Abstract
Background and Aims
A long-running debate centres on whether shade tolerance of tree seedlings is mainly a function of traits maximizing net carbon gain in low light, or of traits minimizing carbon loss. To test these alternatives, leaf display, light-interception efficiency, and simulated net daily carbon gain of juvenile temperate evergreens of differing shade tolerance were measured, and how these variables are influenced by ontogeny was queried.
Methods
The biomass distribution of juveniles (17–740 mm tall) of seven temperate rainforest evergreens growing in low (approx. 4 %) light in the understorey of a second-growth stand was quantified. Daytime and night-time gas exchange rates of leaves were also determined, and crown architecture was recorded digitally. YPLANT was used to model light interception and carbon gain.
Results
An index of species shade tolerance correlated closely with photosynthetic capacities and respiration rates per unit mass of leaves, but only weakly with respiration per unit area. Accumulation of many leaf cohorts by shade-tolerant species meant that their ratios of foliage area to biomass (LAR) decreased more gradually with ontogeny than those of light-demanders, but also increased self-shading; this depressed the foliage silhouette-to-area ratio (STAR), which was used as an index of light-interception efficiency. As a result, displayed leaf area ratio (LARd = LAR × STAR) of large seedlings was not related to species shade tolerance. Self-shading also caused simulated net daily carbon assimilation rates of shade-tolerant species to decrease with ontogeny, leading to a negative correlation of shade tolerance with net daily carbon gain of large (500 mm tall) seedlings in the understorey.
Conclusions
The results suggest that efficiency of energy capture is not an important correlate of shade tolerance in temperate rainforest evergreens. Ontogenetic increases in self-shading largely nullify the potential carbon gain advantages expected to result from low respiration rates and long leaf lifespans in shade-tolerant evergreens. The main advantage of their long-lived leaves is probably in reducing the costs of crown maintenance.
Keywords: Biomass distribution, leaf display, leaf angle, leaf area ratio, leaf lifespan, light-interception efficiency, self-shading, shade tolerance, silhouette-to-area ratio, YPLANT
INTRODUCTION
Understanding the forces controlling forest succession has been a prime goal of ecologists and foresters for >100 years (Warming, 1909). In humid forests, where intervals between stand-destroying disturbances often exceed the lifespan of individual trees, species turnover is thought to be driven mainly by differences in the ability to tolerate shade (Pacala et al., 1994), as light is the resource most diminished by vegetation development and best correlated with juvenile tree survival and growth (Denslow et al., 1990; Finzi and Canham, 2000).
A long-running, evolving debate centres on whether shade tolerance is mainly a function of traits maximizing photosynthetic carbon gain in low light, or of traits minimizing losses (reviewed by Valladares and Niinemets, 2008). The first attempt at a general theory of adaptation to sun and shade proposed that shade tolerance is determined mainly by traits that enhance net energy capture in low light (Givnish, 1988). Shade-tolerant species were therefore expected to develop a large ratio of leaf area to plant biomass (leaf area ratio, LAR) by allocating heavily to leaf production, and to gain more carbon gain per unit leaf area than light-demanding species when grown in low light. However, subsequent comparative studies of young seedlings failed to support most of these predictions. First-year seedlings of shade-tolerant species develop a smaller LAR than light-demanding associates of the same age – reflecting a large initial allocation to roots, and small specific leaf areas (SLA) – as well as growing more slowly even in light as low as 2–5 % of full sun (Kitajima, 1994; Veneklaas and Poorter, 1998; Walters and Reich, 1999). The robust construction (small SLA) of the leaves of shade-tolerant evergreen seedlings is associated with resistance to physical stresses and unattractiveness to natural enemies (Coley and Barone, 1996; Kitajima and Poorter, 2010). These observations seem to support an alternative view that seedling survival in low light depends mainly on ‘conservation of energy rather than the efficiency of its capture’ (Grime, 1965; see also Kitajima, 1994).
Recent work has suggested that ontogeny is important for understanding how traits determine recruitment in shaded environments (Lusk, 2004; Niinemets, 2006; Lusk and Warton, 2007). Although young seedlings of light-demanding species initially outgrow their shade-tolerant associates in all light environments, this advantage is short-lived in low light. A study of four temperate evergreens found that low-light growth of shade-tolerant species underwent more gradually ontogenetic declines than growth of light-demanders (Lusk, 2004), and saplings of light-demanding species in a tropical rainforest were shown to require more light to achieve positive net growth than their shade-tolerant associates (Baltzer and Thomas, 2007). The ontogenetic decline in growth of light-demanding species in low light correlates with decreasing LAR, as a result of short leaf lifespans; in contrast, shade-tolerant species accumulate many leaf cohorts and so their LAR declines more slowly during ontogeny in the juvenile phase (Lusk, 2004). What is less clear is whether the lower light requirements of shade-tolerant saplings simply reflects minimization of lost tissue caused by turnover, herbivores and mechanical damage (King, 1994), or whether higher instantaneous net carbon gain by the seedling crown is also involved (Givnish, 1988).
Few studies of shade tolerance have incorporated the effects of crown architecture on light interception and potential carbon gain. Although carbon gain by whole plants or shoots will in some way be a function of leaf-level gas exchange traits and total leaf area, it will also be influenced by crown architecture and foliage display efficiency (Valladares and Pearcy, 1998; Falster and Westoby, 2003). Pearcy et al. (2004) found that saplings of shade-tolerant species of Psychotria in a Panamanian rainforest had marginally less self-shading and slightly higher light-interception efficiency than their light-demanding congeners. However, their study confounds plastic and constitutive variation in these traits, as species were not compared in a common light environment. Furthermore, simulations of potential carbon gain by juvenile trees have usually ignored the impact of night-time respiration on net daily assimilation. As few field studies have measured night-time respiration, the importance of this component of seedling carbon balance cannot be gauged.
Here, how net daily carbon gain of juvenile evergreens in low light relates to species shade tolerance and to plant size is queried. The natural distributions of juveniles were used to quantify the light requirements of seven co-occurring evergreens in a temperate forest. Juveniles of a range of sizes (17–740 mm tall) growing in a second-growth forest were selected, and gas exchange measurements, hemispherical photography and digital capture of plant architecture used to model light interception and potential carbon gain with the YPLANT software (Pearcy and Yang, 1996). These data were complemented with measurements of night-time respiration of foliage. After harvesting the plants, it was possible to relate biomass distribution, light-interception efficiency and simulated daily net carbon gain to plant height.
