Abstract
Environmental toxicants, such as cadmium and bisphenol A (BPA) are endocrine disruptors. In utero, perinatal or neonatal exposure of BPA to rats affect the male reproductive function, such as the blood-testis barrier (BTB) integrity. This effect of BPA on BTB integrity in immature rats is likely mediated via a loss of gap junction function at the BTB, failing to coordinate tight junction and anchoring junction function at the site to maintain the immunological barrier integrity. This in turn activates the extracellular signal-regulated kinases 1/2 (Erk1/2) downstream and an increase in protein endocytosis, destabilizing the BTB. The cadmium-induced disruption of testicular dysfunction is mediated initially via its effects on the occludin/ZO-1/focal adhesion kinase (FAK) complex at the BTB, causing redistribution of proteins at the Sertoli-Sertoli cell interface, leading to the BTB disruption. The damaging effects of these toxicants to testicular function are mediated by mitogen-activated protein kinases (MAPK) downstream, which in turn perturbs the actin bundling and accelerates the actin-branching activity, causing disruption of the Sertoli cell tight junction (TJ)-barrier function at the BTB and perturbing spermatid adhesion at the apical ectoplasmic specialization (apical ES, a testis-specific anchoring junction type) that leads to premature release of germ cells from the testis. However, the use of specific inhibitors against MAPK was shown to block or delay the cadmium-induced testicular injury, such as BTB disruption and germ cell loss. These findings suggest that there may be a common downstream p38 and/or Erk1/2 MAPK-based signaling pathway involving polarity proteins and actin regulators that is shared between different toxicants that induce male reproductive dysfunction. As such, the use of inhibitors and/or antagonists against specific MAPKs can possibly be used to “manage” the illnesses caused by these toxicants and/or “protect” industrial workers being exposed to high levels of these toxicants in their work environment.
Key words: environmental toxicants, environmental endocrine disrupting compounds, cadmium, bisphenol A, male reproductive function, testis, spermatogenesis, Sertoli cells, germ cells, mitogen activated protein kinase, signal transduction
Introduction
The US Consumer Product Safety Commission and McDonald's recently recalled 12 million cadmium-tainted Shrek drinking glasses (www.cpsc.gov/cpscpub); this is one of the latest incidents consumers are facing regarding environmental toxicants, which have now become an integrated component of our daily life in both developed and developing countries. While the cadmium was found to associate only with the paints found in the Shrek designs printed on the outside of these glasses, the toxicant could leach from the glasses and contaminate other glassware in the dishwashing machine. In light of the toxicity of cadmium and its unusually long half-life in humans, about 20–40 years, a recall appears to be the only means to protect the general public. However, various toxicants are found in virtually all utensils, foods and water in our environment, it is likely that humans, including children, are exposed to these toxicants at high levels that are harmful to our health.1–6 This review is not intended to be exhaustive on the basics of these toxicants or their generally known mechanisms of action since this information can be found in several recent reviews and reports.7–10 Instead, we focus on the destructive effects of several toxicants [e.g., cadmium and bisphenol A (BPA)] on male reproductive functions, such as reducing sperm count in men and rodents11 and by perturbing the blood-testis barrier (BTB) integrity in immature rats.12 In particular, the underlying mechanisms of action will be discussed, since recent findings have shown that these toxicants mediate their effects downstream via a common pathway involving mitogenactivated protein kinases (MAPKs), such as Erk1/2 and p38 MAPK. Thus, future studies should include an examination of whether small molecule inhibitors against these MAPKs can block or reverse the toxicant induced testicular injury. While it is known that these MAPKs are needed for diverse cellular functions and their blockade could have other unwanted side effects, the new information can be used to prepare targeted delivery of small inhibitors, such as to the testis, to prevent testicular injury caused by these toxicants.
Mechanisms of Toxicant-Induced Testicular Damage
Xenoestrogenic endocrine-disrupting compounds.
A number of environmental endocrine-disrupting compounds (EDCs) (Note: EDCs are man-made compounds that impair hormonal function in both humans and animals) are found in the environment as a result of industrial and manufacturing activities. These compound include bisphenol A (BPA)(Fig. 1), polychlorinated biphenyls (PCBs), pesticides, phthalates, polybrominated diphenyl ethers (PBDEs) and others (e.g., cadmium chloride) (reviewed in ref. 3). Herein, we focus our discussion on BPA because of its wide-spread presence in the environment (e.g, utensils, plastics, foods, water bottles) and this compound appears to share the common downstream toxicological signaling pathway of MAPK, similar to the heavy metal environmental toxicant cadmium.
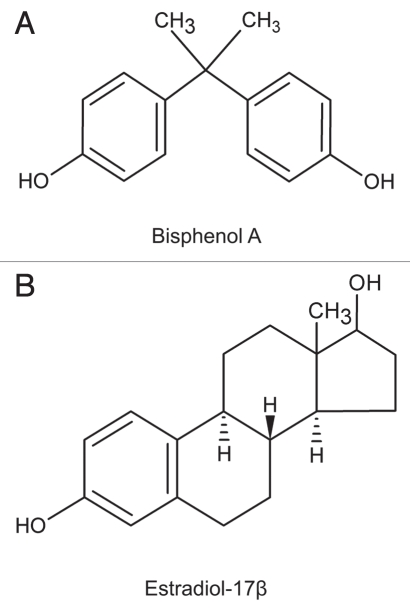
Figure 1.
The structural formulae of bisphenol A (BPA) and estradiol-17β.
Bisphenol A.
Introduction. Bisphenol A [BPA, 2,2-bis (hydroxyphenyl)propane] (Fig. 1), a synthetic non-steroidal estrogen widely used in the manufacture of polycarbonate plastic (e.g., water bottles, baby bottles), epoxy resins (e.g., inside coating in metallic food cans) and as a non-polymer additive to other plastics. BPA leaches from polycarbonate bottles and food containers including canned foods, and thus becomes an integrated part of the food chain.1–2,11,13 While adult animals tolerate acute and high doses of bisphenol (e.g., >2–50 mg/kg b.w./day at multiple doses) without much noticeable phenotypic changes,12 recent studies have shown that in utero, perinatal or neonatal exposure of laboratory animals (rats and mice) to bisphenol is harmful even at a physiologically relevant exposure dose, also known as a “low-dose” (i.e., at a concentration below the current lowest observed effect level, LOAEL, of 50 µg/kg/day for in vivo studies). Harmful effects include disruption of (1) development, differentiation and function of the central nervous system, (2) reproductive function (such as, follicle development), (3) thyroid hormone function, (4) morphology of prostate and mammary glands, (5) meiosis in fetal oocytes and (6) immune system (for recent reviews, see refs. 8 and 13–15). This susceptibility of neonatal animals to BPA versus adults may be related to the lower level of liver enzyme activity that can metabolize BPA to its non-toxic BPA-glucuronide form which is then excreted by the kidney.8 It has been reported that biologically active BPA in neonatal mice following oral administration is indeed ∼10-fold higher than adults.16 Thus, BPA may indeed be harmful to children in humans and additional studies are warranted (see ref. 15).
It is known that BPA exerts its effects by binding to the nuclear steroid receptors: estrogen receptor α (ERα) and ERβ, with about 10-fold higher affinity for ERβ; BPA also binds to androgen receptor and thyroid hormone receptor competitively.13 In Leydig cells, BPA interferes with the expression of steroidogenic enzyme 17α-monooxygenase (17α-hydroxylase/17-20 lyase) (reviewed in ref. 13).
