Abstract
Desmosomes are cell-cell junctions that link to cytoplasmic intermediate filaments, and they are known to mediate robust and stable adhesion in organs such as the skin and heart. Desmosomes are also present between apposing Sertoli cells at the blood-testis barrier, and between Sertoli cells and all germ cells up to, but not including, step 8 spermatids in the seminiferous epithelium. Unfortunately, they remain to be one of the least studied cell junction types in the seminiferous epithelium of the mammalian testis. In this article, we briefly discuss how kinases and the actin cytoskeleton relate to the study of desmosomes in the testis. It is hoped that this information is used to initiate more studies on the biology of the desmosome in the future.
Key words: desmosome, cell junction, testis, Sertoli cell, germ cell, spermatogenesis
Adhesion between Sertoli cells, as well as between Sertoli and germ cells, in the seminiferous epithelium of the mammalian testis is essential for spermatogenesis. Sertoli cells are highly polarized, ‘nurse-like’ epithelial cells that extend upwards from the basement membrane which essentially allows these cells to support between 30 to 50 developing germ cells at any one time throughout the entire seminiferous epithelial cycle.1,2 Another important Sertoli cell feature that is critical for spermatogenesis is the blood-testis barrier (BTB), an ultrastructure comprised of co-existing and mutually interacting junction types [i.e., tight junctions (TJs), basal ectoplasmic specializations (ESs), desmosomes and gap junctions] that basically maintains epithelial cell polarity and integrity3–5 (Fig. 1). The BTB is believed to cyclically restructure so that spermatocytes can enter the adluminal compartment of the seminiferous epithelium, and this is carried out in part by an array of molecules, which include junctional proteins, cytokines, proteases/protease inhibitors, hormones and endocytic/trafficking proteins.6–8 It is behind the BTB in the adluminal compartment that germ cells complete meiosis; thereby developing into mature elongated spermatids. Throughout spermatogenesis, migrating germ cells must also remain attached to Sertoli cells via desmosomes or ESs up until the time they are released from the seminiferous epithelium at late stage VIII of the epithelial cycle when Sertoli cell-spermatid junctions are promptly disassembled.9
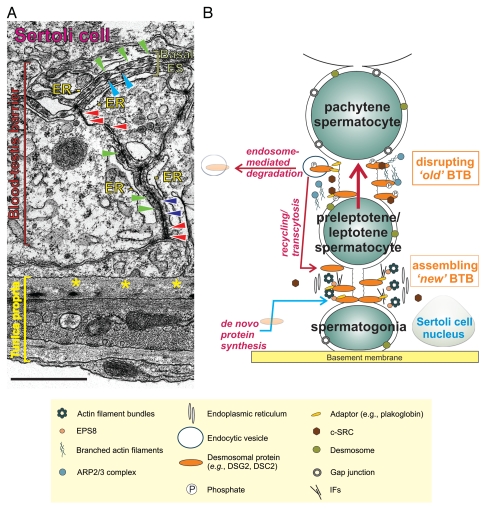
Figure 1.
Morphological features of the desmosome and its intimate relationship with TJs, basal ESs and gap junctions that together constitute the BTB in the mammalian testis. (A) This is an electron micrograph of a cross-section of the seminiferous tubule from an adult rat testis. It shows the seminiferous epithelium, which is composed of Sertoli and developing germ cells resting on the basement membrane (a modified type of extracellular matrix, see yellow asterisks) of the tunica propria. Desmosomes are seen between two Sertoli cells (see red arrowheads); they are typified by the presence of electron dense material. Basal ESs, on the other hand, are typified by the presence of actin filament bundles (see green arrowheads) sandwiched in between cisternae of endoplasmic reticulum (ER) and the Sertoli cell plasma membrane. Basal ESs co-exist with either TJs (“kisses” between apposing Sertoli cell plasma membranes, see blue arrowheads) or gap junctions (see purple arrowheads). Thus, desmosomes are critical structural components that constitute BTB function. Bar = 1 µm. (B) This is a schematic drawing of the seminiferous epithelium illustrating the relative location of the BTB. Desmosomal proteins (e.g., DSG2, DSC2, IFs) at the BTB may be phosphorylated by c-SRC, thereby inducing their internalization via endocytic vesicles. Internalized proteins may be degraded, thereby destabilizing the “old” BTB to facilitate the transit of preleptotene spermatocytes. Additionally, desmosomes also serve as a platform for signal transduction events. For instance, c-SRC -mediated protein phosphorylation may further destabilize the BTB. Internalized proteins may also be recycled and trafficked to the “new” BTB to establish new TJ-fibrils. Coupled with de novo synthesis of TJ proteins, the integrity of the immunological barrier can be maintained during the transit of preleptotene spermatocytes.
