Abstract
Background
Atrial fibrillation (AF) has been associated with myocardial oxidative stress, and antioxidant agents have demonstrated antiarrhythmic benefit in humans. We compared serum markers of oxidation and associated inflammation in individuals with or without AF.
Methods
Serum markers of oxidative stress and inflammation were compared in a cross-sectional, case-control design study of 40 male individuals, with or without persistent or permanent AF, who were matched for age, sex, diabetes, and smoking status, known confounding variables for the measurement of oxidative stress. We used derivatives of reactive oxidative metabolites (DROMs) and ratios of oxidized to reduced glutathione (Eh GSH) and cysteine (Eh CySH) to quantify oxidative stress. We also measured inflammatory markers, including high-sensitivity C-reactive protein, interleukins 1β and 6, and tumor necrosis factor α.
Results
Univariate, conditional logistical regression analysis showed that oxidative stress but not inflammatory markers were statistically associated with AF (P <0.05). The increase in the odds ratios for AF for Eh GSH, Eh CySH, and DROMs were 6.1 (95% CI, 1.3–28.3; P = 0.02), 13.6 (95% CI, 2.5–74.1; P = 0.01), and 15.9 (95% CI, 1.7–153.9; P = 0.02), respectively. There was a stronger correlation between Eh GSH and Eh CySH (r = 0.66) than between Eh GSH and DROMs (r = 0.41). In multivariate analysis corrected for statins and other AF risk factors differing between the groups, the association of AF and oxidative stress remained significant.
Conclusions
These data suggest that oxidative stress markers may have predictive value in AF management.
Atrial fibrillation (AF)5 is by far the most common cardiac arrhythmia. Currently, 2.2 million people in the US have a diagnosis of AF. The pathogenesis of AF is unknown, but studies have supported a role for both oxidative stress and inflammation. Studies of animal and human samples have shown increased myocardial oxidative stress associated with AF (1, 2). Inflammation has a complex relationship with oxidative stress and has also been found to be associated with AF. For example, increased concentration of the inflammatory marker C-reactive protein (CRP) was found to be associated with AF in some studies (3) and has been suggested to be a predictor of the incidence of AF after cardioversion (4) or cardiac surgery (5).
The relative strength of the association of inflammation and oxidative stress markers with AF remains unclear. Therefore, we assessed differences in markers of oxidative stress and inflammation in patients with or without persistent or permanent AF.
Materials and Methods
study population
This cross-sectional, case-control study recruited patients in AF from outpatient clinics at the Atlanta Veterans Affairs Medical Center from May through July 2005 under a protocol approved by the Emory University Institutional Review Board (http://www.clinicaltrials.gov:NCT00142194). Eligible patients were older than 18 years and in persistent or permanent AF at the time of enrollment. Ineligibility criteria included systemic inflammatory diseases, malignant neoplasm, severe stenotic or regurgitant valvular heart disease, New York Heart Association class IV heart failure, hyperthyroidism, uncontrolled hypertension (>180/100 at rest), presence of an illness that may result in death within 1 year, implanted devices designed for the active management of atrial arrhythmias by pacing or defibrillation, and current illicit drug use or alcohol abuse. Eligible patients were identified as they came for previously scheduled clinic visits and invited to enroll in the study.
Control patients were identified at outpatient clinic visits during the same time period and recruited according to the same eligibility and ineligibility criteria, with the exception that control patients were free of current AF and any history of AF. Case patients and controls were matched for variables known to affect the oxidative markers used, age in decades, smoking, and diabetes status (6–8). All study participants gave written informed consent.