BPA-induced MAPK signaling in the testis. A recent study17 has shown that in utero exposure of rats to BPA (0.1 to 200 mg/kg/ day for 7 days from gestational day 14 to birth) led to a transient induction in testicular Erk1 (also known as MAPK 3), but not Erk2, in neonatal rats, most notably in Sertoli cells. This MAPK induction was no longer detected by day 21 postpartum; and the fertility of these rats were not grossly affected. These findings are in agreement with a recent report that treatment of immature rats at age 20–25 days postpartum with BPA (50 mg/kg b.w./day for 6 days) induced a BTB disruption (Note: BTB is assembled in rats by age 15–16 postnatal), but a similar regimen in adult rats (50 mg/kg b.w./day for 5 days) had no apparent effect on the BTB integrity or the fertility.12 Also, this BPA-induced BTB disruption can be reproduced in vitro using Sertoli cell cultures when BPA at 200 µM reversibly perturbed the Sertoli cell tight junction (TJ)-permeability barrier, and this disruption was associated with an induction of p-Erk1 but not p-Erk2.12 Furthermore, BPA also caused a redistribution of several known BTB integral membrane proteins: Cx43, occludin and N-cadherin, but not ZO-1 (a TJ adaptor), moving away from the Sertoli-Sertoli cell interface to the cell cytosol,12 thereby destabilizing the TJ-barrier. In short, these findings suggest that when neonatal rats and perhaps children are exposed to BPA, this toxicant exerts its effects by activating MAPK signaling pathway downstream in the testis.
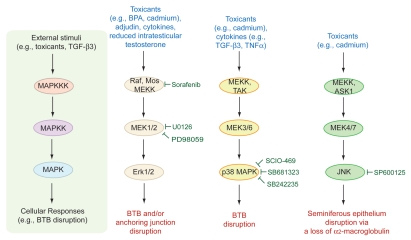
In this regard, the in vivo function of MAPK signaling in spermatogenesis remains unclear from the phenotypes of knockout animals. It is known that Erk1 knockout mice are fertile,18 whereas Erk2 KO mice are embryonic lethal19 so that its role in spermatogenesis and fertility cannot be assessed. Although the former finding seems to suggest that the Erk1 MAPK is not critical for spermatogenesis, this probably resulted from other MAPKs, such as ERK5, JNK and/or p38, superseding Erk1 to perform various necessary functions during spermatogenesis. For instance, it is known that Erk1/2 and other MAPKs (e.g., MEK1, MEK2) are crucial to cell cycle progression in spermatocytes such as chromatin condensation prior to meiosis I.20 Figure 2 illustrates some of the known MAPKs and MAPK-based signaling pathways that are known to be involved in the toxicant-induced BTB and anchoring junction restructuring in the testis, highlighting crucial MAPK molecules that can be tackled to manage toxicant-induced testicular injury, such as the use of small molecule inhibitors to block specific MAPK.
Figure 2.
The three mitogen-activated protein kinase (MAPK) signaling pathways that are known to be involved in the toxicant-induced testicular injury. MAP kinases are a family of Ser/Thr protein kinases found in eukaryotic cells that are well conserved among mammalian species, and they are involved in a number of normal cellular and pathological events including cell proliferation, differentiation, apoptosis, tumorigenesis, cell movement, cell cycle progression and meiosis in germ cells, and junction restructuring.20,69–71 Recent studies have shown that environmental toxicants e.g., cadmium, bisphenol),12,17,41–42,44 cytokines (e.g., TGF-β3, TNFα)72–76 or male contraceptives (e.g., adjudin)73–74 exert their effects to induce either germ cell depletion from the seminiferous epithelium that mimics spermiation or BTB disruption via one of the three MAPKs (i.e. Erk1/2, p38 MAPK and JNK). These findings thus illustrate the possibility that the toxicant-induced testicular injury can be managed by using specific inhibitors to block members of the MAPK signaling molecules. Some of the commonly used inhibitors including a few that are in clinical trials (e.g., SB681323) are indicated in this schematic drawing. The chemical names for these inhibitors are: Sorafenib, 4-[4-{[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino}phenoxy]-N-methyl-pyridine-2-carboxamide; U0126, 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)-butadiene, marketed as Nexavar by Bayer; PD98059, 2′-amino-3′-methoxyflavone; SCIO-469, 2-[6-chloro-5-{[(2R,5S)-4-(4-foluorobenzyl)-2,5-dimethylpiperazin-1-yl]carbonyl}-1-methyl-1H-indol-3-yl]-N,N-dimethyl-2-oxoacetamide; SB681323, a GlaxoSmithKline p38 MAPK inhibitor, its chemical structure has not been released, this p38 MAPK inhibitor is currently in Phases 1 and 2 clinical trials for different pathological conditions, such as neuropathic pain, chronic obstructive pulmonary disease, acute respiratory distress syndrome, rheumatoid arthritis (see www.gsk-clinicalstudyregister.com/protocol_comp_list.jsp;jsessionid=707CC014554CD2B306FE34C4BE56BD27?compound=Sb681323); SB242235, 1-(4-piperidinyl)-4-(4-fluorophenyl)-5-(2-methoxy-4-pyrimidinyl)imidazole; SP600125, anthra(1,9-cd)pyrazol-6(2H)-one.
An emerging new concept on how BPA perturbs the BTB integrity via gap junctions. As noted in a recent study reported by our laboratory, BPA was shown to perturb the integrity of the BTB when administered to neonatal rats on day 20 to 25 postnatal at 50 mg/kg b.w. for 6 doses.12 This in vivo effect was reproduced in vitro using primary Sertoli cells cultured on Matrigelcoated bicameral units with a functional barrier that mimicked the BTB in vivo, in which BPA reversibly perturbed the Sertoli TJ-permeability barrier.12 Although it is known that BPA acts as an endocrine disruptor in multiple organs by mediating its effects via estrogen receptors, androgen receptors, or thyroid hormone receptors (see above), we instead focused on the effects of BPA on BTB components since this ultrastructure is not strictly estrogen- or androgen-dependent. It is noted that the BTB is constituted by coexisting TJ, basal ectoplasmic specialization (ES, a testis-specific adherens junction type21–23), desmosome (also known as desmosome-gap junction because it shares the properties of desmosome and gap junction24) and gap junction (GJ)21,25. Our study first suggested that BPA may perturb the Sertoli cell BTB by blocking GJ communication, since the BPA-induced Sertoli cell TJ-permeability barrier disruption was associated with a redistribution of GJ proteins, such as Cx43, from the Sertoli-Sertoli cell interface to cell cytosol.12 These findings are supported by a more recent study, in which BPA was shown to impede GJ function based on a dye-transfer assay to assess GJ communication.26 Thus, the loss of GJ communication is likely to be an important mechanism for BPA-induced BTB disruption. Indeed, the function of GJ in maintaining the homeostasis of the BTB and the cross-talk of different junction types was demonstrated by the knock-down of Cx43 and plakophilin-2 in Sertoli cells by RNAi, which led to the disruption of TJ-barrier.27
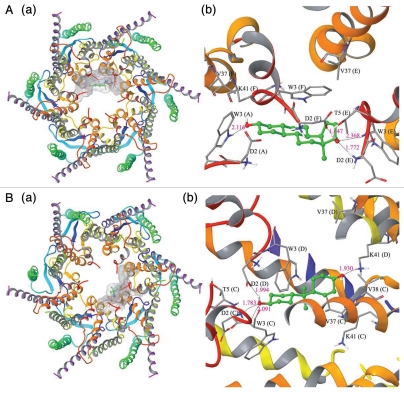
These findings thus warrant the investigation of whether BPA physically interacts with GJ communicating channel. Since the crystal structure of Cx26 at 3.5Å resolution is known28, and Cx26 is also a known GJ-protein in the Sertoli cell BTB27, the likelihood of BPA binding to Cx26-based connexon was assessed by molecular modeling analysis (Fig. 3). This analysis showed the presence of a docking ‘pocket’ in Cx26-based connexon for estradiol-17β (Fig. 3A) and BPA (Fig. 3B), analogous to the docking of these molecules to Cx43-based connexons (data not shown). These results thus further strengthen the notion that BPA directly interacts with GJ, thereby impeding GJ-based communication as demonstrated in a BPA-induced disruption of dye-transfer across the GJ,26 which in turn impedes cross-talk between different junction types at the BTB, destabilizing the BTB integrity (Fig. 4). It is also possible that the interaction of BPA with Cx43-based connexons at the BTB may lead to the activation of MAPK, which in turn perturbs the BTB function in the testis.