MATERIALS AND METHODS
Study area and species
The study was carried out in the lowland forest of Parque Nacional Puyehue (40°39′S, 72°11′W) located in the western foothills of the Andes in south-central Chile. The lowland forests are dominated by broadleaved evergreen trees including Laureliopsis philippiana, Aextoxicon punctatum, Eucryphia cordifolia and Nothofagus dombeyi (Lusk et al., 2006). Plant material for the present study was obtained from a 45-year-old even-aged stand located on an alluvial terrace at 350 m a.s.l., in the Anticura sector of the park. Although the overstorey of this stand was dominated almost exclusively by N. dombeyi, seedlings and saplings of a variety of other species were common in the understorey (Lusk, 2002) At the time of sampling, canopy closure was patchy throughout the stand, as a result of periodic felling of trees for firewood. However, no canopy gaps >20 m2 were present. Measurements with a pair of LAI-2000 canopy analysers (Li-Cor, Lincoln, NE, USA) have indicated canopy openness values of between 2 % and 5 % throughout most of this stand (Lusk, 2004).
Seven common evergreens that differ widely in shade tolerance (Table 1) were chosen. These ranged from species represented by abundant seedlings and saplings beneath intact canopies in old-growth stands (e.g. Myrceugenia planipes, Aextoxicon punctatum), to species that establish mainly in well-lit environments associated with forest margins and recently disturbed areas (e.g. Aristotelia chilensis, Nothofagus dombeyi). As all species belong to different genera, they are referred to henceforth by their generic names.
Table 1.
Taxonomy and ecology of seven rainforest evergreens, Puyehue National Park, Chile
| Size range in present study |
||||||
|---|---|---|---|---|---|---|
| Species | Family | Max ht (m) | Mean leaf lifespan (years, ± s.e.) | %CO10 (50–200 cm tall) | Mass (g) | Height (mm) |
| Aristotelia chilensis (Molina) Stunz | Elaeocarpaceae | 10 | 0·8 ± 0·1 | 3·4 | 0·008–4·690 | 23–590 |
| Nothofagus dombeyi Mirb. (Oerst.) | Nothofagaceae | >45 | 2·1 ± 0·2 | 3·2 | 0·003–11·760 | 38–650 |
| Eucryphia cordifolia Cav. | Cunionaceae | 40 | 2·8 ± 0·2 | 2·1 | 0·009–7·390 | 26–743 |
| Amomyrtus luma (Molina) D.Legrand & Kausel | Myrtaceae | 20 | 3·3 ± 0·3 | 1·7 | 0·033–7·880 | 45–475 |
| Laureliopsis philippiana (Looser) R. Schodde | Atherospermataceae | 35 | 4·7 ± 0·6 | 1·2 | 0·010–9·700 | 17–510 |
| Aextoxicon punctatum Ruiz & Pav. | Aextoxicaceae | 35 | 5·3 ± 0·3 | 1·1 | 0·055–14·920 | 43–597 |
| Myrceugenia planipes (Hook. et Arn.) Berg. | Myrtaceae | 15 | 5·0 ± 0·2 | 0·8 | 0·076–9·110 | 47–530 |
%CO10 = an index of species light requirements, the 10th percentile of the distribution of juveniles in relation to percentage diffuse light availability.
Leaf lifespan
Leaf lifespans of six of the seven species were estimated by monitoring leaf survival over 12 months (Lusk et al., 2008). All leaves were marked on the main stem of five to six 100- to 200-cm-tall saplings of each species, growing at microsites with between 2 % and 5 % canopy openness as measured by the LAI-2000. Plants were relocated 12 months later, and leaf mortality during this period used to estimate average leaf lifespan. As leaf longevities were <1 year on most individuals of Aristotelia, abscission scars were counted to determine mortality of new leaves initiated after the start of the study period. Leaf longevity (year) was estimated as:
where ni = initial number of leaves, nf = final number surviving from ni, and mn = mortality of new leaves initiated since the first census. Static demographic methods were used to estimate leaf lifespans of the other species, Eucryphia (Lusk et al., 2008). The end of previous seasons' extension growth in this species is marked by cataphylls which persist for several years, permitting reconstruction of recent growth history and ready delimitation of foliage cohorts. Mean leaf lifespan of Eucryphia was estimated by inspecting ten saplings growing at microsites with between 2 % and 5 % canopy openness.
Determining species light requirements
Distributions of juvenile trees were quantified in relation to canopy openness measurements made with a pair of LAI-2000 canopy analysers (Li-Cor; Lusk and Piper, 2007). One instrument was used to take measurements at each sampling point, while the other, placed at the centre of a 2-ha clearing, was programmed to take readings at 30-s intervals. Integration of data from the two instruments enabled estimation of percentage diffuse irradiance at each sampling point within the forest, equivalent to percentage canopy openness over the quasi-hemispherical (148°) field of view perceived by the LAI-2000 sensors. Measurements were made on overcast days, using the full 148° field of view, over a period of about 4 years from 2000 to 2003. Measurements with the LAI-2000 are a good surrogate of spatial variation in mean daily photosynthetic photon flux density within a stand (Machado and Reich, 1999).
Sampling was carried out on a series of parallel transects run through old-growth stands, including tree-fall gaps of varied sizes. Transects were spaced at least 20 m apart, the angle and number depending on terrain, ease of access and proximity to forest margins. At 1608 sample points spaced at random intervals (2–10 m apart) along transects, canopy openness measurements were made at 200 cm height with the LAI-2000. Presence of juvenile trees 50–200 cm tall was recorded in a circular plot of 1-m diameter, centred on the sample point. Although up to 20 individuals of some species were found in some plots, only presence or absence data are used in the present analysis. One of the species (Eucryphia) frequently reproduces by basal shoots and root suckers as well as seedlings (Donoso et al., 1985). For the purposes of evaluating light requirements in the present study, however, only juveniles of seedling origin were counted.
The 10th percentile of the distribution of each species in relation to light availability (percentage canopy openness) was used as an approximation of the lowest light levels tolerated by each species (Table 1). This parameter, referred to hereafter as %CO10, is an inversion of traditional shade tolerance ratings (Donoso, 1989); i.e. shade-tolerant taxa such as Myrceugenia have low %CO10, and light-demanders such as Aristotelia score high (Table 1).
Selection of plant material
Seedlings and juveniles <750 mm tall were chosen haphazardly in the second-growth stand (Table 2). A pair of canopy analysers (LAI-2000) was used to quantify canopy openness within a quasi-hemispherical field of view (148°) immediately above the apex of each tagged seedling. Percentage canopy openness was calculated by referring measurements made within the forest to simultaneous readings taken in a clearing of approx. 1 ha.