data collected
Data were collected by interviews of study participants, review of Department of Veterans Affairs hospital and clinic charts, telemetry recordings, and electrocardiograms. The presence or absence of AF was confirmed on the basis of an electrocardiogram done at the time of enrollment. A single blood draw was performed at the time of enrollment, and the blood sample was analyzed in the Emory Biomarkers Core Laboratory for markers of oxidative stress and inflammation. All study participants underwent testing for oxidative stress and inflammatory markers. No adverse events occurred, and no test results were indeterminate or excluded. Markers used to measure oxidative stress were ratios of oxidized to reduced glutathione (Eh GSH) and cysteine (Eh CySH) in plasma (thiol ratios) (6) and derivatives of reactive oxygen metabolites (DROMs) (9). Detailed methods to prevent rapid oxidation of samples have been delineated previously (10). Briefly, blood was collected from an antecubital vein and transferred immediately to a microcentrifuge tube containing 0.5 mL of a preservation solution of 100 mmol/L serine · borate (pH 8.5) containing (per mL) 0.5 mg sodium heparin, 1 mg bathophenanthroline disulfonate sodium salt, and 2 mg iodoacetic acid. Use of this procedure minimizes autoxidation and hemolysis (10). All blood was drawn between 7:30 AM and 3:00 PM in nonfasting patients. After centrifugation to remove blood cells, aliquots (200 μL) were transferred to tubes containing 200 μL of 10% (wt/vol) perchloric acid containing 0.2 mol/L boric acid and 10 μmol/L γ-Glu-Glu as an internal calibrator. Samples were stored at −80 °C for <2 months before further processing to form N-dansyl derivatives and analysis by HPLC with fluorescence detection. Reduced glutathione, cysteine, and cystine concentrations in plasma were >1000 times the level of detection (approximately 1 nmol/L). Oxidized glutathione concentrations were approximately 10 times this limit. Previous data have shown stable measurements with storage for this length of time (10). Metabolites were identified by coelution with calibrators, and quantification was obtained by integration relative to the internal calibrator. Samples from control and AF patients were treated identically. Laboratory technicians were blinded with respect to the clinical data.
The redox states (Eh) of the thiol/disulfide pools were calculated with the Nernst equation:
where Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred, and F is the Faraday constant. The standard potential Eo used for the glutathione and cysteine redox couples was −264 mV and −250 mV, respectively (10). Less negative Eh numbers imply a more oxidized state. DROMs were measured in Carr units, with higher values indicating increased oxidative stress. DROMs (Diacron International) and inflammatory markers [high-sensitivity CRP (hsCRP; Life Diagnostics) and interleukin (IL)-1β, IL-6, and tumor necrosis factor α (TNFα; all from R&D Systems)] were measured using commercially available reagent sets. Intraassay CVs were <1% at −156 and <1% at −120 mV for Eh GSH; 5.0% at −100 and 4.5% at −60 mV for Eh CySH; 0.2% at 300 and 2.3% at 550 Carr units for DROMs; 10.1% at 0.2 and 5.2% at 10 ng/L for IL-1β; 5.1% at 1 and 3.6% at 8 mg/L for hsCRP; 20.9% at 3.2 and 6.2% at 50 ng/L for IL-6; and 11.9% at 2 and 7.3 at 50 ng/L for TNFα.
DATA ANALYSIS
Statistical analyses were performed with SAS software 9.1 (SAS Institute). We compared baseline characteristics of AF patients and their matched controls with a paired t-test for continuous variables [expressed as mean (SD)], and Fisher exact test for categorical variables. Marker data were presented as the mean (SD), except as noted. All statistical tests were 2-tailed, and significance was assumed at P ≤0.05. We assessed correlations between markers of inflammation and oxidative stress with Spear-man rank-order correlation coefficients. All oxidative and inflammatory markers were examined as predictors of AF occurrence in single-variate conditional logistic regression models. Parameter estimates for each oxidative and inflammatory marker were scaled so that reported odds ratios corresponded to approximate interquartile range increases. The approximate increases in the measured units needed to move from the 25th to 75th percentile were 20 mV, 10 mV, 70 Carr, 0.35 ng/L, 3 ng/L, 5 ng/L, and 3 mg/L for Eh GSH, Eh CySH, DROMs, IL-1β, IL-6, TNFα, and hsCRP, respectively. Multivariate conditional logistic regression models were used to examine the association between each oxidative marker and the presence of AF while controlling for inflammatory markers, hypertension, statins, and congestive heart failure. Statins were assessed as predictors of AF presence and in linear regression models as predictors of Eh GSH. All patients had been on statins for at least 1 month.
Results
We compared a total of 40 individuals with or without persistent or permanent AF. Table 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol53/issue9 compares the demographics of study patients and their controls. The mean (range) age of AF patients was 74.8 (58–86) years. Five AF patients (25%) had adult-onset diabetes, and all were male and nonsmokers. The mean length of AF was 10.1 years, with a median (SD) of 6.4 (13.3) years. In nonmatched variables, hypertension and heart failure were slightly more common in the AF group. For all other variables, the populations were statistically similar (P >0.05).