Figure 3.
The docked complex of estradiol-17β (A:a, b) and BPA (B:a, b) with a Cx26-based GJ communicating channel. The Panel a in A or B illustrates the top view of the corresponding docked complex and Panel b in A or B illustrates the ball and stick model for the interaction residues with the protein secondary structure representation. The crystal structure of human Cx26 is known at 3.5Å resolution.28 The protein crystal structure of Cx26 was retrieved from Protein Data Bank (PDB ID: 2ZW3). The initial requirement of docking study is the preparation of protein and ligand to have optimized structures. Cx26 protein structure was prepared using the Maestro 9.0 protein preparation wizard; bond orders assigned, and hydrogen added appropriately. Exhaustive sampling method was used for the optimization of the hydrogen bonding network, in which the orientation of hydroxyl groups, amide groups of Asn and Gln, and imidazole ring of His residues were optimized. The root mean square deviation (RMSD, to be used to measure the scalar distance between atoms of the same type of two structures) of atom displacement for terminating the minimization was set to be 0.30Å. The minimization was carried out with OPLS2005 force field. The ligand structures of estradiol-17β (CID: 5757) and bisphenol A (CID: 6623) were retrieved from NCBI-PubChem database. LigPrep (Version 2.3, Schrödinger, LLC, New York, NY, 2009) was used to generate 3D structure with correct chiralities for each ligand used in this study. Grid files represent the volume of the receptor that can be searched for ligand docking. We have set the grid box on the centriod of all the residues in the Cx26 and no constraints were selected. The Standard Precision (SP) mode of Glide (Version 5.5, Schrödinger, LLC, New York, NY, 2009) was used for the flexible ligand docking. The best binding conformation was selected as one which has the lowest docking energy from the generated docking solutions. Docking of estradiol-17β into the Cx26-based GJ channel shows strong interactions with the pore lining residues such as tryptophan (Trp, W) and aspartic acid (Asp, D). The amino acid residues such as Asp2 (Chain E and F) and Trp3 (Chain A and E) were involved in multiple hydrogen bond formation with estradiol-17β (Aa, b). The backbone nitrogen atom of Asp2 (Chain E) and side chain carboxyl oxygen of Asp2 (Chain F) makes hydrogen bond interaction with estradiol-17β in the bond distances of 1.772Å and 1.647Å, respectively. Whereas the side chain nitrogen of Trp3 (Chain A) and backbone nitrogen atom of Trp3 (Chain E) were also involved in hydrogen bond formation in the bond distance of 2.116Å and 2.368Å, respectively. In addition to hydrogen bonds, Asp2 (Chain A), Trp3 (Chain F), Val37 (Chain E and F), Thr5 (Chain E) and Lys41 (Chain F) were involved in non-bonded interaction to further stabilize the interaction. The docking score of this binding conformation was −5.36 kcal/mol. The binding site surface area of Cx26 and ligand were 1230.776 Å and 273.880Å, respectively. This docking result shows that estradiol-17β binds with residues of chain A, E and F only that are lining the GJ channel. Hence, we conclude that additional unit of estradiol-17β is also required for binding with monomeric unit of other chains, which leads to blockage of the gating of channel Cx26. Bisphenol A docking result reveals that pore lining residues such as Asp2 (Chain C and D), Trp3 (Chain C) and Lys41 (Chain D) of Cx26 were involved in hydrogen bond formation in the bond distance of 1.783Å, 1.994Å, 2.091Å and 1.930Å. respectively (Ba,b). The non-bonded interactions were also observed in the residues of Trp3 (Chain D), Thr5 (Chain C), Val37 (Chain C and D), Val38 (Chain C) and Lys41 (Chain C) with bisphenol A. The binding site surface area of Cx26 and bisphenol A were 1029.645Å and 254.604Å, respectively. The docking score for this binding complex was −4.47kcal/mol. It is interesting to note that Asp2, Trp3, Val37 and Lys41 residues of both Chain C and D were involved in the interactions and thus it creates an empty space in the pore region. Hence an additional unit of bisphenol A is also required to bind with other monomeric chains of Cx26 to block the passage of chemical molecules through the pore. In this study, we observed that both estradiol-17β and bisphenol A interact with pore lining residues of N-terminal helix (NTH) and first transmembrane helix (TMH1) of Cx26 protein. We also observed that each of the compounds is forming four hydrogen bonds with the Cx26. Hence, we conclude that both the compounds can regulate or inhibit the functions of Cx26 by binding with pore region of the protein. Asn (N), asparagine; Asp (D), aspartic acid; Gln (Q), gluatamine; His (H), histidine; Lys (K), lysine; Thr (T), threonine; Trp (W), tryptophan; Val (V), valine. The “numeral” after an amino acid residue represents the sequence number from the N-terminus.
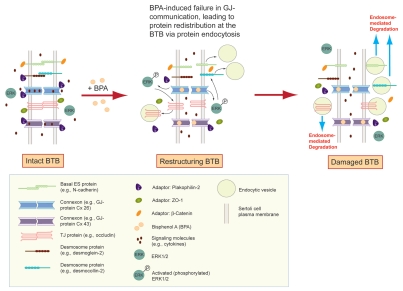
Figure 4.
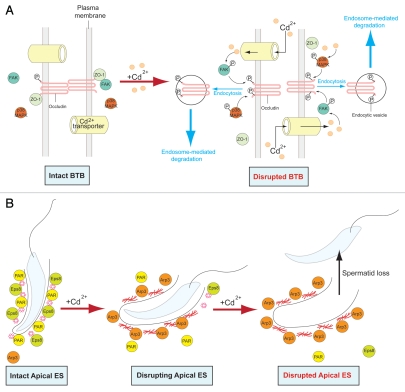
A schematic drawing illustrating an emerging mechanism of action of BPA in disrupting the BTB integrity via its effects on gap junction, which in turn perturbs the proper protein distribution at the tight junction and basal ES. The left panel represents an intact BTB in the rat testis. The presence of bisphenol A, such as when immature rats or the Sertoli cell epithelium in vitro are exposed to this environmental toxicant, however, blocks the GJ communication (e.g., Cx43-based GJ), leading to changes in protein re-distribution at the BTB via an increase in protein endocytosis (middle panel),12,27 thereby causing a disruption of the BTB (right panel).