Table 2.
Gas exchange characteristics of juveniles (20–100 cm tall) of seven rainforest evergreens growing in low light (2·5–6 % canopy openness), Puyehue National Park, Chile
| Dark respiration (μmol m−2 s−1) |
Dark respiration (nmol g−1 s−1) |
||||
|---|---|---|---|---|---|
| Species | Amax (μmol m−2 s−1) | Day | Night | Day | Night |
| Aristotelia | 5·1 ± 0·3 | –0·33 ± 0·06 | –0·32 ± 0·06 | –8·8 ± 1·9 | –8·5 ± 1·6 |
| Nothofagus | 4·6 ± 0·2 | –0·41 ± 0·04 | –0·38 ± 0·03 | –7·3 ± 1·1 | –7·2 ± 1·1 |
| Eucryphia | 3·7 ± 0·2 | –0·29 ± 0·03 | –0·28 ± 0·05 | –3·3 ± 0·3 | –3·3 ± 0·7 |
| Amomyrtus | 3·4 ± 0·2 | –0·29 ± 0·02 | –0·32 ± 0·02 | –3·1 ± 0·4 | –3·5 ± 0·6 |
| Laureliopsis | 3·4 ± 0·2 | –0·35 ± 0·04 | –0·30 ± 0·04 | –4·4 ± 0·7 | –3·6 ± 0·5 |
| Aextoxicon | 2·5 ± 0·2 | –0·21 ± 0·03 | –0·20 ± 0·02 | –2·2 ± 0·3 | –2·1 ± 0·2 |
| Myrceugenia | 3·2 ± 0·3 | –0·31 ± 0·03 | –0·32 ± 0·04 | –2·9 ± 0·3 | –3·0 ± 0·3 |
Photosynthesis data show means and s.e. of measurements on one leaf of each of seven to ten plants. Respiration data show means and s.e. of measurements on five replicate plants, averaged over three days and nights in December 2008. Leaf respiration is expressed on both area and mass bases. Day-to-day variation in respiration rates and measurement temperatures is given in Supplementary Data Table S2, available online.
Gas exchange measurements
Simulation of C gain in YPLANT requires measurements of photosynthetic capacity (Amax) and dark respiration rates (Rd). A CIRAS-1 gas analyser fitted with a 2·5-cm2 leaf chamber (PP Systems, Hitchin, UK) was used to estimate Amax and Rd on five 250- to 1000-mm-tall juveniles of each species, growing at microsites with 3–6 % canopy openness. A tungsten light source was used to measure photosynthesis at 20 °C on one fully expanded leaf of each plant. Measurements were carried out between 0900 and 1300 h in September 2006, early in the growing season. Net photosynthesis was measured at PAR of approx. 500 µmol m−2 s−1, which has been found sufficient to saturate shade leaves of most Chilean rainforest species (Lusk, 2002).
Respiration was measured at ambient daytime and night-time temperatures during the middle of the growing season. Temporal variation in respiration rates can be considerable, reflecting the influence of carbon gain during the previous 24 h, as well as the direct effect of temperature variation (Amthor, 1994). To take into account such variation, respiration of leaves of five replicate plants of each species we measured, on three days and three nights over a 6-d period in December 2008. The same leaves were re-measured on each date. Daytime measurements were made between 1030 and 1330 h; night-time measurements between 2230 and 0130 h. Immediately after completing measurements, the leaves were harvested in order to calculate gas exchange rates per unit mass. Leaf area was measured photographically, and leaves were then dried to a constant weight at 60 °C. The relatively large standard errors of the respiration measurements (Table 2) reflect the small signal-to-noise ratio inherent in measuring small fluxes.
It was assumed that Amax and Rd per area were independent of plant size. Although this was not verified for the species studied, other studies have reported minimal changes in photosynthetic capacity per unit area during seedling ontogeny, despite increasing leaf mass per area (Poorter and Pothmann, 1992; Tjoelker et al., 1998). It was assumed that leaf respiration rates per area did not change with ontogeny, implying declining mass-specific leaf respiration with increasing size (Walters et al., 1993).
For simplicity's sake, it was also assumed that Amax and Rd per area did not change with leaf age or position. Although empirical studies show relatively rapid declines in Amax of sun leaves (e.g. Hikosaka et al., 1994; Ackerly and Bazzaz, 1995), Sims and Pearcy (1991) reported only very gradual declines in both Amax and Rd of leaves which developed in a shaded environment; furthermore, the ratio of Amax to Rd of shade-grown foliage remained roughly constant as leaves aged. The assumption of invariant gas exchange parameters in the understorey plants may therefore involve relatively minor errors in estimates of net daily carbon gain.
Hemispherical photography
A Nikon Coolpix digital camera (Nikon Corporation, Japan) with a 182° fisheye adapter was used to take a hemispherical photograph directly above each seedling, orienting the top of the camera towards north. Analysis of hemispherical photographs indicated that canopy openness above the selected seedlings ranged from 2·3 % to 5·6 %, including small but significant differences between species (P < 0·001). Since the aim of the present study was to compare species under standardized conditions, a composite light environment derived using canopy structure information obtained from analysing the set of 159 photographs taken above the seedlings was created (Falster and Westoby, 2003). This was equivalent to 4·0 % canopy openness (Supplementary Data Table S1, available online). Photographs were analysed using the gap light analyser software package (Frazer et al., 1999). Fractional canopy openness (0–1) was calculated for 20 altitude and eight azimuth classes in each photograph. Mean openness in each angle class was calculated for each photograph by averaging openness across all azimuth classes, and an average openness for the habitat was calculating by averaging across all photographs for each angle class. This made it possible for a habitat average indirect site factor (Pearcy and Yang, 1996) to be calculated and the average time series of direct photon flux density (PFD) interception to be estimated for any day of the year. A single average light regime was calculated for the summer solstice (21 December), as the gas exchange measurements were made at ambient temperatures during this period of the growing season.
Digital capture of plant architecture
Each seedling was excavated carefully, removing a sod of sufficient width and depth to include the root system, after cutting through any intruding coarse roots from neighbouring plants. The seedling was transplanted to a pot of sufficient size to accommodate the excavated sod, and the seedling's orientation was indicated by marking the former position of north on the side of the pot. Potted plants were then taken to a field station, and architecture digitized within 36 h.