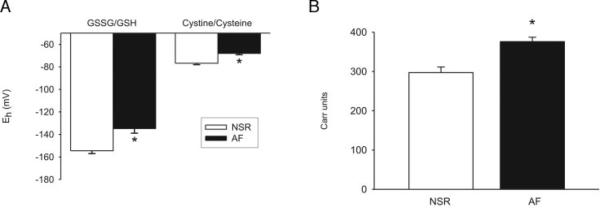
All measures of oxidative stress were significantly increased in AF patients compared with controls. Thiol ratios in the AF group were significantly more oxidized (i.e., negative) than in the controls (P <0.001; Fig. 1A). The AF group showed more oxidation, with a mean (SD) Eh GSH of −133 (21) mV (median, −143 mV; interassay CV, 15.8%) and Eh CySH of −68 (6) mV (median, −67 mV; CV, 8.8%) compared with the control group, which had a mean (SD) Eh GSH of −154 (12) mV (median, −156 mV; CV, 7.8%) and Eh CySH of −77 (6) mV (median, −76; CV, 7.8%). Consistent with the thiol results, the DROMs also showed more oxidation in the AF group [388 (54) Carr units; median, 370 Carr units; CV, 13.9%] than the controls [310 (44) Carr units; median, 308 Carr units; CV, 14.2%; P <0.001; Fig. 1B].
Fig. 1.
Oxidative stress markers between subjects with and without AF. (A), comparison of oxidized (GSSG) to reduced (GSH) glutathione and oxidized (cystine) to reduced cysteine thiol couples in subjects with AF (black columns) as compared with matched controls in normal sinus rhythm (NSR; open columns). (B), DROMs in the AF group (black columns) as compared with the controls in NSR (open columns). Data are presented as mean ± SE; * indicates a P <0.05.
The inflammatory markers IL-1β, IL-6, TNFα, and hsCRP were mildly, but insignificantly, increased in the AF group compared with controls. Mean (SD) values for IL-1β, IL-6, TNFα, and hsCRP in the AF group were 0.5 (0.8) ng/L (median 0.3), 5.5 (3.9) ng/L (median 4.2), 6.5 (8.1) ng/L (median 3.8), and 5.1 (3.8) mg/L (median 4.5) compared with 0.4 (0.4) ng/L (median 0.3), 3.9 (1.6) ng/L (median 3.6), 5.5 (3.4) ng/L (median 4.7), and 3.6 (3.1) μg/mL (median 2.6) for the control group, respectively.
The relationship of oxidative stress and inflammatory markers to AF was analyzed in single-exposure conditional logistical models. The odds ratios for AF were computed based on an interquartile range increase for each single marker, comparing the risk of AF in individuals at the 25th percentile to those at the 75th percentile. Single-exposure model odds ratios were controlled for matching variables of age, sex, smoking, and diabetes status but otherwise were unadjusted. Oxidative stress markers, Eh GSH, Eh CySH, and DROMs all predicted AF with odds ratios of 6.1 (95% CI, 1.3–28.3; P = 0.02), 13.6 (95% CI, 2.5–74.1; P = 0.01), and 15.9 (95% CI, 1.7–153.9; P = 0.02), respectively. None of the odds ratios for any of the 4 inflammatory markers measured were statistically significant (Fig. 2).
Fig. 2.
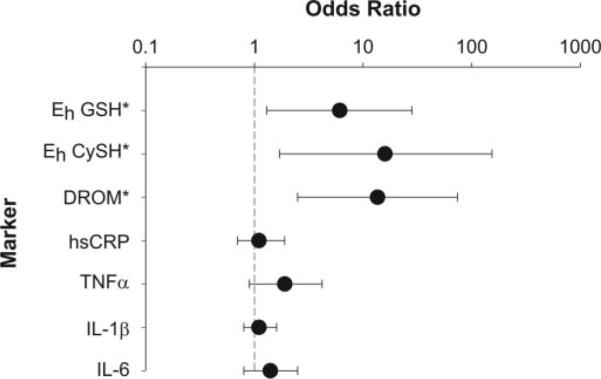
The univariate odd ratios for AF as a function of an interquartile increase in various markers. *, Eh GSH, Eh CySH, and DROMs significant at P ≤0.02.
