Abstract
Background and Aims
Vegetation has long been recognized to protect the soil from erosion. Understanding species differences in root morphology and functional traits is an important step to assess which species and species mixtures may provide erosion control. Furthermore, extending classification of plant functional types towards root traits may be a useful procedure in understanding important root functions.
Methods
In this study, pioneer data on traits of alpine plant species, i.e. plant height and shoot biomass, root depth, horizontal root spreading, root length, diameter, tensile strength, plant age and root biomass, from a disturbed site in the Swiss Alps are presented. The applicability of three classifications of plant functional types (PFTs), i.e. life form, growth form and root type, was examined for above- and below-ground plant traits.
Key Results
Plant traits differed considerably among species even of the same life form, e.g. in the case of total root length by more than two orders of magnitude. Within the same root diameter, species differed significantly in tensile strength: some species (Geum reptans and Luzula spicata) had roots more than twice as strong as those of other species. Species of different life forms provided different root functions (e.g. root depth and horizontal root spreading) that may be important for soil physical processes. All classifications of PFTs were helpful to categorize plant traits; however, the PFTs according to root type explained total root length far better than the other PFTs.
Conclusions
The results of the study illustrate the remarkable differences between root traits of alpine plants, some of which cannot be assessed from simple morphological inspection, e.g. tensile strength. PFT classification based on root traits seems useful to categorize plant traits, even though some patterns are better explained at the individual species level.
Keywords: Growth rings, plant diversity, plant functional types, PFTs, ski slope, soil erosion, tensile strength, alpine plants
INTRODUCTION
The integrity of a persistent vegetation cover with intact root systems is crucial for soil stability in alpine ecosystems (Körner, 2003). Species diversity or the presence of a specific species may be key factors in forming a plant cover that provides an effective protection from soil erosion (e.g. Bautista et al., 2007; Reubens et al., 2007; Stokes et al., 2009). Vegetation cover mainly intercepts raindrops, enhances the infiltration, transpires soil water, provides additional surface roughness and adds organic material to the soil (Gyssels et al., 2005). Root systems physically bind soil particles and affect important soil properties such as aggregate stability, infiltration capacity, bulk density, soil texture, organic and chemical content and shear strength (Amezketa, 1999; Gyssels et al., 2005; Reubens et al., 2007). Even though the role of vegetation for erosion control has been demonstrated in numerous studies, especially in Agriculture and the Mediterranean region (e.g. Mattia et al., 2005; Bautista et al., 2007; Zuazo and Pleguezuelo, 2008), research above the treeline and on root morphology of alpine plant species and their mechanical properties is limited (but see Daubenmire, 1941; Kutschera and Sobotik, 1997; Polomski and Kuhn, 1998, for qualitative descriptions of the structure and distribution of root systems of some alpine species, and see Jochimsen, 1983; Jonasson and Callaghan, 1992, for data on root tensile strength).
The potential of alpine plant diversity in increasing topsoil aggregate stability and reducing surface erosion above the treeline has recently been shown in Pohl et al. (2009) and Martin (2010a). Pohl et al. (2009) showed that an increase in plant species richness significantly increased the topsoil aggregate stability. Fine roots (e.g. graminoid roots) made the largest contribution to aggregate stability. However, in their study the beneficial effects of higher species richness could not be assigned to only one specific functional group, but to the combination of several groups, since plant species showing the highest correlations with species richness belonged to different functional groups. Likewise, no single species was correlated with sediment yield in the study of Martin (2010a); only the combination of different species and growth forms could explain the positive diversity effect. In both studies a higher number of root types was assumed to be responsible for the positive effect on aggregate stability and surface erosion. Nevertheless, detailed information on root morphology and mechanical characteristics of alpine plants of different functional groups was lacking.
Diverse plant species may contribute to soil aggregation and erosion control by their differences in root functional traits such as root biomass, their vertical and horizontal distribution in the soil, diameters of fine and coarse roots and their branching patterns (Amezketa, 1999; Gyssels and Poesen, 2003). Another parameter that is important to quantify the plant's anchorage in the ground against external forces which could cause failure is tensile strength (Ennos and Pellerin, 2000). Tensile strength represents the maximum stress a root can withstand before failing (Smith et al., 2000). First, direct applied horizontal (erosive) forces may set the roots to tension. Roots will have to withstand compression or bending and loading in tension, which are transferred to the soil. Secondly, a high root tensile strength will prevent root systems from being uprooted, e.g. through grazing (Ennos and Pellerin, 2000). Thus, a high tensile strength will contribute to hold aggregates together physically and will enable the plant to withstand uprooting and to remain anchored in the soil, e.g. during grazing, intense rainfall (Ennos and Pellerin, 2000) or at times of soil movement (Jonasson and Callaghan, 1992).