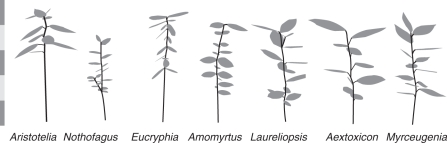
Digital capture of plant architecture (Hanan and Room, 1997; Falster and Westoby, 2003) is much less time-consuming than the manual methods often used in conjunction with YPLANT. The three-dimensional (3-D) leaf arrangement of each seedling was recorded using a FASTRAK® 3D-digitizer (Polhemus, Colchester, VT, USA), in conjunction with the software package FLORADIG (CSIRO Entomology, Brisbane, Australia). The digitizer includes a magnetic signal receiver and pointer, allowing the user to record the 3-D spatial co-ordinates of the pointer within a hemisphere of 3-m diameter from the receiver. Individual plants are reconstructed virtually by recording a series of point co-ordinates, and the relevant connectivity between points. Stem segments (and petioles, if present) are characterized by their elevation angle, azimuth, length and diameter. Individual leaves are characterized by their length together with the azimuth and elevation angle of two vectors on the lamina surface. Examples are shown in Fig. 1.
Fig. 1.
Crown reconstructions of selected seedlings of seven temperate rainforest evergreens, using YPLANT. Crown architecture was described in three dimensions using a magnetic digitizer. Each segment of the scale on the left = 50 mm.
Modelling light interception and carbon gain
The YPLANT software (Pearcy and Yang, 1996) was used to model light interception and net daily carbon gain at the summer solstice. Simulations using YPLANT have been shown to correspond well with actual measurements of light interception and C gain (Valladares and Pearcy, 1998; Naumburg et al., 2001).The 3-D description of leaf arrangement recorded for each seedling in FLORADIG was converted to the appropriate YPLANT format using a program written in the C programming language (Falster and Westoby, 2003).
YPLANT inputs are the geometry of leaf arrangement, a description of leaf shape, physiological parameters for leaf photosynthetic capacity and respiration rate, and a description of canopy structure above the plant to permit estimation of the light regime provided by hemispherical photography. A solar movement submodel allows estimation of PFD (μmol photons m−2 s−1) incident on each leaf surface at different times of day. A submodel for potential photosynthesis allows the resulting assimilation rate to be estimated, using PFD response curves generated from species-specific measurements of gross photosynthetic capacity and dark respiration rate (Table 2), using the method described by Thornley (1976). As mitochondrial respiration is strongly inhibited by light (Villar et al., 1995; Atkin et al., 1998), the daytime respiration rate was estimated as being 40 % of the measured daytime Rd (Pearcy et al., 2004).
YPLANT 3·1 enables the user to simulate light interception and carbon gain under a range of atmospheric conditions. This is achieved by varying the atmospheric transmission coefficient Rs/Ro, where Ro is the global solar irradiance at the top of the atmosphere, and Rs is the global solar irradiance at the top of the canopy (Roderick et al., 2001). When Rs/Ro is high (i.e. clear sky) most irradiance arrives as direct beams, whereas diffuse light predominates under overcast conditions, when this coefficient is low. Carbon gain was simulated under both clear and overcast skies, setting Rs/Ro at 0·3 to mimic overcast conditions, and at 0·79 to reproduce the effect of a clear sky (Roderick et al., 2001). Under sunny conditions, plants are periodically illuminated by direct light (‘sunflecks’) when the position of the solar disc coincides with canopy openings, but receive only small amounts of diffuse light between sunflecks.
Data on mean daily sunshine hours were used to weight the output from simulations under clear and overcast scenarios, thus obtaining overall estimates of net daily carbon gain at the summer solstice (Lusk et al., 2011). As data on sunshine hours were not available for Puyehue, data from Puerto Montt (41°28′S, 72°56′W), the nearest meteorological station that does measure this parameter (Dirección Meteorológica de Chile, unpubl. data) were used. These data indicated an average of 6·5 sunshine hours per day in December. As there are about 14·9 h of total daylight at the summer solstice in Puyehue, the clear-sky simulations were weighted by 6·5/14·9 = 0·436, and the overcast simulations by 8·4/14·9 = 0·564.
Biomass distribution, crown architecture and light-interception efficiency
As light interception by plant crowns is determined by leaf inclination angles as well as overlap among leaves (i.e. self-shading), YPLANT was used to estimate both these parameters. YPLANT output includes leaf area projected towards each of 160 sectors of the hemisphere (20 elevation classes × 8 azimuth classes) without taking into account overlap of leaves, and leaf area displayed towards each sector, i.e. the effective area for light interception (Pearcy and Yang, 1996). The mean leaf angle of a plant crown, weighted by the size of individual leaves, can be estimated as arccosine (PAV/LA), where PAV = leaf area projected towards the vertical, and LA = actual leaf area of the plant (Pearcy et al., 2004). The self-shaded fraction of the crown leaf area was estimated as (PA – DA)/PA, where PA = projected leaf area and DA = displayed leaf area. This parameter was averaged for the 80 uppermost sectors of the hemisphere, as most direct light came from elevations >45°, with most sunflecks occurring between 0830 and 1530 h. The foliage silhouette-to-area-ratio or STAR (Carter and Smith, 1985; Delagrange et al., 2006) was used as an index of light-interception efficiency; this was calculated as the ratio of displayed to total leaf area. Like self-shading, STAR was averaged for the 80 uppermost sectors of the hemisphere.
After harvesting plants, the LAR of each plant was calculated by dividing total leaf area by plant dry weight. LAR is frequently measured in comparative plant ecology because it is an obvious determinant of whole-plant carbon gain potential, and because it is frequently correlated with seedling growth rates in short-term experiments, especially in low light (Poorter and Van der Werf, 1998). A new parameter was calculated to take into account the effects of self-shading and leaf angles on the effective LAR that plants actually display: called the displayed leaf area ratio (LARd), it was calculated as the product of LAR and STAR.
Data integration
The 24-h net C gain of the foliage of each seedling wasestimated at the summer solstice, when there are 14·9 decimal hours of daylight at the latitude of Puyehue (40°39′S). YPLANT estimates net daytime assimilation per unit leaf area: from this dark respiration × foliage was subtracted during the 9·1 h of darkness at the summer solstice, estimated as night-time Rd × 60 × 60 × 9·1.