Collectively, recent studies suggest that BPA affects the BTB primarily by targeting GJ (or also desmosome-gap junction), and this mechanism is facilitated by the unique structure of the BTB. In general, blood-tissue barriers (e.g., the blood-brain barrier and the blood-retina barrier) are constituted by endothelial tight junctions of the microvessels inside the corresponding organs, such as the brain and the eye. Unlike these blood-tissue barriers, the BTB is constituted by coexisting TJ, basal ES, desmosome and GJ between Sertoli cells near the basement membrane21,29, while the microvessels in the interstitium contribute relatively little barrier function to the BTB21,30 similar to the myoid cell layer in the tunica propria in primates.31 The physiological significance of these coexisting junctions at the BTB is not clear. However, recent studies have shown that a disruption of GJ27 or desmosome32 by the knock-down of their components using specific siRNA duplexes by RNAi can impede the Sertoli cell BTB integrity, illustrating that these junctions likely provide the necessary cross-talk between different junction types at the BTB to maintain its integrity. For instance as previously mentioned, it was shown that a loss of Cx43 and plakophilin-2 expression by their knockdown using RNAi led to a loss of proper distribution of occludin and ZO-1 at the Sertoli-Sertoli cell interface, moving from the cell surface to cell cytosol via an increase in protein endocytosis, disrupting the Sertoli cell TJ-permeability barrier.27 Similarly, the simultaneous knockdown of desmoglein-2 and desmocollin-2 also led to a redistribution of ZO-1 and CAR from the Sertoli cell surface to cell cytosol, thereby disrupting the Sertoli cell-TJ barrier.32 Thus, the blockage of GJ communication by BPA26 (see also Fig. 3) is likely to destruct the necessary cross-talk of cell junctions at the BTB, causing an increase in the kinetics of protein endocytosis at the BTB, thereby perturbing the barrier function (see the hypothetical pathway shown in Fig. 4).
In short, the above discussion illustrate that the GJ at the BTB is one of the cellular targets of BPA and environmental toxicants. These findings should be further explored to examine if a blockade of GJ communication by using inhibitors would render the Sertoli cell TJ-permeability barrier insensitive to the BPA treatment. Such studies can open a new window of opportunity to therapeutically manage BPA-induced damage to the reproductive function in children.
Cadmium.
Introduction. Cadmium is a heavy metal and an environmental toxicant used for manufacturing paints for plastics, ceramics and glasses; and for making nickel-cadmium battery and electric cables when alloyed with copper.11 Cadmium is also as an endocrine disrupting compound (EDC) that interferes with the synthesis and the regulation of several hormones in both females and males.33 Besides its disruptive effects on Leydig cell steroidogenesis34, cadmium also modifies several hormone levels in the hypothalamic-pituitary-testicular axis35, such as testosterone, LH and FSH. Similar to BPA, cadmium is an integrated component of the food chain due to industrial activities, such as smelting and refining of metals that contaminate our foods and water, and municipal waste incineration which release cadmium to the atmosphere as cadmium oxide, chloride or sulfide11,33. While the cadmium levels in the air and drinking water are relatively low (i.e., ∼0.04 µg/m3 and <1 µg/L, respectively), an average person ingests ∼30 µg cadmium/day via food and water, which is then absorbed by the stomach or intestines; smokers who consume ∼1 pack cigarettes per day absorb an additional 1–3 µg of cadmium through their lungs10 (for additional information, see www.e-b-i.net/ebi/comtaminants/cadmium.html). Unfortunately, cadmium has an exceedingly long biological half-life of ∼20–40 years in humans so that it can accumulate in the body over a long period of time, particularly in the kidneys and the liver.33 Cadmium is also known to cause a lot of illnesses including cancers in various organs, such as kidney, prostate and breast7,36 besides disrupting male reproductive function.4,11,33
While the damaging effect of cadmium in the testis by causing necrosis is known for more than five decades37–39, its mechanism of action and the cellular targets in the testis are not known until recent years. The first report illustrating that the BTB is an early target of cadmium toxicity in the testis appeared in 1970.40 Subsequent studies have shown that following the administration of an acute dose (3 mg/kg b.w.) of cadmium chloride in adult rats, damage of microvessels was preceded by the loss of BTB integrity41–42, with a destruction of TJ-associated microfilaments in Sertoli cells.43
Cadmium-induced MAPK signaling in the testis. Similar to the action of BPA as discussed in the previous section, cadmium-induced toxicity also involves MAPK pathways. The disruption of the BTB, which occurs at ∼14–16 hr after cadmium administration, was shown to be mediated via an activation of the p38 MAPK.44 This finding is important because it illustrates for the first time that cadmium-induced toxicity in the testis can possibly be therapeutically managed by using specific inhibitors against MAPK. Indeed, the use of a specific p38 MAPK inhibitor, such as SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4pyridyl)-1H-imidazole] was shown to delay and block, at least in part, the cadmium-induced BTB disruption in vivo.44 These findings were confirmed in two follow-up studies.41–42 In addition, another MAPK member c-Jun N-terminal kinase (JNK) was also shown to play a role in cadmium toxicity. The inhibition of JNK by DMAP (6-dimethylaminopurine, a JNK inhibitor) worsened cadmium-induced testicular injury as shown by accelerated germ cell loss, which might be due to the decreased production of the non-specific protease inhibitor α2-macroglobulin.41 These studies also noted the irreversible nature of cadmium-induced germ cell loss from the seminiferous epithelium, as well as BTB disruption, following the administration of an acute dose (such as a single dose of 3 mg/kg b.w.).41–42
New insights on the mechanism of cadmium toxicity: The role of cell polarity. Prior to the visible damage to the BTB and the loss of germ cell from the epithelium (e.g. at ∼6-hr after cadmium administration), many of the elongated spermatids became mis-oriented. This may reflect a loss of cell polarity (see Fig. 5) following cadmium treatment, as opposed to the properly oriented developing spermatids with their heads pointing towards the basement membrane in the seminiferous epithelium of normal testes (Fig. 5). This loss of cell polarity in elongating spermatids becomes more extensive by 16-hr after cadmium treatment (Fig. 5) and thereafter, germ cells were shown to begin their depletion from the seminiferous epithelium.41 Cell polarity of developing spermatids in the seminiferous epithelium is conferred by a specialized testis-specific anchoring junction known as apical ectoplasmic specialization (apical ES)45 (Fig. 6), which appears in developing step 8 through step 19 spermatids in the rat. Once the apical ES appears, it is the only anchoring device found between Sertoli cells and the developing spermatids, which is also used to maintain their proper orientation, so as to maximize the number of spermatids that can be packed in a limited cross section area of the seminiferous epithelium.21,45
Figure 5.
