Abstract
Benner, Ernest J. (University of Washington School of Medicine, Seattle), John V. Bennett, Jean L. Brodie, and William M. M. Kirby. Inactivation of cephalothin and cephaloridine by Staphylococcus aureus. J. Bacteriol. 90:1599–1604. 1965.—Marked differences were observed in the susceptibility of penicillinase-producing staphylococci to cephalothin and cephaloridine. All of 100 strains of penicillin G-resistant Staphylococcus aureus, with the use of a large inoculum, were found to be susceptible to 2 μg/ml of cephalothin, whereas only 50% were susceptible to this concentration of cephaloridine, and 15% required 15 μg/ml or more for inhibition. In contrast, penicillin G-sensitive strains were more susceptible to cephaloridine and did not show the marked inoculum effect observed with the cephaloridine-resistant strains. These differences were due to a much greater destruction of cephaloridine than of cephalothin by staphylococcal penicillinase. Cephaloridine-resistant staphyloccoci were stronger penicillinase producers than were susceptible strains, and the resistant strains were found to inactivate cephaloridine by hydrolysis of the β-lactam ring. In population studies, cephaloridine-resistant cells differed from methicillin-resistant cells in that they decreased in numbers as the drug concentration was increased, and the survivors in higher drug concentrations were no more resistant than was the parent strain. Treatment with acriflavine eliminated resistance of the cells to both penicillin G and cephaloridine. It was concluded that cephaloridine resistance was due to hydrolysis by penicillinase, and that this was related to the pyridine ring substitution in the cephalosporanic acid nucleus.
Full text
PDF
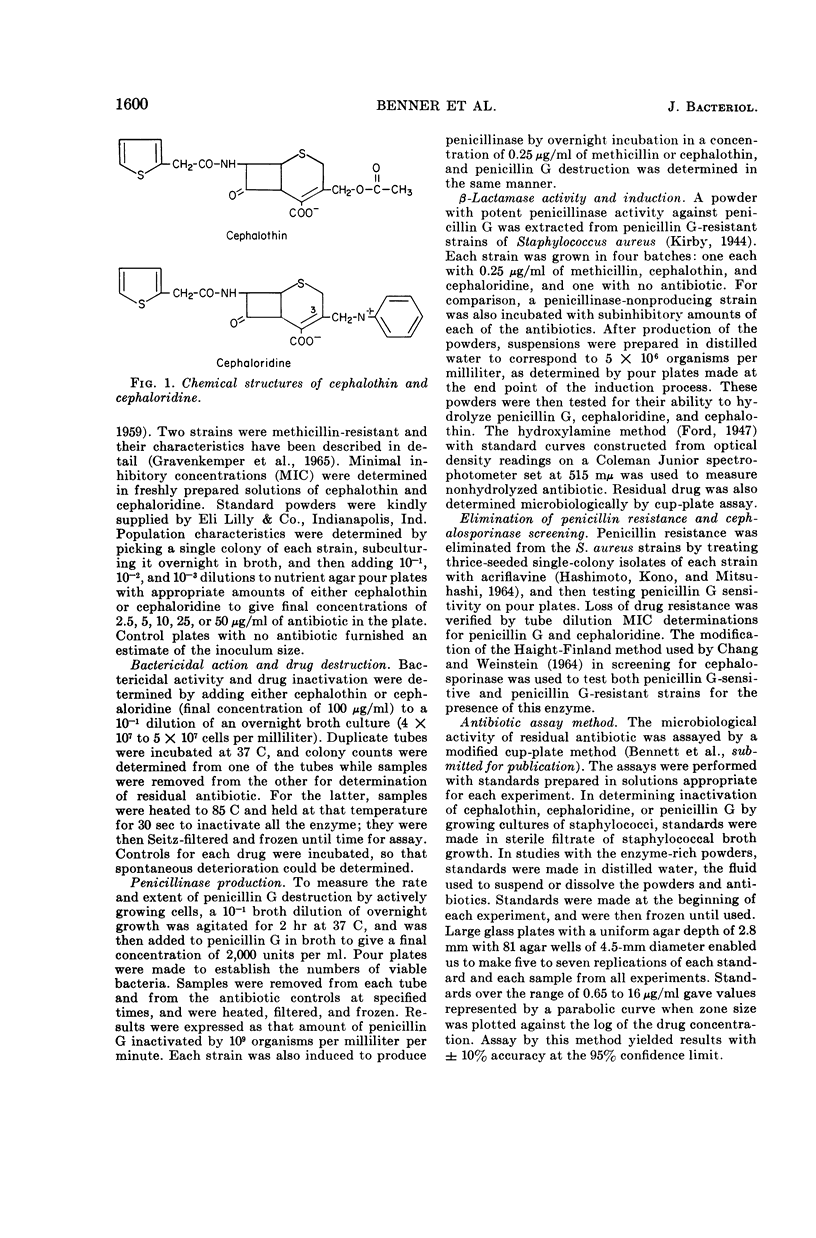
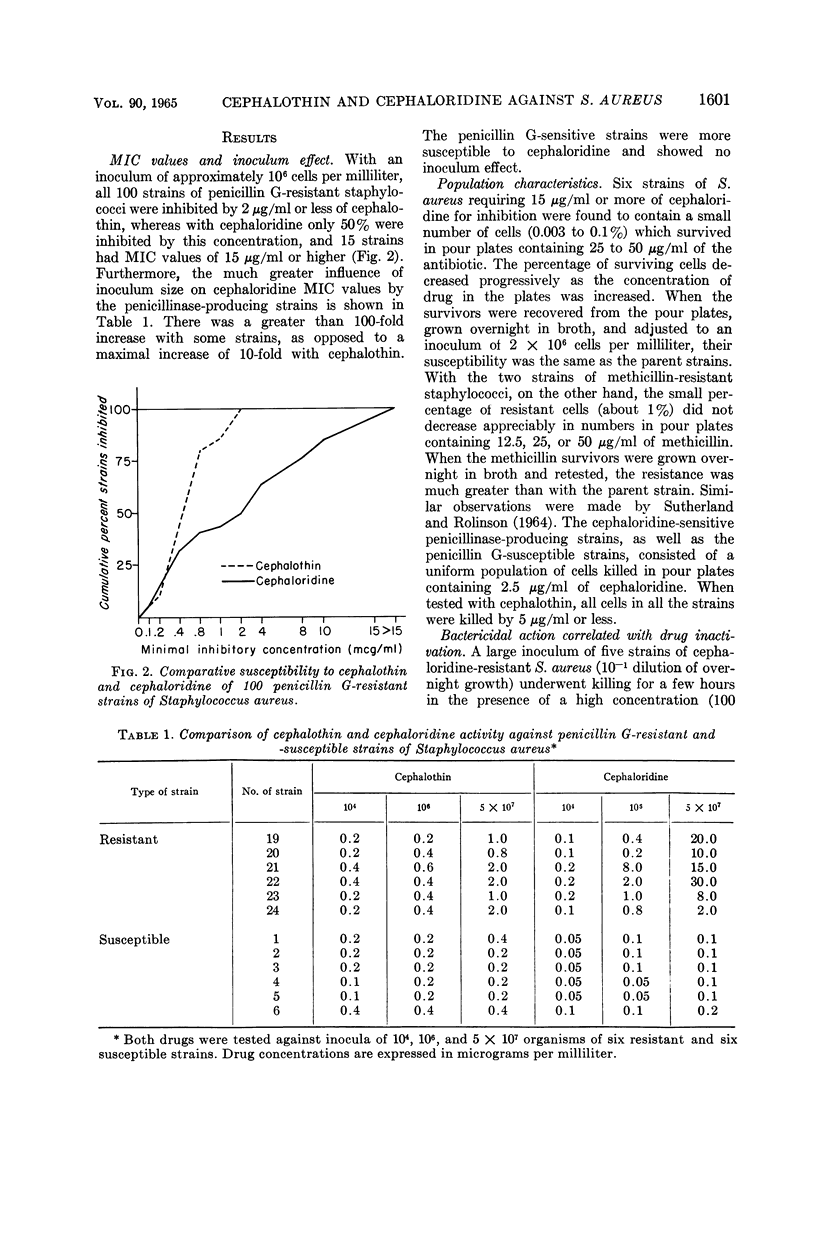
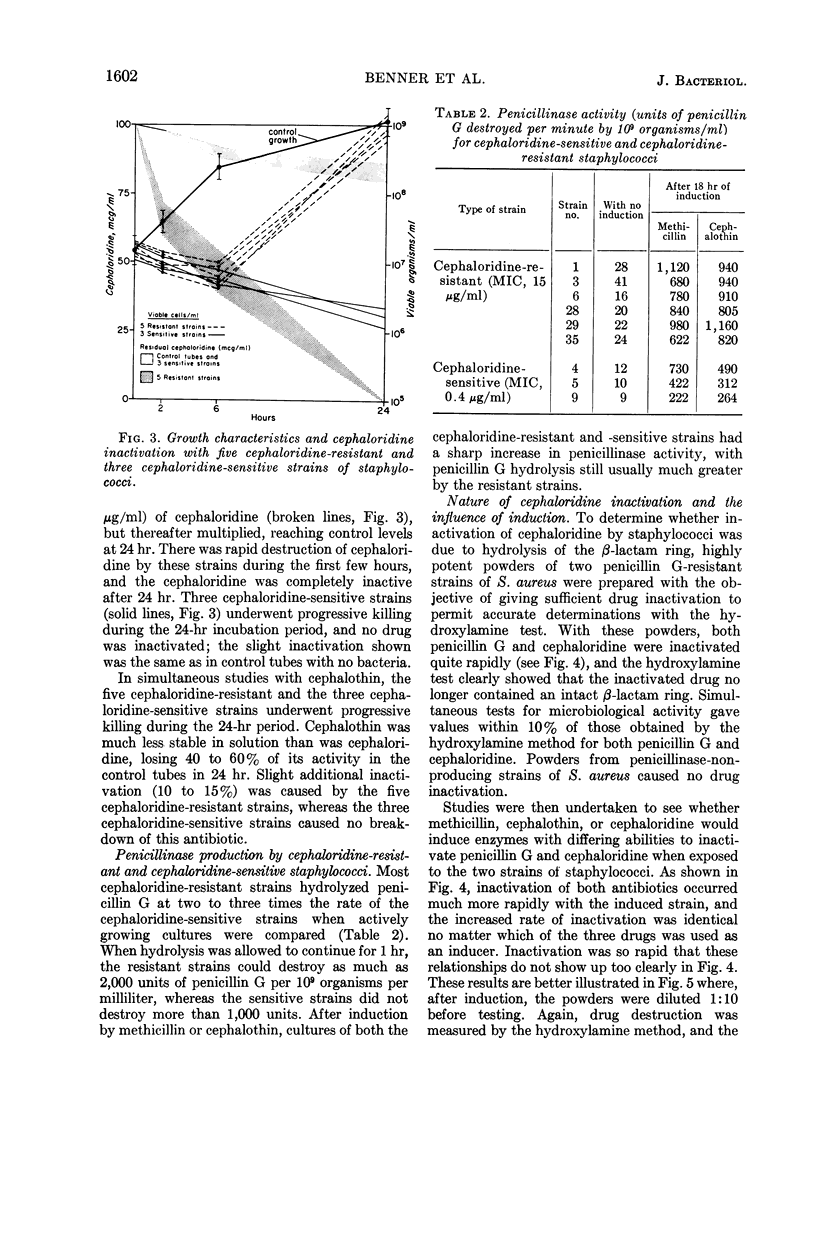
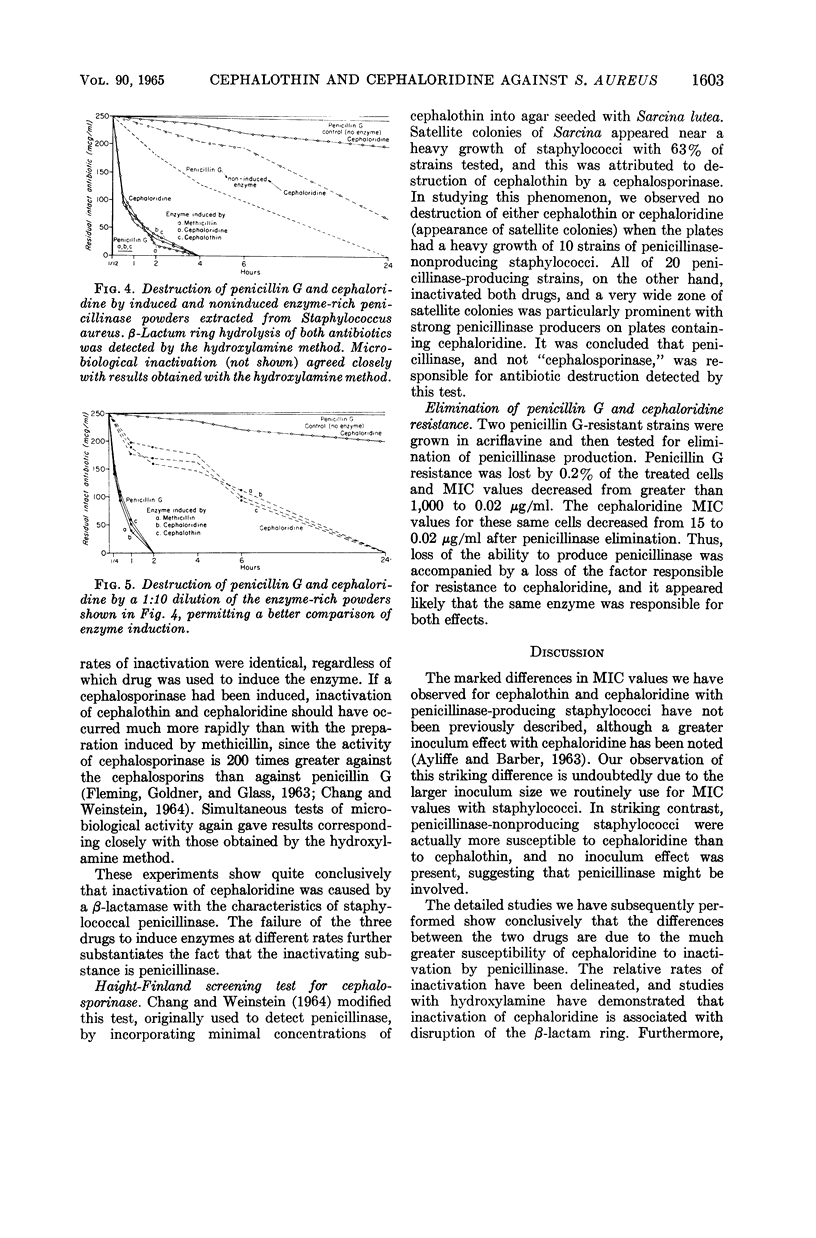

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYLIFFE G. A., BARBER M. Inactivation of benzylpenicillin and methicillin by hospital staphylococci. Br Med J. 1963 Jul 27;2(5351):202–205. doi: 10.1136/bmj.2.5351.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER A. W., PERRY D. M., KIRBY W. M. Single-disk antibiotic-sensitivity testing of staphylococci; an analysis of technique and results. AMA Arch Intern Med. 1959 Aug;104(2):208–216. doi: 10.1001/archinte.1959.00270080034004. [DOI] [PubMed] [Google Scholar]