Regression analysis implied a positive association between the degree of oxidative stress and the odds ratio for AF. Fig. 3 shows the calculated relationship of Eh GSH to the odds ratio for AF based on the parameters determined in the multivariate logistical regression analysis. A change in Eh GSH of 15 mV implies an approximately 4-fold increase in the odds ratio of AF. Spearman correlation coefficients revealed a statistically significant correlation between Eh GSH and Eh CySH (r = 0.66; P <0.01), whereas the relationship of thiol ratios to DROMs was weaker (r = 0.41 or 0.38, respectively). For the most part, oxidative stress markers were independent of inflammatory markers, except for the case of a statistically significant positive correlation between IL-6 and DROMs (r = 0.38; P = 0.02) and a negative correlation between TNFα and Eh CySH (r =−0.42; P = 0.01). On the other hand, most inflammatory markers showed significant correlation between each other. Statin use was negatively correlated with AF, with an odds ratio of 0.2 (95% CI, 0.05–0.99; P = 0.05). Moreover, linear regression analysis revealed that statins were associated with a 14.3 mV (95% CI, 0.8 –27.8) decrease in the oxidative stress marker Eh GSH.
Fig. 3.
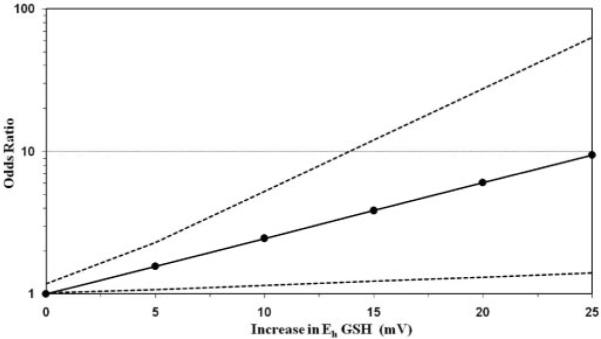
The odds ratio for AF for a given change in Eh GSH. The dashed lines indicate 95% CIs.
The sample size restricted the number of variables we could examine simultaneously in multivariate models. Nevertheless, we did examine all the oxidative stress markers while controlling for various subsets of possible confounders: hypertension, congestive heart failure, statin use, and the 4 inflammatory markers. In each of these multivariate models, the association of AF and more oxidized thiol ratios remained statistically significant.
Discussion
Oxidative stress has been implicated in the pathogenesis of AF. We found that oxidative stress markers differed between patients with and those without persistent or permanent AF. Increases in interquartile range across all markers of oxidative stress strongly and significantly correlated with increased risk of AF, an association that persisted even after correction for differences in hypertension, congestive heart failure, and statin use between the 2 groups, the former 2 conditions predisposing to AF (11, 12).
A complex association exists between oxidative stress and inflammation. To evaluate this further, we compared the correlations between markers in our study. Inflammatory markers were not strongly correlated with oxidative stress markers. Consistent with the idea that thiol ratios best represent the redox states of the hydrophilic phase, whereas DROMs more likely measure the redox state of a lipid phase, there was a stronger relationship between thiol ratios than between thiol ratios and DROMs. Because statins are postulated to have antioxidant activity and have been associated with a decreased incidence of AF (13, 14), we analyzed the relationship between use of statins and AF among patients in our study. Consistent with previous findings, statin use was negatively correlated with AF. Moreover, patients on statins showed less oxidation.
The strong correlation of AF with oxidative stress markers may suggest novel measures to predict the onset of AF and the efficacy of treatment. According to parameters derived from the regression analysis, the odds ratio for AF was predicted to increase as a function of oxidative stress. This relationship was consistent with the effects of a decade increase in age, the presence of diabetes or hypertension, or smoking on Eh GSH and AF risk (6–8). The mechanisms whereby oxidative stress may contribute to AF are unknown, but there is evidence that oxidants can affect ion channel activity (15). Recently, we have shown that the cardiac sodium channel (SCN5a) promoter region contains an NF-κB response element that could lead to Na+ channel transcriptional regulation by an NF-κB-dependent mechanism (16).