Statistical analysis
A mixed-model ANOVA was used to test for species and diel (day–night) effects on leaf respiration rates, as well as day-to-day (date) variation. This analysis was carried out in PASW Statistics 18 (IBM Corporation, Somers, NY, USA), using the following model, after log-transforming respiration data:
GLM Respiration BY Species Replicate Date Diel
/RANDOM = Replicate
/DESIGN = Species Replicate(Species) Date Diel(Date)
Analysis of covariance was used to test effects of species and plant height on biomass distribution, crown architecture and net daily carbon gain variables. Ordinary least-squares regression was used to test the influence of plant height on each of these parameters for individual species. Ordinary least squares regression is appropriate when the primary interest is in predicting one variable from another, rather than describing scaling relationships (Sokal and Rolf, 1995; Warton and Weber, 2002).
RESULTS
Leaf lifespan
Species mean leaf lifespans ranged from 0·8 to 5·3 years (Table 1), and were strongly negatively correlated with species light requirements, as indexed by %CO10 (R = –0·96, P < 0·001).
Gas exchange
Net photosynthetic capacity varied about 2-fold among species (Table 2) and was closely related to species light requirements (Table 1). ANOVA showed that this interspecific variation was statistically significant (P < 0·0001).
Leaf dark respiration was closely related to species light requirements on a mass basis, but only weakly so on an area basis (Table 2). ANOVA showed significant interspecific and day-to-day variation in respiration rates (P = 0·009 and P < 0·0001, respectively), but no significant difference between daytime and night-time measurements (P = 0·556), despite the substantial difference in average measurement temperatures (20·2 vs. 15·3°C).
Biomass distribution
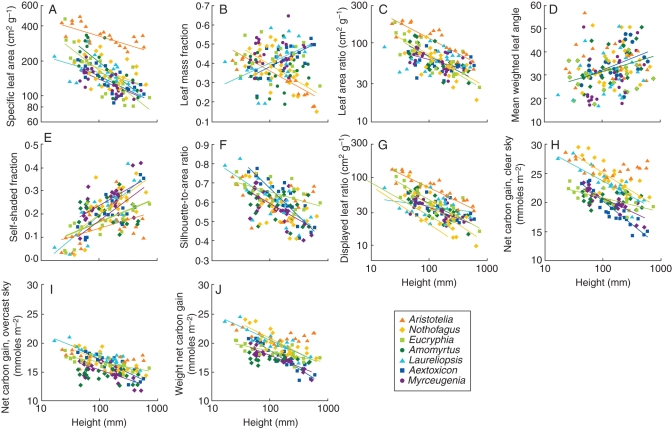
There was highly significant interspecific variation in SLA (Table 3), although this is largely attributable to the very large SLA of light-demanding Aristotelia (Fig. 2A). Overall, species light requirements were not significantly related to SLA of either small or large seedlings (Table 4). SLA of all species declined significantly with increasing plant height (Fig. 2A), and there was significant interspecific variation in this ontogenetic trend (Table 3).
Table 3.
ANCOVA testing effects of species and plant height on biomass distribution, crown architecture and net daily carbon gain variables
| Species |
(log) Height |
Species × (log) height |
||||
|---|---|---|---|---|---|---|
| F-value | P | F-value | P | F-value | P | |
| Specific leaf area | 2·94 | 0·010 | 112·45 | <0·001 | 3·27 | 0·005 |
| Leaf mass fraction | 7·14 | <0·001 | 0·15 | 0·702 | 8·86 | <0·001 |
| Leaf area ratio | 8·20 | <0·001 | 95·58 | <0·001 | 6·64 | <0·001 |
| Mean weighted leaf angle | 2·65 | 0·018 | 6·66 | 0·011 | n/a | n/a |
| Self-shaded fraction | 5·25 | <0·001 | 83·73 | <0·001 | 5·83 | <0·001 |
| Mean STAR | 5·17 | <0·001 | 87·35 | <0·001 | 5·41 | <0·001 |
| LARd | 4·24 | 0·001 | 158·28 | <0·001 | 3·50 | 0·003 |
| Net daily C gain, clear sky | 10·97 | <0·001 | 177·32 | <0·001 | 11·10 | <0·001 |
| Net daily C gain, overcast | 8·81 | <0·001 | 154·16 | <0·001 | 7·56 | <0·001 |
| Net daily C gain, weighted | 15·07 | <0·001 | 255·95 | <0·001 | 14·38 | <0·001 |
The analysis of mean weighted leaf angle was re-run after removal of the non-significant interaction term (P = 0·273).
Fig. 2.
Relationships of biomass distribution, leaf display and net daily carbon gain parameters with height of juvenile rainforest trees, with species as indicated in the key in (E). Lines show regressions that are significant at P = 0·05. Regression statistics are given in Supplementary Data Table S3, available online.
Table 4.
Correlations (Pearson's R) of species light requirements (%CO10) with biomass distribution, leaf display and simulated net carbon gain parameters of small and large seedlings growing in a second-growth temperate rainforest
| Variable | 50 mm | 500 mm |
|---|---|---|
| Specific leaf area | 0·51 | 0·47 |
| Leaf mass fraction | 0·57 | –0·94 |
| Leaf area ratio | 0·82 | –0·12 |
| Mean weighted leaf angle | 0·07 | –0·44 |
| Self-shaded fraction | –0·06 | –0·31 |
| Mean STAR | –0·37 | 0·54 |
| LARd | 0·81 | 0·26 |
| Net daily C gain, clear sky | 0·63 | 0·79 |
| Net daily C gain, overcast | 0·05 | 0·70 |
| Net daily C gain, weighted average | 0·41 | 0·78 |
Correlations significant at P = 0·05 shown in bold. Values of parameters at 50 and 500 mm were estimated from regression lines in Fig. 6, or as local averages when regressions were not significant.
Leaf mass fraction (LMF) did not differ significantly across species overall, but there was striking interspecific variation in the relationship of LMF with plant height (Table 3). LMF of the two most light-demanding species was initially high, but fell steeply with increasing plant size (Fig. 2B). LMF of two of the most shade-tolerant species (Laureliopsis and Aextoxicon) showed the opposite trend, rising steeply with increasing size. The other three species showed no significant trend in LMF.
There was significant interspecific variation in the relationship of LAR with plant height (Table 3). The LAR of light-demanding and intermediate species was initially large (>80), and declined significantly with increasing plant size (Fig. 2C). In contrast, LAR of two of the most shade-tolerant species (Aextoxicon and Laureliopsis) was not significantly related to height. As a result, although LAR of small seedlings was strongly correlated with species %CO10 values, this pattern disappeared in the larger size classes (Table 4).