- CHANG T. W., WEINSTEIN L. ISOLATION, CHARACTERIZATION, AND DISTRIBUTION OF CEPHALOSPORINASE. Antimicrob Agents Chemother (Bethesda) 1963;161:278–282. [PubMed] [Google Scholar]
- ERIKSEN K. R., ERICHSEN I. INACTIVATION OF METHICILLIN, OXACILLIN, CLOXACILLIN, AND CEPHALOTHIN BY STAPHYLOCOCCAL PENICILLINASE. Acta Pathol Microbiol Scand. 1964;62:399–408. doi: 10.1111/apm.1964.62.3.399. [DOI] [PubMed] [Google Scholar]
- FLEMING P. C., GOLDNER M., GLASS D. G. Observations on the nature, distribution, and significance of cephalosporinase. Lancet. 1963 Jun 29;1(7296):1399–1401. doi: 10.1016/s0140-6736(63)92051-8. [DOI] [PubMed] [Google Scholar]
- GRAVENKEMPER C. F., BRODIE J. L., KIRBY W. M. RESISTANCE OF COAGULASE-POSITIVE STAPHYLOCOCCI TO METHICILLIN AND OXACILLIN. J Bacteriol. 1965 Apr;89:1005–1010. doi: 10.1128/jb.89.4.1005-1010.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO H., KONO K., MITSUHASHI S. ELIMINATION OF PENICILLIN RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1964 Jul;88:261–262. doi: 10.1128/jb.88.1.261-262.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX R., SMITH J. T. Stability of methiciilin and cloxacillin to staphylococcal penicillinase. Br Med J. 1963 Jul 27;2(5351):205–207. doi: 10.1136/bmj.2.5351.