In our study, we showed an inverse relationship between statin use and oxidative stress. Statins are thought to have antioxidant properties attributable at least in part to prevention of NADPH oxidase–induced oxygen free radical production (17). Recently, increased NADPH oxidase activity has been associated with AF in humans (2). Our findings are consistent with reports that statins prevent electrical remodeling in rapid pacing-induced AF (18) and experimentally induced sterile pericarditis (19) in canine models and that statins reduce AF burden after cardiac (13) or noncardiac surgeries (20). Moreover, our findings are consistent with a recent report of statins preventing recurrence of AF after cardioversion (14).
Because inflammation has been associated with AF and oxidative stress, we also measured and compared inflammatory markers in our patient and control groups, but we did not find an association. This result is consistent with findings of several other groups investigating the use of CRP to predict postoperative AF (20, 21). Conway et al. (22) found that CRP predicted only initial but not long-term cardioversion success. Conversely, other reports suggest a correlation of inflammatory markers with AF. There is a well-documented increase in AF incidence after cardiac surgery, and this increase in AF correlates temporally with the peak elevation in CRP concentrations (23). Moreover, in 2 trials, patients with high CRP concentrations were more likely to develop AF (3, 5). A recent metaanalysis of 16 trials does suggest a relationship between inflammation and persistent or permanent AF (24). In one trial, IL-6 but not CRP or TNFα predicted postoperative AF (25). The concomitant lack of increase of IL-1β, IL-6, and CRP is consistent with the known roles of these ILs as synergistic upstream stimuli for CRP production (26).
Because our patients had persistent or permanent AF, the association of AF with oxidative stress but not inflammatory markers could represent a more prominent role for oxidative stress relative to inflammation in the maintenance rather than the initiation of AF. Alternatively, our findings may be attributable to differences in postoperative and nonoperative AF, lack of sensitivity given our high baseline CRP concentrations compared to other trials, or the limited power of the study to detect a relationship. The concentrations of IL-6 and TNFα in our study participants were comparable with baseline concentrations in a recent report, however, a finding that suggests that our patients were not substantially different in inflammatory state from those in other trials (25).
Our results do not speak to the role of oxidative stress in the initiation of AF nor do they rule out a potential role for inflammation in the initiation and/or maintenance of AF. Interestingly, cardiac surgery has also been reported to increase oxidative stress as measured by thiol ratios in the plasma and myocardium (27), and supplementing postoperative patients with ascorbate, a known antioxidant, cuts rates of AF more than 2-fold (28).
Despite the novel finding of an association of oxidative stress markers with AF, this study has several limitations. First is the small sample size. Nevertheless, based on the oxidative stress measure with the weakest association to AF (Eh GSH), only 13 individuals in each group would have been necessary to have a 90% power to detect the observed difference in means with a 2-sided test. Although our sample size was sufficient to show differences between groups in oxidative stress markers, the sample size was too small to allow firm conclusions about the relationship between inflammatory markers and AF, given their smaller means and higher SDs. Moreover, all participants in this study were males and predominantly white. The results may not hold true for females or individuals of other races. The effect of medications other than statins or the duration of AF on the strength of association between oxidative stress and AF were not evaluated in this study. The net number of medications was not different between the 2 groups. This study does not make clear the source of oxidative stress or how oxidative stress is related to AF. As noted above, AF has been associated with cardiac oxidative stress, but a recent trial suggests that oxidative stress in AF may be more widespread (29). Therefore, it is possible that systemic oxidative stress contributes to AF risk and, once AF is established, local cardiac oxidative stress reinforces the risk (1, 2). Interestingly, congestive heart failure and hypertension are associated with oxidative stress, perhaps contributing to their unequal distribution between the 2 groups (30, 31). Finally, we measured marker concentrations during usual clinical hours and without fasting, mimicking the most common clinical scenario. Measuring at other times or under other conditions may affect the results, but there is no known diurnal variation in DROM concentrations. There does appear to be diurnal variation of plasma-reduced thiols related to meals in animals (32), but the effect of this variation on the ratio of oxidized to reduced thiols is unknown. In preliminary studies, diurnal variations in Eh GSH and Eh CySH were too small to explain our results and have peaks that are separated by 6–7 h, suggesting that the differences that we observed were not the result of these variations alone.
In conclusion, our results suggest that further prospective research is warranted to examine the predictive value of oxidative stress markers in the management of AF.