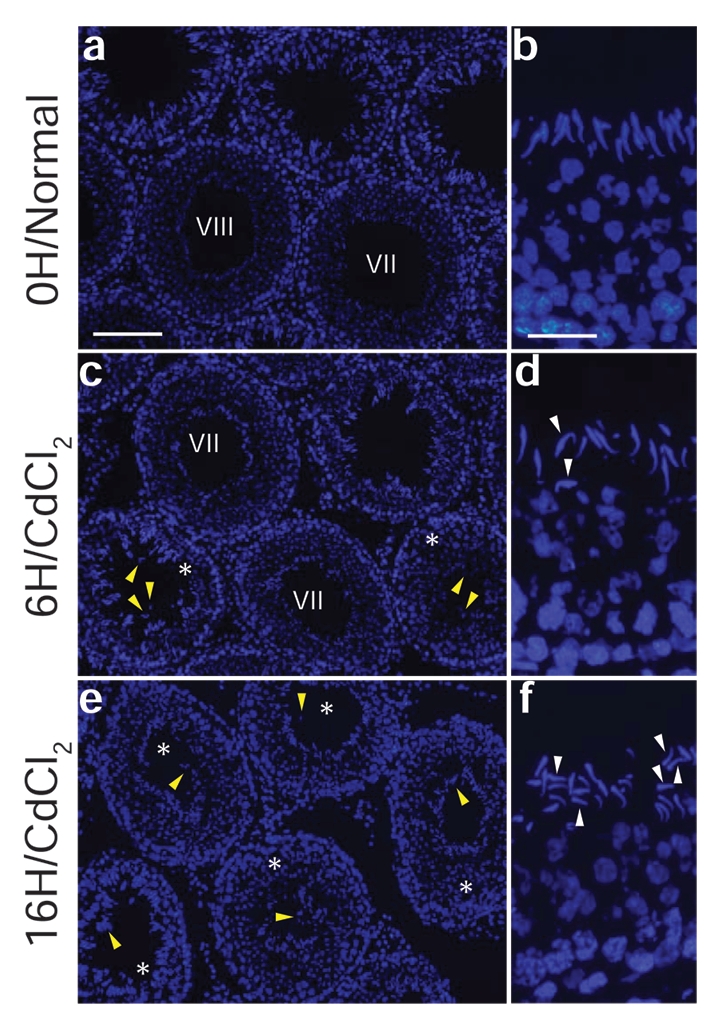
Cadmium chloride-induced mis-orientation and loss of cell polarity in developing spermatids prior to their premature depletion from the seminiferous epithelium in adult rats. Adult rats (∼300 gm body weight, n = 3 rats for each group including control) were treated with saline (0.9% NaCl) at time 0 (a, b) versus CdCl2 (3 mg/kg b.w., administered via i.p.) and terminated by 6 hr (c, d) or 16 hr (e, f) thereafter. Germ cells were shown to deplete from the seminiferous epithelium prematurely following cadmium exposure in tubules (marked with an asterisk) versus control testes (see yellow arrowheads in c and e versus a). In control rats at time 0, developing spermatids are properly oriented with the heads of spermatids pointing towards the basement membrane (see b) in the seminiferous epithelium. At 6 hr after cadmium exposure, some spermatids began to lose their cell polarity with improper orientation (see white arrowheads in d). Significantly more spermatids were mis-oriented by 16 hr (f, see white arrowheads), prior to their premature depletion from the epithelium. Scale bars: a, 120 µm (also applies to c and e); b, 25 µm (also applies to b and f).
Figure 6.
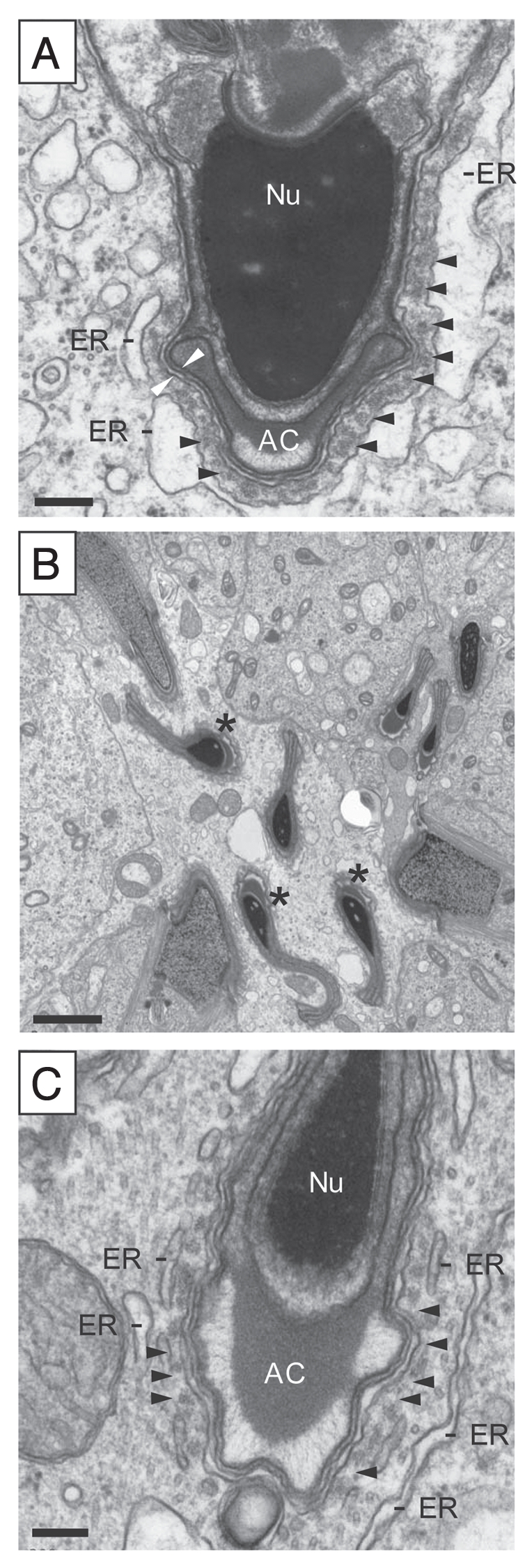
Adjudin-induced loss of orientation and cell polarity in developing spermatids (see ref. 46). In normal rat testes (A), developing spermatids (from steps 8 through 19) remain attached to the Sertoli cell in the seminiferous epithelium via apical ectoplasmic specialization (apical ES) which also confers cell polarity by maintaining proper orientation with the head of the spermatid pointing towards the basement membrane. The apical ES is typified by the presence of actin filament bundles (see black arrowheads) sandwiched between the plasma membrane of the Sertoli cell and the endoplasmic reticulum (ER). The two opposing white arrowheads illustrate the plasma membranes of the Sertoli cell and the elongating spermatid. Nu, nucleus; AC, acrosome. In rats treated with a single dose of adjudin (50 mg/kg b.w., by gavage, a chemical known to induce premature loss of spermatids from the epithelium) for 12 hr (B), spermatids lose their cell polarity and proper orientation, with some spermatids pointing to the opposite direction instead of towards the basement membrane (see asterisks in B, illustrating mis-oriented elongating spermatids). This observation is analogous to rats receiving a single dose of cadmium shown in Figure 5. When a mis-oriented spermatid was magnified as shown in (C), it was noted that the actin filament bundles became disrupted and less organized (see arrowheads in C), possibly the result of an increase in actin branching activity via an activation of Arp2/3 (proteins that regulate actin nucleation)(see Figs. 7 and 8), so that these spermatids can be depleted from the epithelium. Scale bars: (A), 0.1 µm; (B), 2 µm; and (C), 0.1 µm.