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby W. M. EXTRACTION OF A HIGHLY POTENT PENICILLIN INACTIVATOR FROM PENICILLIN RESISTANT STAPHYLOCOCCI. Science. 1944 Jun 2;99(2579):452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- MUGGLETON P. W., O'CALLAGHAN C. H., STEVENS W. K. LABORATORY EVALUATIOON OF A NEW ANTIBIOTIC--CEPHALORIDINE (CEPORIN). Br Med J. 1964 Nov 14;2(5419):1234–1237. doi: 10.1136/bmj.2.5419.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURDOCH J. M., SPEIRS C. F., GEDDES A. M., WALLACE E. T. CLINICAL TRIAL OF CEPHALORIDINE (CEPORIN), A NEW BROAD-SPECTRUM ANTIBIOTIC DERIVED FROM CEPHALOSPORIN C. Br Med J. 1964 Nov 14;2(5419):1238–1240. doi: 10.1136/bmj.2.5419.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART G. T., HOLT R. J. LABORATORY AND CLINICAL RESULTS WITH CEPHALORIDINE. Lancet. 1964 Dec 19;2(7373):1305–1309. doi: 10.1016/s0140-6736(64)91102-x. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND R., ROLINSON G. N. CHARACTERISTICS OF METHICILLIN-RESISTANT STAPHYLOCOCCI. J Bacteriol. 1964 Apr;87:887–899. doi: 10.1128/jb.87.4.887-899.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