Numerous studies from the past two decades have described many important structural molecules that constitute Sertoli-Sertoli and/or Sertoli-germ cell adhesion in the mammalian testis; these include, but are not limited to, classic cadherins, protocadherins, nectins, integrins, junctional adhesion molecules and the coxsackie and adenovirus receptor (CAR).6,10,11 For instance, studies have reported N-cadherin to be a basal ES protein functioning in the maintenance of BTB integrity but also in its restructuring which is needed for spermatocytes to traverse this barrier. We arrive at this conclusion because cytokines (e.g., transforming growth factor-β, TGF-β) can trigger Sertoli cell N-cadherin endocytosis in vitro via a clathrin-dependent mechanism,12,13 which can ‘loosen’ or disassemble at least in part the basal ES. Unfortunately, there are relatively fewer studies that investigate the biology behind desmosomes in the testis. Consequently, our understanding of the spermatogenic process and the maintenance of fertility remain incomplete without additional information on this junction type. In this article, we discuss a few key points as they relate to the biology of desmosomes in the seminiferous epithelium, in particular why this junction type should be investigated in future studies, what are some of the more important open questions that can be addressed through basic experiments and how this information can be used to develop safe non-hormonal male contraceptives. For in-depth background information, interested readers are asked to refer to references 14–17.
Desmosomes were originally studied in the testis by the late Lonnie Russell who described these intermediate filament-based structures as ‘desmosome-like’ (alternatively coined as ‘desmosome-gap’) because ultrastructurally they did not seem to resemble the robust and Ca2+-independent (i.e., hyper-adhesive) desmosomes that were found in the skin or heart.18 Today, we know that desmosome-like junctions, which are found between Sertoli cells at the BTB and between Sertoli cells and all germ cells up to (but not including) step 8 (elongating) spermatids (Fig. 1), are comprised of many of the same proteins (e.g., desmogleins, desmocollins, plakoglobin, plakophilins and desmoplakins) that constitute desmosomes in other organs;14 and because of this, they should be called as such. Moreover, desmogleins and desmocollins, either alone or with connexins (gap junction proteins), cannot form functional hemichannels. Irrespective of these important and recent findings, a key difference between desmosomes in the skin, for example, and desmosomes in the testis may be the rate at which these junctions are restructured in vivo. This may ultimately control the extent of their organization because it makes little physiological sense for rapidly migrating cells (i.e., germ cells) to assemble robust and stable adhesive contacts. (In this context, it is worth noting that adhesion conferred by desmosomes in vitro is considerably weaker than that conferred by apical ESs;19 this is in contrast to other epithelia where desmosomes reinforce adherens junctions.20) In addition, the organization, as well as the regulation, of different junction types within an epithelium is highly variable among different tissues. For example, Sertoli-Sertoli and Sertoli-germ cell desmosomes co-exist with gap junctions; and Sertoli-Sertoli cell desmosomes at the BTB are further juxtaposed with TJs and basal ESs5,6 (Fig. 1). As such, other junction types may directly or indirectly affect the adhesive function of desmosomes in the testis. To summarize, these are just two ways in which testicular desmosomes may differ from conventional desmosomes, but at this point additional research is needed to define more concretely how testicular desmosomes differ from their counterpart. For example, it is not known if mature desmosomes between Sertoli cells or between Sertoli and germ cells are hyper-adhesive. Culturing Sertoli cells in low Ca2+ medium should provide new insights.