The early disruption of spermatid polarity and orientation following cadmium treatment (see Fig. 5) is reminiscent of germ cell depletion induced by another chemical known as adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] (Fig. 6). In this respect, a previous study showed that adjudin treatment led to a loss of cell polarity protein partitioning-defective protein 6 (PAR6) at the apical ES, rendering these spermatids incapable of orientating themselves properly with their heads pointing toward the basement membrane,46 triggering their eventual depletion from the epithelium. Thus, cadmium-induced loss of cell polarity may also involve the down-regulation of polarity proteins, which remains to be investigated.
Furthermore, the disruption of homeostasis in the actin filament network surrounding the apical ES may also contribute to the loss of spermatid polarity following cadmium treatment. One of the major actin nucleation machineries Arp2/3 complex, as well as its activator N-WASP (neuronal Wiskott-Aldrich syndrome protein),47–48 are important for the proper orientation of elongated spermatids in the seminiferous epithelium. This was demonstrated by the inhibition of N-WASP using wiskostatin49, which led to spermatid mis-orientation50 (see Fig. 7). When N-WASP binds and activates the Arp2/3 complex51, the entire activated Arp2/3/N-WASP protein complex can bind to the lateral side of an existing actin filament and serve as the nucleation site for a new actin branch, thereby forming a branched actin network in cells. While actin branching is necessary for changes in cell shape in response to the environmental stimuli (e.g., stress, growth factors, cytokines, infection), it is also necessary for spermatid development during spermiogenesis. For instance, elongating spermatids transform their cell shape and also move progressively in the epithelium near the basement membrane towards the luminal edge of the seminiferous tubule so that spermiation can take place at stage VIII of the seminiferous epithelium cycle.
Figure 7.
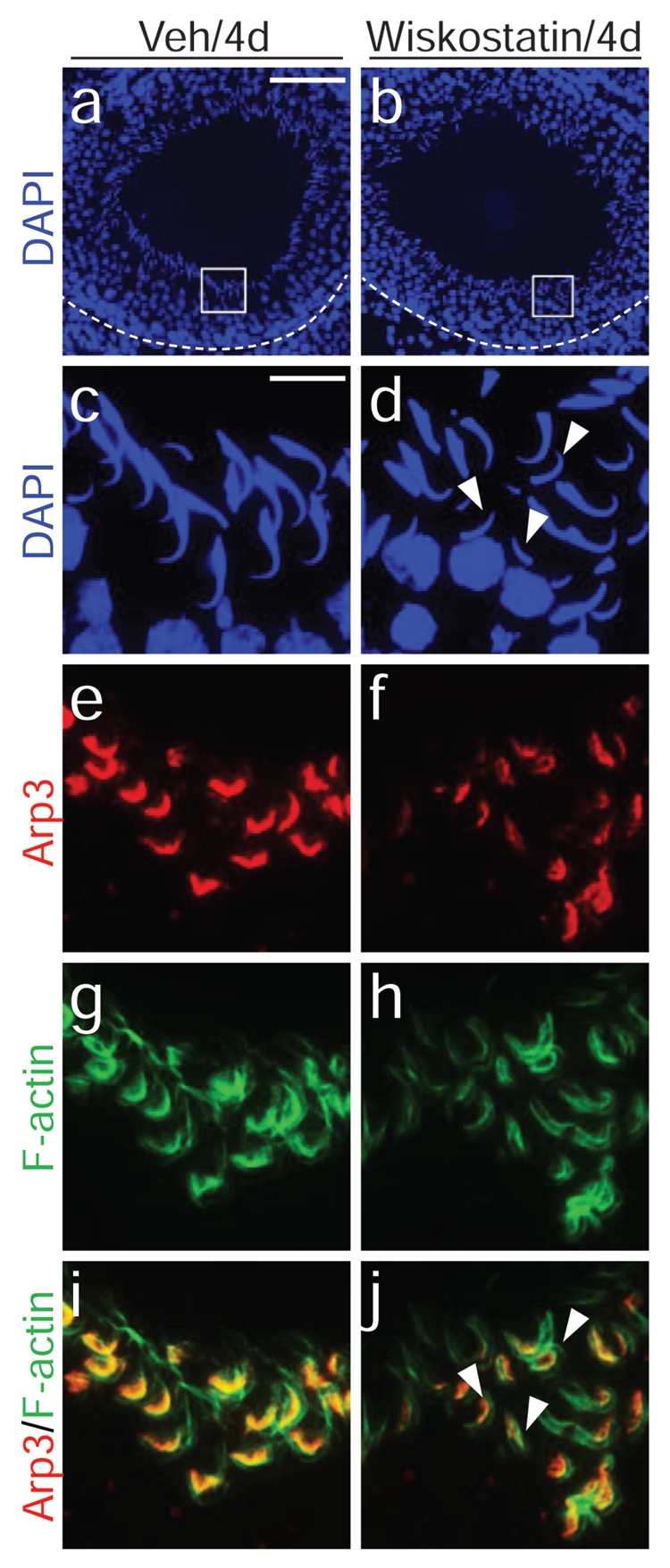
A disruption of proper actin nucleation by Arp2/3 using an inhibitor against N-WASP, an activator of Arp2/3, induces mis-orientation and loss of polarity in developing spermatids (see ref. 50). The left panel, a, c, e, g and i, illustrates rats treated with vehicle control (0.15% DMSO in PBS) via intratesticular administration as described in reference 50. Elongating spermatids were properly oriented in the seminiferous epithelium (a, c) on day 4 post-treatment, with Arp3 found in the concave side of the spermatid heads (e, red) whereas F-actin was found around the entire spermatid heads at the apical ES (g, green); the co-localization of Arp3 and F-actin is shown in i (orange). However, after wiskostatin (an inhibitor of N-WASP) treatment which perturbs Arp2/3-mediated actin nucleation to generate proper actin-branching at the apical ES which facilitates proper cell polarity and orientation, many elongating spermatids became misoriented without pointing towards the basement membrane (see white arrowheads in d versus properly oriented spermatids shown in c). The Arp3 (f) and the actin network (h) appeared diminished and less organized (f), which were also evident in the merged image shown in j. Scale bars: a, 100 µm, also applies to b; c, 15 µm, also applies to d–j.
In this context, it is of interest to note that FAK, a non-receptor protein tyrosine kinase52, is an integrated component of the occludin-ZO-1 complex at the BTB.53 This FAK-occludin-ZO-1 protein complex also appears to be the primary target of cadmium toxicity in the testis.53 For instance, a knockdown of FAK by RNAi using FAK-specific siRNA duplexes was shown to render the Sertoli cell TJ-permeability barrier unresponsive to cadmium-induced disruption.53 In short, these findings suggest that the FAK-occludin-ZO-1 complex is one of the cellular targets of cadmium toxicity in the testis and a disruption of this protein complex, such as by RNAi to knock-down FAK, can render the Sertoli cell BTB insensitive to cadmium toxicity. As such, this protein complex is also a therapeutic target to manage toxicant-induced male reproductive dysfunction.