Crown architecture and light interception
Mean weighted leaf angles of individual plants fell mainly between 20° and 50° (Fig. 2D). Average leaf angles differed significantly across species, and tended to increase overall with plant size, although neither of these effects were strong (Table 3). Leaf angles of Aextoxicon and Amomyrtus increased significantly with increasing plant size, but the other five species showed no significant pattern (Fig. 2D). Leaf angles of neither small nor large seedlings were significantly correlated with species light requirements (Table 4).
Increasing size brought increasing self-shading in all species except Amomyrtus (Fig. 2E), and there was significant interspecific variation in this ontogenetic trend (Table 3). Self-shading of small seedlings was low (<0·2) across all species, but interspecific differences became more evident in the larger size classes, with the highest (>0·3) found in large individuals of shade-tolerant Laureliopsis, Aextoxicon and Myrceugenia, and light-demanding Nothofagus (Fig. 2D). However, self-shading of neither small nor large seedlings was significantly correlated with species light requirements (Table 4).
The pattern in light-interception efficiency (STAR) was essentially the mirror image of that seen in self-shading. STAR of most species decreased significantly with increasing size, with the exception of light-demanding Aristotelia and mid-tolerant Amomyrtus (Fig. 2F). Although STAR of small seedlings was uniformly high (>0·65) across species, interspecific differences were more evident in the larger size classes, with the smallest values (<0·45) found in large individuals of shade-tolerant Laureliopsis, Aextoxicon and Myrceugenia, and light-demanding Nothofagus (Fig. 2F). STAR of neither small nor large seedlings was significantly correlated with species light requirements (Table 4).
Displayed leaf area ratio (LARd) of all species decreased significantly with increasing plant size (Fig. 2G), but there was significant interspecific variation in this trend (Table 3). LARd of small seedlings was strongly correlated with species %CO10 values, but this pattern grew progressively weaker with increasing plant size (Table 4).
Net daily carbon gain
All species were predicted to have higher net daily carbon gain under the clear-sky scenario than under overcast conditions (Fig. 2H, I). Under both scenarios, average carbon gain differed significantly across species and declined with increasing plant size; there was also significant interspecific variation in this ontogenetic trend (Table 3). Species rank order of net carbon gain by small seedlings was somewhat dependent on atmospheric conditions (Fig. 2I); light-demanders tended to gain more carbon under the clear-sky scenario (Fig. 2H), but this pattern was absent under the overcast scenario where species' differences were minimal (Fig. 2I and Table 4). Atmospheric conditions had less influence on interspecific variation of net carbon gain by large seedlings; light-demanders tended to gain more carbon than shade-tolerant species under both clear-sky and overcast scenarios (Fig. 2H, I), although this was statistically significant only for the former (Table 4). When the averages of clear-sky and overcast outputs were weighted by sunshine hours, small seedlings showed no conclusive pattern but net daily carbon gain of large seedlings was significantly positively correlated with %CO10 (Fig. 2J and Table 4), i.e. negatively correlated with species shade tolerance.
DISCUSSION
The data suggest that, unlike plastic responses to shade, adaptation to shade in evergreens does not involve enhanced light-interception efficiency (Grime, 1965). Crown silhouette-to-area ratios were not systematically related to shade tolerance, and in the larger size classes the most shade-tolerant species had low STAR values (Fig. 2F). This is a consequence of the increasing self-shading attending the accumulation of four to six leaf cohorts by the shade-tolerant species (Table 1 and Fig. 2E). Neither have other studies provided any convincing evidence that shade-tolerant species intercept light particularly efficiently. A study of five deciduous species found no relationship between light-interception efficiency and shade tolerance (Delagrange et al., 2006). Although a YPLANT-based comparative study reported that shade-tolerant Psychotria species had higher light interception efficiencies than light-demanding congeners (Pearcy et al., 2004), this finding could be largely a reflection of plastic (rather than inherent) variation in crown architecture, as light environments were not standardized across species. Plants in general respond plastically to shade by reducing leaf overlap and/or leaf angles, resulting in increased light-interception efficiency (McMillen and McClendon, 1979; Valladares and Pearcy, 1998; Gálvez and Pearcy, 2003; Delagrange et al., 2006). The pattern reported for Psychotria spp. thus likely reflects the fact that light-demanding species were sampled in brighter light environments than their shade-tolerant congeners (Pearcy et al., 2004, fig. 4).
Although most species of low to intermediate shade tolerance underwent only minor ontogenetic declines in light-interception efficiency, the exception presented by Nothofagus requires some explanation (Fig. 2F). The low STAR values of large individuals of light-demanding Nothofagus reflected the accumulation of large numbers of leaves, but this was associated with the small size of individual leaves (Fig. 1) rather than retention of many leaf cohorts. Although a mean lifespan of only about 2 years for this species was calculated (Table 1), large, highly branched Nothofagus juveniles had >100 leaves, leading to heavy self-shading (Fig. 2E). A study of 38 Australian sclerophyll evergreens reported that self-shading within shoots was strongly negatively correlated with leaf size, and positively correlated with leaf number (Falster and Westoby, 2003).
Variation in leaf inclination angles contributed little to species differences in light-interception efficiency (Fig. 2D). This agrees well with Delagrange et al. (2006), who found that leaf angles of five deciduous species compared in common light environments were not related to reputed shade tolerance. In contrast, Pearcy et al. (2004) reported that slightly flatter leaf angles contributed to shade-tolerant Psychotria species' advantage in light-interception efficiency over their light-demanding congeners. However, as mentioned above, this reported variation in both leaf angles and light-interception efficiency is probably attributable to the lack of standardization of light environments in the study in question. Shade-tolerant species of Psychotria were on average sampled at shadier microsites than light-demanders, and leaf angles are known to respond plastically to light availability, with sun leaves being more steeply inclined than shade leaves (McMillen and McClendon, 1979; Valladares and Pearcy, 1998; Delagrange et al., 2006).
The present results suggest that night-time respiration is a sizeable component of seedling carbon balance. In view of the well-known responsiveness of dark respiration to short-term changes in temperature, dark respiration rates might be expected to be lower at night. However, night-time and daytime dark respiration rates were statistically indistinguishable (Table 2), despite the approx. 5° C difference between average midday and midnight temperatures. As mitochondrial respiration is strongly inhibited in the light (Villar et al., 1995; Atkin et al., 1998), total night-time respiratory losses by the plants studied should therefore exceed actual respiration during the daytime, even at the summer solstice when nights are shortest. We are aware of few studies reporting comparable field measurements of both daytime and night-time leaf respiration at ambient temperatures. However, a study of Quercus ilex in a low-rainfall Mediterranean environment reported that daytime and night-time dark respiration shared a common response to temperature (Zaragoza-Castells et al., 2008). As a result, dark respiration rates were higher during the day, in sharp contrast to the present own findings. More research would help clarify whether these two divergent results can be ascribed to environmental differences between the study sites, or to other causes.