Besides functioning in cell-cell adhesion, desmosomes also provide important platforms for signal transduction events that downstream control many different aspects of cell function because several protein kinases have been found to associate with desmosomal proteins. For instance, conventional protein kinase C (PKC) family members are known to phosphorylate desmosomal proteins.21–23 While this can affect desmosome assembly and disassembly because phosphorylation per se can elicit discrete changes in protein-protein interactions and protein localization,16 it can also result in more global changes in cell function. In the testis, the process of germ cell differentiation may be regulated in part by transient and cyclic changes in adhesion and de-adhesion which occur as germ cells traverse the seminiferous epithelium throughout spermatogenesis. In other words, as Sertoli cells re-establish interactions with newly migrated germ cells in vivo, ‘nurse-like’ Sertoli cells may produce signals to stimulate germ cell differentiation/development—thereby balancing proliferation and apoptosis which essentially determines the number of germ cells that Sertoli cells can physiologically support within the confines of the seminiferous epithelium. Desmosomes may also prevent aberrant proliferation of germ cells, which might otherwise culminate in testicular tumorigenesis, and these hypotheses should be tested in future studies. Furthermore, SRC, a non-receptor tyrosine kinase, is also an important player in desmosome dynamics, as was recently shown in Sertoli cells in vitro. We previously reported that simultaneous knockdown of desmoglein-2 (DSG2) and desmocollin-2 (DSC2) in cultured Sertoli cells by RNA interference (RNAi) resulted in an increase in the rate at which CAR (a transmembrane protein with semi-defined roles in TJ and adherens junction dynamics, it links indirectly to actin filaments24,25) was endocytosed and that this was possibly mediated by SRC.14 SRC is well known to control cell proliferation, adhesion and migration, and actin dynamics;26,27 it also co-immunoprecipitates with DSG2 and CAR in the testis14,28 (Fig. 1). These results illustrate that crosstalk between junctional proteins converges downstream on a network of kinases that may facilitate endocytic vesicle-mediated protein trafficking events. These results also provide a novel starting point for addressing how kinases regulate desmosome dynamics in the seminiferous epithelium. For example, it would be important to determine whether TGF-β or interleukin-1α (IL-1α, see discussion below) triggers internalization of desmosomal cadherins in Sertoli-germ cell co-cultures, whether this affects Sertoli-germ cell adhesion, and finally whether a decrease in SRC (by RNAi, expression of dominant-negative SRC, or possibly by treatment with a specific SRC family inhibitor) blocks these cellular events.
Conventionally speaking, desmosomes are known to attach to intermediate filaments (IFs, Fig. 1); however, recent studies have also unexpectedly implicated actin and actin-associated proteins (e.g., RHOA, SRC) in desmosome dynamics.29–32 A good example of the involvement of the actin cytoskeleton in desmosome dynamics is evidenced by the loss of adhesion between keratinocytes (i.e., acantholysis) that were earlier treated with pemphigus vulgaris (PV, an autoimmune skin blistering disorder) IgG;33 here, the actin network was found to be severely disrupted. At the cellular level, PV IgG binds to the DSG3 ectodomain, resulting in its clathrin- and dynamin-independent endocytosis and consequently in desmosome destabilization.34 Moreover, PV IgG was found to target RHOA. Specifically, RHOA activation abrogated PV IgG-induced desmosome disassembly.35 In support of these findings, a disruption in actin polymerization also resulted in a striking increase in DSG3 internalization,33 illustrating that stable desmosomal adhesion relies largely on the intactness of the actin cytoskeleton. In the testis, actin filaments are confined largely to the apical and basal ES where they are oriented unipolarly, hexagonally packed and not branched (Fig. 1). Thus, desmosomes at the BTB are under the control of the actin cytoskeleton, whose remodeling from late stage VIII to XI of the seminiferous epithelial cycle facilitates junction disassembly and germ cell movement across the barrier. Interestingly, a recent study has demonstrated IL-1α to profoundly disrupt the Sertoli cell actin cytoskeleton in vitro and in vivo.36,37 Because PV pathogenesis has been linked to a disruption of the actin cytoskeleton and desmosomes, and several ILs were found to be elevated in sera from individuals with PV; it is important that the levels of different desmosomal proteins be investigated following IL-1α treatment. The involvement of SRC in these cellular events should also be investigated. On a final note, IFs did not appear to associate with desmosomes in germ cells, suggesting that they may associate weakly with actin filaments to facilitate germ cell movement across the seminiferous epithelium.35
Herein, we have briefly highlighted two potentially important directions (i.e., the roles of kinases and the actin cytoskeleton) in the study of desmosomes in the future. Their study is, and will continue to be, of great significance to our field because it will help us better understand how a Sertoli cell can maintain adhesion with another Sertoli cell, as well as with several germ cells, in the seminiferous epithelium. After all, this forms the basis of spermatogenesis, and a disruption in desmosomal adhesion may lead to transient or even permanent sterility, as this would eradicate most germ cells from the seminiferous epithelium. From a clinical perspective, testicular desmosomes may be a unique and appealing target for non-hormonal contraceptive development, and their adhesion may be easy to perturb if we can determine how they differ from desmosomes in other organs. For example, targeting desmosomes within developing germ cells, which do not appear to link to IFs, may be an interesting approach that may result in germ cell sloughing from the seminiferous epithelium as this would leave Sertoli cells largely unscathed. It is hoped that more studies are initiated to better understand the biology of desmosomes in the testis.