Taken collectively, these findings suggest that cadmium may initially alter the proper function of the polarity proteins as well as the actin-nucleation/branching activity, altering cell polarity in Sertoli cells near the basement membrane of the seminiferous tubules, which in turn activates MAPK downstream that leads to a disruption of the BTB. This mechanism may also be used by cadmium to perturb the cell polarity of developing spermatids, disrupting the cell polarity protein function and proper actin branching activity at the actin ES, perturbing spermatid polarity and orientation in the seminiferous epithelium behind the BTB (see Fig. 8). This, in turn, contributes to germ cell depletion from the seminiferous epithelium prematurely, thereby reducing sperm counts in the affected individuals. This hypothetical mechanism of action also reveals there are multiple targets that can be tackled to manage cadmium-induced testicular injury, which should be pursued in future studies.
Figure 8.
A schematic drawing illustrating the likely mechanism of action of environmental toxicants (e.g., cadmium) that causes disruption of the BTB and premature germ cell loss from the seminiferous epithelium, thereby reducing sperm count and leading to male reproductive dysfunction. In (A), the left panel illustrates an intact and functional BTB in the rat testis. The entry of cadmium into the testis via drug transporter SLC39A8 in mice55 can affect FAK in the FAK/occludin-ZO-1 protein complex,53,77 such as changes in the phosphorylation status of the occludin-ZO-1 protein complex,77 causing their re-distribution, such as mediated by enhanced endocytosis of occludin in the rat Sertoli cell BTB,53 this thus destabilizes the BTB integrity, leading a loss of BTB integrity (see right panel). This postulate was confirmed by the observation that a loss of FAK function by RNAi would render the Sertoli cell epithelium less susceptible to cadmium.77 In (B), the left panel illustrating an intact apical ES at the Sertoli cell-elongating spermatid interface with the abundant presence of Par-based proteins (e.g., Par6)46 and Eps8,78 which facilitate the maintenance of the actin filament bundles to confer apical ES integrity. The presence of cadmium (e.g., CdCl2) which reaches the apical compartment of the seminiferous epithelium via the SLC39A8 transporter (or other drug transporters) likely activates Arp3, and this activation Arp3 is recently shown to induce the formation of actin branching network, replacing the actin filament bundles at the apical ES50 (middle panel). This, in turn, destabilizes the apical ES, leading to premature loss of spermatids from the seminiferous epithelium (right panel), analogous to spermiation.
Regulation of cadmium entry to the seminiferous epithelium behind the BTB. Cadmium appears to enter the seminiferous epithelium behind the BTB in the mouse testis via the drug transporter SLC39A8, also known as ZIP8 (a zinc transporter that is highly expressed in human T cells54) (see Fig. 8). This drug transporter is found in Sertoli cells, but is most notably associated with microvessels in the interstitium.55–56 It is noted that the mRNA of ZIP8 is the most abundant in lung and kidney, and its steady-state mRNA level in the liver is similar to that in the testis.55 However, besides the zinc cation transporter ZIP8, it is also possible that cadmium and other heavy metals (e.g., zinc) traverse a blood-tissue barrier including the BTB in the testis via other drug transporters (e.g., influx pump transporters57, such as OCT family cation transporters58–59) or by macropinocytosis.60 Indeed, recent studies have shown that Sertoli cells express a large number of drug transporters, including efflux and influx pump transporters, in the testis.30,61–62 For instance, p-glycoprotein (an efflux pump protein) is an integrated component of the JAM-A-, claudin-11- and occludin-based protein complexes at the BTB.62 This efflux pump apparently plays a role in regulating the types of drugs, heavy metals and/or environmental toxicants that can traverse the BTB to exert their effects in developing germ cells behind the BTB. Recent studies have shown that other proteins, such as tissue plasminogen activator, can also mediate the transport of heavy metals (e.g., zinc) across a cell epithelium via its interaction with drug transporter ZIP4.63 The role of these drug transporters in cadmium and/or BPA toxicity in the male reproductive function should be carefully evaluated in future studies.
Alleviating Toxicant-Induced Testicular Damage by Specific Inhibitor
As discussed above and summarized in Figure 3, specific MAPK MAPK42,44 inhibitors (e.g., SB202190 that blocks p38 and DMAP that blocks JNK41) that are delivered specifically to the testis via intratesticular administration can indeed interfere with cadmium-induced injury in the testis. Thus, environmental toxicant-induced male reproductive dysfunction can be therapeutically ‘managed’. It is obvious that local administration of MAPK inhibitors to the testis is not a viable option, these inhibitors, however, can be administered using nanotechnology, such as the use of a modified FSH mutant protein64 for targeted delivery since FSH receptor is limited to the Sertoli cells in the mammalian testis. There are recent advances in the field using inhibitors against different MAPKs to treat various pathological conditions, mostly notably cancer, heart disease and pulmonary disease.65–68 While many of these studies are in the stage of laboratory-based in vitro testing and studies,65,67 several of these MAPK inhibitors or related kinase inhibitors have moved onto clinical studies.66,68 Some of these clinical studies are now at the stages of data collection and analysis prior to the publication in peer-reviewed journals regarding their use to treat different illnesses ranging from late stage cancers to heart disease and inflammatory disorders (see www.ciscrp.org, www.nih.gov or www.cancer.gov for selected and additional references).
Concluding Remarks and Future Perspectves
Herein we have critically discussed some of the latest developments in the field regarding the role of MAPK in the cadmium- and BPA-induced damage to the testis. It is increasingly clear that the BTB, while it is one of the tightest blood-tissue barriers in the mammalian body, is the primary cellular target of cadmium (and perhaps BPA as well, at least in immature rats12) wherein cadmium initiates its “assault” (e.g., by disrupting the TJ-permeability barrier), to be followed by a cascade of events (e.g., germ cell depletion from the epithelium) that leads to testicular injury.33 And within the BTB ultrastructure, the FAK/occludin/ZO-1 protein complex is one of the primary molecular targets of cadmium.53,77 Furthermore, BPA and cadmium were shown to activate MAPK downstream of the FAK/occludin/ZO-1 protein complex at the BTB, which is constituted by coexisting TJ, basal ES, desmosome and gap junction between adjacent Sertoli cells near the basement membrane in the seminiferous tubule, and the use of specific inhibitors against some members of the MAPK family was effective to block and/or delay the toxicant-induced testicular damage, such as the BTB disruption (see Figs. 2, 4 and 8). Furthermore, the cadmium-induced testicular injury may also involve a disruption of the polarity proteins and the likely participation of FAK (see Fig. 8). These findings thus illustrate that much research is needed to address the possibility of manipulating the activity of MAPK, FAK and/or polarity proteins by using small molecule specific inhibitors and/ or activators to manage the toxicant-induced injury in the testes.
Acknowledgements
Studies in the authors' laboratory were supported by grants from the National Institutes of Health (National Institute of Child Health and Human Development, R01 HD056034 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.; and R03 HD061401 to D.D.M.). P.P.M. and M.J. acknowledge receipt of financial support from the Department of Biotechnology (No. BT/BI/03/015/2002) and Department of Information Technology (No. DIT/R&D/BIO/15(9)/2007), Government of India, New Delhi, India. We are also indebted to many investigators in the field whose work has inspired us in our studies on the impact of environmental toxicants on male reproductive biology in the past decade. However, due to the space constraint, many of these earlier studies could not be cited. Readers are encouraged to refer to these earlier important studies and findings in the literature and in the recent reviews cited here.
