repeated domain within mammalian alpha-synemin that interacts directly with talin. Exp Cell Res. 2008;314:1839–1849. doi: 10.1016/j.yexcr.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 50.Sun N, Critchley DR, Paulin D, Li Z, Robson RM. Human alpha-synemin interacts directly with vinculin and metavinculin. Biochem J. 2008;409:657–667. doi: 10.1042/BJ20071188. [DOI] [PubMed] [Google Scholar]
- 51.Sun N, Huiatt TW, Paulin D, Li Z, Robson RM. Synemin interacts with the LIM domain protein zyxin and is essential for cell adhesion and migration. Exp Cell Res. 2010;316:491–505. doi: 10.1016/j.yexcr.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Lee CH, Coulombe PA. Self-organization of keratin intermediate filaments into cross-linked networks. J Cell Biol. 2009;186:409–421. doi: 10.1083/jcb.200810196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Woll S, Windoffer R, Leube RE. p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J Cell Biol. 2007;177:795–807. doi: 10.1083/jcb.200703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ku NO, Azhar S, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease causing mutation. J Biol Chem. 2002;277:10775–10782. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- 56.Menon MB, Schwermann J, Singh AK, Franz-Wachtel M, Pabst O, Seidler U, et al. p38 MAP kinase and MAPKAP kinases MK2/3 cooperatively phosphorylate epithelial keratins. J Biol Chem. 2010;285:33242–33251. doi: 10.1074/jbc.M110.132357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toivola DM, Strnad P, Habtezion A, Omary MB. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010;20:79–91. doi: 10.1016/j.tcb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omary MB, Ku NO, Liao J, Price D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem. 1998;31:105–140. [PubMed] [Google Scholar]
- 59.Flitney EW, Kuczmarski ER, Adam SA, Goldman RD. Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodeling of keratin intermediate filaments. FASEB J. 2009;23:2110–2119. doi: 10.1096/fj.08-124453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner NS, Windoffer R, Strnad P, Grund C, Leube RE, Magin TM. Epidermolysis bullosa simplex-type mutations alter the dynamics of the keratin cytoskeleton and reveal a contribution of actin to the transport of keratin subunits. Mol Biol Cell. 2004;15:990–1002. doi: 10.1091/mbc.E03-09-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ku NO, Michie SA, Soetikno RM, Resurreccion EZ, Broome RL, Oshima RG, et al. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest. 1996;98:1034–1046. doi: 10.1172/JCI118864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snider NT, Weerasinghe SV, Herrmann H, Omary MB. Keratin hypersumoylation alters filament dynamics and is a marker for human liver disease and keratin mutation. J Biol Chem. 2011;286:2273–2284. doi: 10.1074/jbc.M110.171314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaminsky R, Denison C, Bening-Abu-Shach U, Chisholm AD, Gygi SP, Broday L. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev Cell. 2009;17:724–735. doi: 10.1016/j.devcel.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolsch A. Untersuchungen des Keratinfilament-Turnover in lebenden Zellen. Mainz: Johannes Gutenberg-University; 2008. pp. 1–189. (Ger). [Google Scholar]
- 67.Paramio JM, Jorcano JL. Transient transfections and heterokaryons as tools for the analysis of keratin IF dynamics. Methods Mol Biol. 2001;161:189–197. doi: 10.1385/1-59259-051-9:189. [DOI] [PubMed] [Google Scholar]