Acknowledgements
Research in the authors' laboratory is supported by NICHD, NIH (R03 HD061401 to D.D.M.; R01 HD056034, R01 HD056034-02S1 and U54 HD029990 to C.Y.C.).
References
- 1.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, et al., editors. The Physiology of Reproduction. New York, NY: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 2.Kerr JB, Loveland KL, O'Bryan MK, de Kretser DM. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. New York, NY: Elsevier; 2006. pp. 827–947. [Google Scholar]
- 3.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 4.Setchell BP, Waites GMB. The blood-testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology. Baltimore, MD: Williams and Wilkens; 1975. pp. 143–172. (1975) [Google Scholar]
- 5.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 6.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MWM, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: an emerging concept of regulation. Cytokine Growth Factor Rev. 2009;20:329–338. doi: 10.1016/j.cytogfr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–36. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germ cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech. 2010;73:409–494. doi: 10.1002/jemt.20786. [DOI] [PubMed] [Google Scholar]
- 12.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia W, Wong EWP, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lie PPY, Cheng CY, Mruk DD. The desmoglein-2/desmocollin-2/Src kinase protein complex regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2009;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lie PP, Cheng CY, Mruk DD. The biology of the desmosome-like junction: a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 17.Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1:a002543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- 19.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 20.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 21.Sheu HM, Kitajima Y, Yaoita H. Involvement of protein kinase C in translocation of desmoplakins from cytosol to plasma membrane during desmosome formation in human squamous cell carcinoma cells grown in low to normal calcium concentration. Exp Cell Res. 1989;185:176–190. doi: 10.1016/0014-4827(89)90047-5. [DOI] [PubMed] [Google Scholar]
- 22.Denning MF, Guy SG, Ellerbroek SM, Norvell SM, Kowalczyk AP, Green KJ. The expression of desmoglein isoforms in cultured human keratinocytes is regulated by calcium, serum, and protein kinase C. Exp Cell Res. 1998;239:50–59. doi: 10.1006/excr.1997.3890. [DOI] [PubMed] [Google Scholar]
- 23.van Hengel J, Gohon L, Bruyneel E, Vermeulen S, Cornelissen M, Mareel M, Roy F. Protein kinase C activation upregulates intercellular adhesion of a-catenin-negative human colon cancer cell variants via induction of desmosomes. J Cell Biol. 1997;137:1103–1116. doi: 10.1083/jcb.137.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. In: Tracy S, Oberste MS, Drescher KM, editors. Group B Coxsackieviruses. Current Topics in Microbiology and Immunology. Berlin, Germany: Springer-Verlag Berlin; 2007. pp. 67–87. [DOI] [PubMed] [Google Scholar]
- 25.Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57:869–882. doi: 10.1016/j.addr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death and cancer. Phamacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 27.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–1392. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LT, Huen AC, Thorne ME, Gaudry CA. Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J Cell Biol. 2005;171:1045–1059. doi: 10.1083/jcb.200510038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennings JM, Tucker DK, Kottke MD, Saito M, Delva E, Hanakawa Y, Amagai M, Kowalczyk AP. Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can be reversed by expression of exogenous Dsg3. J Invest Dermatol. 2010;131:706–718. doi: 10.1038/jid.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics and homeostasis. In: Nelson WJ, Fuchs E, editors. Cold Spring Harbor Perspectives in Biology. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2010. p. a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 33.Gliem M, Heupel WM, Spindler V, Harms GS, Waschke J. Actin reorganization contributes to loss of cell adhesion in pemphigus vulgaris. Am J Physiol Cell Physiol. 2010;299:C606–C613. doi: 10.1152/ajpcell.00075.2010. [DOI] [PubMed] [Google Scholar]
- 34.Delva E, Jennings JM, Calkins CC, Kottke MD, Faundez V, Kowalczyk A. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrinand dynamin-independent mechanism. J Biol Chem. 2008;283:18303–18313. doi: 10.1074/jbc.M710046200. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D. (2006) Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin-1α (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–454. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lie PP, Cheng CY, Mruk DD. Interleukin-1α is a regulator of the blood-testis barrier. FASEB J. 2010 doi: 10.1096/fj.10-169995. [DOI] [PMC free article] [PubMed] [Google Scholar]