References
- 1.Groff T. Bisphenol A: invisible pollution. Curr Opin Pediatr. 2010;22:524–529. doi: 10.1097/MOP.0b013e32833b03f8. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg LN, Chauhoud IHJj, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meeker JD. Exposure to environmental endocrine disrupting compounds and men's health. Maturitas. 2010;66:236–241. doi: 10.1016/j.maturitas.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 2010;56:147–167. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 5.Nava-Hernandez MP, et al. Lead-, cadmium-, and arsenic-induced DNA damage in rat germinal cells. DNA Cell Biol. 2009;28:241–248. doi: 10.1089/dna.2009.0860. [DOI] [PubMed] [Google Scholar]
- 6.Sekizawa J. Low-dose effects of bisphenol A: A serious threat to human health? J Toxicol Sci. 2008;33:389–403. doi: 10.2131/jts.33.389. [DOI] [PubMed] [Google Scholar]
- 7.Huff J, Lunn RM, Waalkes MP, Tomatis L, Infante PF. Cadmium-induced cancers in animals and in humans. Int J Occup Environ Health. 2007;13:202–212. doi: 10.1179/oeh.2007.13.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapin RE, Adams J, Boekelheide K, Gray L, Jr, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 9.Zhou T, Jia X, Chapin RE, Maronpot RR, Harris MW, Liu J, et al. Cadmium at a non-toxic dose alters gene expression in mouse testes. Toxicol Lett. 2004;154:191–200. doi: 10.1016/j.toxlet.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 10.ATSDR, author. Cadmium toxicity—Case Studies in Environmental Medicine. Atlanta, GA: Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 11.Wong EWP, et al. Cell junctions in the testis as targets for toxicants. In: McQueen CA, editor. Comprehensive Toxicology. 2nd Edition. Vol. 11. Oxford: Academic Press, Elseiver; 2010. pp. 167–188. Reproductive and Endocrine Toxicology. Hoyer PB, Richburg JH, eds. [Google Scholar]
- 12.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetherill YB, et al. In vitro molecualr mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JA, Welshon WV, Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod Toxicol. 2008;25:169–176. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thuillier R, Manku G, Wang Y, Culty M. Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microsc Res Tech. 2009;72:773–786. doi: 10.1002/jemt.20756. [DOI] [PubMed] [Google Scholar]
- 18.Pages G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, et al. Defective thymocyte maturation in p44 MAP kinase (Erk1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Li W, Wu J, Germann UA, Su MS, Kuida K, Boucher DM. Extracellular signal-regualted kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci USA. 2003;100:12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lie PPY, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: A biochemical model. Trends Biochem Sci. 2009;34:366–373. doi: 10.1016/j.tibs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- 25.Vogl A, Vaid K, Guttman J. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science+Business Media, LLC; 2008. pp. 186–211. [Google Scholar]
- 26.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of blood-testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci USA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S, et al. Structure of the Cx26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 29.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 30.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science+Business Media, LLC; 2008. pp. 212–233. [DOI] [PubMed] [Google Scholar]
- 31.Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973;175:639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- 32.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laskey JW, Phelps PV. Effect of cadmium and other metal cations on in vitro Leydig cell testosterone production. Toxicol Appl Pharmacol. 1991;108:296–306. doi: 10.1016/0041-008x(91)90119-y. [DOI] [PubMed] [Google Scholar]
- 35.Lafuente A, Gonzalez-Carracedo A, Romero A, Cano P, Esquifino AI. Cadmium exposure differntially modifies the circadian patterns of norepinephrine at the median eminence and plasma LH, FSH and testosterone levels. Toxicol Lett. 2004;146:175–182. doi: 10.1016/j.toxlet.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Waalkes M, Coogan T, Barter R. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit Rev Toxicol. 1992;22:175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- 37.Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956;177:1036–1037. doi: 10.1038/1771036b0. [DOI] [PubMed] [Google Scholar]
- 38.Parizek J. Sterilization of the male by cadmium salts. J Reprod Fertil. 1960;1:294–309. [Google Scholar]
- 39.Chiquoine D. Observations on the early events of cadmium necrosis of the testis. Anat Rec. 1964;149:23–36. doi: 10.1002/ar.1091490104. [DOI] [PubMed] [Google Scholar]
- 40.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 41.Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 42.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 43.Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 44.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 45.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: A friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/ Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nature Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 48.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson JR, Bickford LC, Morgan D, Kim AS, Ouerfelli O, Kirschner MW, Rosen MK. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nature Struct Mol Biol. 2004;11:747–755. doi: 10.1038/nsmb796. [DOI] [PubMed] [Google Scholar]
- 50.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 52.Cheng CY, Mruk DD. Regulation of blood-testis barrier dynamics by focal adhesion kinase (FAK). An unexpected turn of events. Cell Cycle. 2009;8:3493–3439. doi: 10.4161/cc.8.21.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siu ER, et al. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence TNF-γ expression in activated human T cells. J Leukoc Biol. 2010;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalton TP, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He L, Wang BH, Hay EB, Nebert DW. Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol Appl Pharmacol. 2009;238:250–257. doi: 10.1016/j.taap.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright SH. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol Appl Pharmacol. 2005;204:309–319. doi: 10.1016/j.taap.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Lee WK, Wolff NA, Thevenod F. Organic cation transporters: Physiology, toxicology and special focus on ethidium as a novel substrate. Curr Drug Metab. 2009;10:617–631. doi: 10.2174/138920009789375360. [DOI] [PubMed] [Google Scholar]
- 59.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, et al. Mechanism of IFNγ-induced endocytosis of tight junction proteins: Myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melaine N, Liénard MO, Dorval I, Le Goascogne C, Lejeune H, Jégou B. Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod. 2002;67:1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- 62.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emmetsberger J, Mirrione MM, Zhou C, Fernandez-Monreal M, Siddiq MM, Ji K, Tsirka SE. Tissue plasminogen activator alters intracellular sequestration of zinc through interaction with the transporter ZIP4. J Neurosci. 2010;30:6538–6547. doi: 10.1523/JNEUROSCI.6250-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nature Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 65.Bracht C, Hauser DR, Schattel V, Albrecht W, Laufer SA. Synthesis and biological testing of N-aminoimidazole-based p38β MAP kinase inhibitors. ChemMedChem. 2010;5:1134–1142. doi: 10.1002/cmdc.201000114. [DOI] [PubMed] [Google Scholar]
- 66.LoRusso PM, Krishnamurthi SS, Rinehart JJ, Nabell LM, Malburg L, Chapman PB, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin Cancer Res. 2010;16:1924–1937. doi: 10.1158/1078-0432.CCR-09-1883. [DOI] [PubMed] [Google Scholar]
- 67.Sarkar S, Mazumdar A, Dash R, Sarkar D, Fisher PB, Mandal M. ZD6474, a dual tyrosine kinase inhibitor of EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/ PI3-K and induces apoptosis in breast cancer cells. Cancer Biol Ther. 2010;9:592–603. doi: 10.4161/cbt.9.8.11103. [DOI] [PubMed] [Google Scholar]
- 68.Singh D, Smyth L, Borrill Z, Sweeney L, Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 69.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 70.Li MWM, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009;15:159–168. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: A review of recent data. Dev Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 73.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 74.Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 75.Li MWM, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, et al. TNFa reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 76.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 77.Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2003;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]