Ontogenetic increases in self-shading nullified the expected low-light carbon gain advantages of low respiration rates in shade-tolerant species. Although Rd was only weakly related to light requirements of the seven study species (Table 2), shade-tolerant species in general have lower dark respiration rates and leaf-level compensation points than their light-demanding associates (e.g. Lusk, 2002; Baltzer and Thomas, 2007), leading to the expectation of that the former should have higher instantaneous net carbon gain in low light. However, this expectation was not supported by simulations incorporating the effects of self-shading at crown level, which generally indicated lower net daily carbon gain by the shade-tolerant species. Simulations were carried out in moderate shade (approx. 4·0 % light availability), and the rank order of net daily carbon gain by the study species would undoubtedly be different in deeper shade under dense rainforest canopies, where seedlings generally receive <2 % of full sunlight (Chazdon and Fetcher, 1984; Lusk et al., 2006). However, even under the overcast scenario, in which average PFD reaching the plants was estimated at only approx. 22 % of that penetrating the understorey on clear days, net daily carbon gain of large seedlings still tended to be lowest in shade-tolerant species (Fig. 2I), reflecting the considerable self-shading associated with the accumulation of four to six leaf cohorts.
The long leaf lifespans of the shade-tolerant evergreens did not result in them displaying larger effective leaf areas in low light than their light-demanding counterparts. As reported previously for a subset of these species (Lusk, 2004), LAR of shade-tolerant species declined more gradually with size than those of light-demanding taxa (Fig. 2C). However, because the ontogenetic decrease in crown silhouette-to-area ratio tended to be steepest in shade-tolerant species (Fig. 2F), LARd of 500-mm-tall juveniles was not correlated with species light requirements (Table 4). Accumulation of multiple leaf cohorts thus brings diminishing returns for displayed leaf area, with increasing self-shading apparently nullifying the expected advantages of long leaf lifespans for maximization of light interception by shade-tolerant species. The present findings therefore seem consistent with the view that the main advantage of long leaf lifespans in shade-tolerant evergreens is in reducing the costs of crown maintenance (King, 1994; Walters and Reich, 1999), rather than in maximizing light capture.
In conclusion, as far as we are aware, this is the first study to compare light interception and simulate net daily carbon gain by evergreens of differing shade tolerance in a standardized understorey environment. Integration of data on gas exchange, biomass distribution and crown architecture indicated that the shade-tolerant evergreens studied were not particularly efficient at harvesting light or fixing carbon in the understorey; this was especially true of large seedlings that bore several leaf cohorts, resulting in considerable self-shading. Rather, they appear to succeed by incrementally constructing persistent crowns that require only modest annual carbon allocation to replace losses due to foliage turnover, herbivory and mechanical damage.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Bob Pearcy and Remko Duursma for helpful advice on YPLANT, Tanja Lenz for running the simulations, and the Australian Research Council for support through DP0878209.
LITERATURE CITED
- Ackerly DD, Bazzaz FA. Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia. 1995;101:289–298. doi: 10.1007/BF00328814. [DOI] [PubMed] [Google Scholar]
- Amthor JS. Higher plant respiration and it relationships to photosynthesis. In: Schulze ED, Caldwell MM, editors. Ecophysiology of photosynthesis. Berlin: Springer Verlag; 1994. pp. 71–101. [Google Scholar]
- Atkin OK, Evans JR, Siebke K. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Australian Journal of Plant Physiology. 1998;25:437–443. [Google Scholar]
- Baltzer JL, Thomas SC. Determinants of whole-plant light requirements in Bornean rain forest tree saplings. Journal of Ecology. 2007;95:1208–1221. [Google Scholar]
- Carter GA, Smith WK. Influence of shoot structure on light interception and photosynthesis in conifers. Plant Physiology. 1985;79:1038–1043. doi: 10.1104/pp.79.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazdon RL, Fetcher N. Photosynthetic light environments in a lowland tropical rain forest in Costa Rica. Journal of Ecology. 1984;72:553–564. [Google Scholar]
- Coley PD, Barone JA. Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics. 1996;27:305–335. [Google Scholar]
- Delagrange S, Montpied P, Dreyer E, Messier C, Sinoquet H. Does shade improve light interception efficiency? A comparison among seedlings from shade-tolerant and -intolerant temperate deciduous tree species. New Phytologist. 2006;172:293–304. doi: 10.1111/j.1469-8137.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- Denslow JS, Schultz JC, Vitousek PM, Strain BR. Growth responses of tropical shrubs to treefall gap environments. Ecology. 1990;71:165–179. [Google Scholar]
- Donoso C. Antecedentes básicos para la silvicultura del tipo forestal siempreverde. Bosque. 1989;10:37–53. [Google Scholar]
- Donoso C, Escobar B, Urrutia J. Estructura y estrategias regenerativas de un bosque virgen de Ulmo (Eucryphia cordifolia Cav.)-Tepa (Laurelia philippiana Phil.) Looser en Chiloé, Chile. Revista Chilena de Historia Natural. 1985;58:171–186. [Google Scholar]
- Falster DS, Westoby M. Leaf size and angle vary widely across species: what consequences for light interception? New Phytologist. 2003;158:509–525. doi: 10.1046/j.1469-8137.2003.00765.x. [DOI] [PubMed] [Google Scholar]
- Finzi AC, Canham CD. Sapling growth in response to light and nitrogen availability in a southern New England forest. Forest Ecology and Management. 2000;131:153–165. [Google Scholar]
- Frazer GW, Canham CD, Lertzman KP. Gap Light Analyzer (GLA), Version 2·0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. 1999 Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York. [Google Scholar]
- Gálvez D, Pearcy RW. Petiole twisting in the crowns of Psychotria limonensis: implications for light interception and daily carbon gain. Oecologia. 2003;135:22–29. doi: 10.1007/s00442-002-1158-3. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Adaptation to sun and shade: a whole-plant perspective. Australian Journal of Plant Physiology. 1988;15:63–92. [Google Scholar]
- Grime JP. Shade tolerance in flowering plants. Nature. 1965;208:161–163. [Google Scholar]