Supplementary Material
Acknowledgments
Grant/funding support: This work was supported by National Institutes of Health Grants HL39006, HL77398, and HL73753; a Department of Veterans Affairs Merit Grant (to S.C.D.); an American Heart Association Established Investigator Award (to S.C.D.); a grant from Pfizer, Inc. (to S.C.D.); the Emory University School of Medicine Medical Student Summer Research Program (R.B.N.); and the Health Services Research and Development Program, Atlanta Veterans Affairs Medical Center.
Footnotes
Nonstandard abbreviations: AF, atrial fibrillation; CRP, C-reactive protein; DROMs, derivatives of reactive oxidative metabolites; Eh GSH, oxidized to reduced glutathione; Eh CySH, oxidized to reduced cysteine; hsCRP, high-sensitivity CRP; IL, interleukin; TNFα, tumor necrosis factor α.
Financial disclosures: S.C.D. is the principal investigator in a trial funded by Pfizer, Inc., to investigate the use of atorvastatin to prevent recurrences of AF after cardioversion. S.C.D. and D.P.J., Emory University, and the Department of Veterans Affairs have filed a provisional patent, Oxidative Stress Markers Predict Atrial Fibrillation (60/835,074), based on this work.
References
- 1.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulus L, Fukai T, et al. Atrial fibrillation increases production of super-oxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–73. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 2.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–36. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 3.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe E, Arakawa T, Uchiyama T, Kodama I, Hishida H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. 2006;108:346–53. doi: 10.1016/j.ijcard.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann Thorac Surg. 2005;79:1530–5. doi: 10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 7.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–8. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 9.Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178:115–21. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, et al. Glutathione measurement in human plasma: evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 13.Marin F, Pascual DA, Roldan V, Arribas JM, Ahumada M, Tornel PL, et al. Statins and postoperative risk of atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2006;97:55–60. doi: 10.1016/j.amjcard.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 14.Ozaydin M, Varol E, Aslan SM, Kucuktepe Z, Dogan A, Ozturk M, et al. Effect of atorvastatin on the recurrence rates of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2006;97:1490–3. doi: 10.1016/j.amjcard.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, et al. Oxidative mediated lipid peroxidation recapitulates proar-rhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–9. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 16.Shang LL, Dudley SC., Jr. Tandem promoters and developmentally regulated 5′ and 3′ mRNA untranslated regions of the mouse scn5a cardiac sodium channel. J Biol Chem. 2005;280:933–40. doi: 10.1074/jbc.M409977200. [DOI] [PubMed] [Google Scholar]
- 17.Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–5. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 18.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–9. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62:105–11. doi: 10.1016/j.cardiores.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–7. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 21.Zarauza J, Rodriguez Lera MJ, Farinas Alvarez C, Hernando JP, Ceballos B, Gutierrez B, et al. Relationship between C-reactive protein level and early recurrence of atrial fibrillation after electrical cardioversion. Rev Esp Cardiol. 2006;59:125–9. [PubMed] [Google Scholar]
- 22.Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–82. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 23.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 24.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–49. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 25.Ishida K, Kimura F, Imamaki M, Ishida A, Shimura H, Kohno H, et al. Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur J Cardiothoracic Surg. 2006;29:501–5. doi: 10.1016/j.ejcts.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Ganter U, Arcone R, Toniatti C, Morrone G, Ciliberto G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J. 1989;8:3773–9. doi: 10.1002/j.1460-2075.1989.tb08554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vecchi E, Pala MG, Di Credico G, Agape V, Paolini G, Bonini PA, et al. Relation between left ventricular function and oxidative stress in patients undergoing bypass surgery. Heart. 1998;79:242–7. doi: 10.1136/hrt.79.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 29.Guazzi M, Belletti S, Bianco E, Lenatti L, Maurizio GD. Endothelial dysfunction and exercise performance in lone atrial fibrillation or associated with hypertension or diabetes. Different results with cardioversion. Am J Physiol. 2006;291:H921–8. doi: 10.1152/ajpheart.00986.2005. [DOI] [PubMed] [Google Scholar]
- 30.Choudhary G, Dudley SC., Jr. Heart failure, oxidative stress, and ion channel modulation. Congest Heart Fail. 2002;8:148–55. doi: 10.1111/j.1527-5299.2002.00716.x. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 32.Adams JD, Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983;227:749–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.