Associated with the lack of understanding of alpine root systems, only a few studies have suggested for root traits that functional classifications of species with similar traits can be useful (Jonasson and Callaghan, 1992; Guerrero-Campo and Fitter, 2001; Guerrero-Campo et al., 2006). Although classifications into plant functional types (PFTs) have received considerable attention for many years (e.g. Körner, 1993; Lavorel et al., 1997; Cornelissen et al., 1999), PFTs other than the familiar classification into graminoids, forbs and shrubs may be more rewarding when questions about soil stabilization or erosion control are considered.
In this study, we (1) analyse above- and below-ground plant traits of 13 alpine pioneer species from different families, life forms and growth forms, and (2) classify species into PFTs based on these measured traits. We studied species from a topographically adjusted machine-graded alpine ski slope for two reasons. First, functional root systems are particularly important in disturbed areas that are prone to the loss of soil particles, nutrients, organic matter and plant seeds during heavy rainfall (Isselin-Nondedeu et al., 2006; Zuazo and Pleguezuelo, 2008). The disturbance often involves long-lasting damage to the vegetation and the upper soil (Wipf et al., 2005; Krautzer et al., 2006; Delgado et al., 2007). Secondly, plants from that site could be analysed as individual specimens because plants could develop with no or little influence of neighbours. Investigated traits included plant height and shoot biomass, root depth, horizontal root spreading, root length, diameter, tensile strength, plant age and root biomass.
It was assumed that biodiversity effects on soil aggregation will most probably be functional when traits differ between plant species. Based on that assumption, we hypothesize that plant traits differ significantly even between species of the same life form (graminoids, forbs and shrubs), and, more specifically, that the trait tensile strength can differ significantly between species that have the same root diameter. Furthermore, we hypothesize that a PFT classification into root traits may be more useful than using the most frequent PFT classification into graminoids, forbs and shrubs when the aim is to describe functional differences of root systems among plant species.
MATERIALS AND METHODS
Study site
The study on root characteristics was conducted on a machine-graded ski slope at 2625 m a.s.l. at the Piz Corvatsch (46° 26′N, 9°50′E, Upper Engadin, Switzerland). The climate is alpine, with mean annual rainfall of 850 mm and a mean temperature of the warmest months July and August of 5·4 °C (estimated temperature according to a lapse rate of 0·60 K per 100 m according to Körner, 2003, from station Piz Corvatsch, 3315 m a.s.l.). The ski slope was machine-graded in 1980 in alpine sedge grassland (Caricetum curvulae Rübel 1911) on siliceous bedrock and is usually covered with snow from the beginning of November until the end of May. The slope is northwest facing and has an inclination of 25°. Since the aim was to study the growth characteristics of plant roots that have grown without the influence of neighbours, we selected a study site of 25 × 25 m with homogeneous sparse vegetation (cover of 25 %). Therefore, the measured plant characteristics may be representative of these species in a stressed environment, which may be harsher than the typical environment of the species. The most abundant species [including the rarer Geum reptans L. (Rosaceae)] are listed in Table 1, and rarer species (<5 individuals in the entire area) comprised Achillea nana L. (Asteraceae), Bartsia alpina L. (Scrophulariaceae), Carex curvula All. S.L. (Cyperaceae), Cirsium spinosissimum (L.) Scop. (Asteraceae), Doronicum clusii (All.) Tausch (Asteraceae), G. reptans, Linaria alpina (L.) Miller S.L. (Scrophulariaceae), Salix glaucosericea Floderus (Salicaceae), Salix retusa L. (Salicaceae) and Senecio incanus L. S.L. (Asteraceae).
Table 1.