- Hanan JS, Room PM. Practical aspects of virtual plant research. In: Michalewicz MT, editor. Plants to ecosystems: advances in computational life sciences. Melbourne, Australia: CSIRO; 1997. pp. 28–44. [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. American Journal of Botany. 1994;97:451–457. doi: 10.1007/BF00325881. [DOI] [PubMed] [Google Scholar]
- King DA. Influence of light level on the growth and morphology of saplings in a Panamanian forest. American Journal of Botany. 1994;81:948–957. [Google Scholar]
- Kitajima K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia. 1994;98:419–428. doi: 10.1007/BF00324232. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Poorter L. Tissue-level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytologist. 2010;186:708–721. doi: 10.1111/j.1469-8137.2010.03212.x. [DOI] [PubMed] [Google Scholar]
- Lusk CH. Leaf area accumulation helps juvenile evergreen trees tolerate shade in a temperate rainforest. Oecologia. 2002;132:188–196. doi: 10.1007/s00442-002-0974-9. [DOI] [PubMed] [Google Scholar]
- Lusk CH. Leaf area and growth of juvenile temperate evergreens in low light: species of contrasting shade tolerance change rank during ontogeny. Functional Ecology. 2004;18:820–828. [Google Scholar]
- Lusk CH, Piper FI. Seedling size influences relationships of shade tolerance with carbohydrate storage patterns in a temperate rainforest. Functional Ecology. 2007;21:78–86. [Google Scholar]
- Lusk CH, Warton DI. Global meta-analysis shows that relationships of leaf mass per area with species shade tolerance depend on leaf habit and ontogeny. New Phytologist. 2007;176:764–774. doi: 10.1111/j.1469-8137.2007.02264.x. [DOI] [PubMed] [Google Scholar]
- Lusk CH, Chazdon RL, Hofmann G. A bounded null model explains juvenile tree community structure along light availability gradients in a temperate rain forest. Oikos. 2006;112:131–137. [Google Scholar]
- Lusk CH, Falster DS, Jara-Vergara CK, Jimenez-Castillo M, Saldana A. Ontogenetic variation in light requirements of juvenile rainforest evergreens. Functional Ecology. 2008;22:454–459. [Google Scholar]
- Lusk CH, Sendall K, Kooyman R. Latitude, solar elevation angles and gap-regenerating rain forest pioneers. Journal of Ecology. 2011;99:491–502. [Google Scholar]
- McMillen GG, McClendon JH. Leaf angle: an adaptive feature of sun and shade leaves. Botanical Gazette. 1979;140:437–442. [Google Scholar]
- Machado JL, Reich PB. Evaluation of several measures of canopy openness as predictors of photosynthetic photon flux density in deeply shaded conifer-dominated forest understory. Canadian Journal of Forest Research. 1999;29:1438–1444. [Google Scholar]
- Naumburg E, Ellsworth DS, Pearcy RW. Crown carbon gain and elevated [CO2] responses of understory saplings with differing allometry and architecture. Functional Ecology. 2001;15:263–273. [Google Scholar]
- Niinemets Ü. The controversy over traits conferring shade-tolerance in trees: ontogenetic changes revisited. Journal of Ecology. 2006;94:464–470. [Google Scholar]
- Pacala SW, Canham CD, Silander JA, Kobe RK. Sapling growth as a function of resources in a north temperate forest. Canadian Journal of Forest Research. 1994;24:2172–2183. [Google Scholar]
- Pearcy RW, Yang WM. A three-dimensional crown architecture model for assessment of light capture and carbon gain by understory plants. Oecologia. 1996;108:1–12. doi: 10.1007/BF00333208. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Valladares F, Wright SJ, de Paulis EL. A functional analysis of the crown architecture of tropical forest Psychotria species: do species vary in light capture efficiency and consequently in carbon gain and growth? Oecologia. 2004;139:163–177. doi: 10.1007/s00442-004-1496-4. [DOI] [PubMed] [Google Scholar]
- Poorter H, Pothmann P. Growth and carbon economy of a fast-growing and a slow-growing grass species as dependent on ontogeny. New Phytologist. 1992;120:159–166. [Google Scholar]
- Poorter H, Van der Werf A. Is inherent variation in RGR determined by LAR at low irradiance and by NAR at high irradiance? In. In: Lambers H, Poorter H, Van Vuren MMI, editors. Inherent variation in plant growth: physiological mechanisms and ecological consequences. Leiden, The Netherlands: Backhuys; 1998. pp. 309–336. [Google Scholar]
- Roderick ML, Farquhar GD, Berry SL, Noble IR. On the direct effect of clouds and atmospheric particles on the productivity and structure of vegetation. Oecologia. 2001;129:21–30. doi: 10.1007/s004420100760. [DOI] [PubMed] [Google Scholar]
- Sims DA, Pearcy RW. Photosynthesis and respiration in Alocasia macrorrhiza following transfers to high and low light. Oecologia. 1991;86:447–453. doi: 10.1007/BF00317615. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rolf FJ. Biometry. 3rd edn. New York, NY: Freeman and Company; 1995. [Google Scholar]
- Thornley JHM. Mathematical models in plant physiology. London: Academic Press; 1976. [Google Scholar]
- Tjoelker M, Oleksyn J, Reich PB. Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO2 and temperature. Tree Physiology. 1998;18:715–726. doi: 10.1093/treephys/18.11.715. [DOI] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology and Systematics. 2008;39:237–257. [Google Scholar]
- Valladares F, Pearcy RW. The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia. 1998;114:1–10. doi: 10.1007/s004420050413. [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Poorter L. Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In: Lambers H, Poorter H, van Vuuren MMI, editors. Inherent variation in plant growth: physiological mechanisms and ecological consequences. Leiden: Backhuys Publishers; 1998. pp. 337–361. [Google Scholar]
- Villar R, Held AA, Merino J. Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiology. 1995;107:421–427. doi: 10.1104/pp.107.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MB, Reich PB. Low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broad-leaved evergreen species differ? New Phytologist. 1999;143:143–154. [Google Scholar]
- Walters MB, Kruger EL, Reich PB. Relative growth-rate in relation to physiological and morphological traits for northern hardwood tree seedlings: species, light environment and ontogenic considerations. Oecologia. 1993;96:219–231. doi: 10.1007/BF00317735. [DOI] [PubMed] [Google Scholar]
- Warming E. Oecology of plants: an introduction to the study of plant communities. Oxford: Clarendon Press; 1909. [Google Scholar]
- Warton DI, Weber NC. Common slope tests for bivariate structural relationships. Biometrical Journal. 2002;44:161–174. [Google Scholar]
- Zaragoza-Castells J, Sánchez-Gómez D, Hartley IP, et al. Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of acclimation in both high-light and shaded habitats. Functional Ecology. 2008;22:172–184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.