Plant age, above- and below-ground characteristics of 13 plant species from a machine-graded ski slope at 2625 m a.s.l. in the order of their life form
| Biomass (g dry matter) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Family | Life form | Growth form | Root type | n | Age (years) | Shoots | Roots | Horizontal root spread (cm) | Total root length (cm) | Mean root diameter (mm) |
| Arabis caerulea All. | Brassicaceae | Forb | Sem | Tap | 5 | 5·6 (0·8) | 0·48 (0·17) | 0·04 (0·01) | 0 | 31·0 (4·8) | 2·4 (0·3) |
| Campanula scheuchzeri Vill. | Campanulaceae | Forb | Sem | Lateral | 5 | 8·5 (0·4) | 0·48 (0·08) | 0·20 (0·09) | 70·0 (9·0) | 854·7 (279·5) | 1·4 (0·1) |
| Geum reptans L. | Rosaceae | Forb | Sem | Tap | 2 | 14·5 (2·4) | 1·50 (0·46) | 1·72 (0·41) | 30·0 (0) | 159·1 (5·9) | 2·3 (0·2) |
| Leucanthemopsis alpina (L.) Heywood | Asteraceae | Forb | Sem | Lateral | 5 | 2·8 (0·2) | 0·12 (0·03) | 0·10 (0·03) | 11·0 (1·0) | 568·3 (159·6) | 1·0 (0·03) |
| Ranunculus glacialis L. | Ranunculaceae | Forb | Sem | Lateral | 5 | NA | 0·19 (0·09) | 0·37 (0·13) | 74·7 (12·6) | 732·7 (201·3) | 1·0 (0·05) |
| Trifolium badium Schreb. | Fabaceae | Forb | Scap | Lateral | 5 | 3·4 (0·2) | 0·96 (0·23) | 0·25 (0·09) | 38·4 (5·0) | 809·0 (250·4) | 0·7 (0·01) |
| Veronica alpina L. | Scrophulariaceae | Forb | Scap | Lateral | 5 | NA | 0·16 (0·07) | 0·05 (0·01) | 71·0 (10·0) | 546·7 (107·7) | 0·5 (0·02) |
| Luzula alpinopilosa (Chaix) Breistr. | Juncaceae | Graminoid | Caesp | Fibrous | 5 | NA | 0·76 (0·19) | 0·92 (0·17) | NA | 16470·0 (4520·9) | 0·9 (0·02) |
| Luzula spicata (L.) DC. | Juncaceae | Graminoid | Caesp | Fibrous | 5 | NA | 1·44 (0·36) | 0·41 (0·12) | NA | 17402·3 (6749·8) | 0·9 (0·01) |
| Poa alpina L. | Poaceae | Graminoid | Caesp | Fibrous | 5 | NA | 0·45 (0·15) | 0·26 (0·06) | NA | 3117·9 (1171·0) | 0·8 (0·1) |
| Trisetum spicatum (L.) Richter | Poaceae | Graminoid | Caesp | Fibrous | 5 | NA | 0·90 (0·25) | 0·39 (0·25) | 38·8 (3·3) | 5660·6 (3412·5) | 0·9 (0·02) |
| Salix breviserrata Floderus | Salicaceae | Shrub | Caesp | Plate | 5 | 9·8 (0·3) | 0·45 (0·04) | 0·76 (0·19) | 96·8 (11·4) | 1014·5 (145·9) | 1·3 (0·03) |
| Salix herbacea L. | Salicaceae | Shrub | Rept | Plate | 5 | 8·4 (0·4) | 0·30 (0·06) | 0·24 (0·06) | 63·6 (14·3) | 450·8 (110·3) | 1·2 (0·03) |
Values are means with s.e.m. in parentheses. Within a column, the highest values are in bold. Growth form abbreviations are: caesp, caespitosa; scap, scaposa; sem, semi-rosulata; and rept, reptantia. n is the number of sampled plant individuals; NA, not analysed.
Measurements
Plant sampling and root excavation
A total of 62 individuals representing 13 alpine plant species were sampled to study their morphology. Since the aim of the study was to compare a large number of the most abundant species commonly found on such degraded sites, the replication of individuals was limited to five individuals per plant species (except for the rarer G. reptans for which only two individuals could be sampled) and they were excavated between August and September 2006 (Table 1, see section on ‘Tensile strength’ below for additional plants sampled). For graminoids and forbs, average, mature-sized individuals were selected. For shrubs, for practical reasons, we selected small individuals before they started to produce clonal complexes, which may have resulted in an underestimation of parameters such as root system size and age. The measured early establishment characteristics of young shrubs may not be representative for older individuals.
Shoot and root traits
Plant height was measured as inflorescence height before cutting off the above-ground biomass at the root collar. Above-ground biomass was oven-dried at 105 °C and weighed. The entire root systems were carefully dry excavated using small tools. First, lateral roots were exposed and the complete horizontal root spreading was measured. It amounted to a maximal 120 cm in the case of two specimens of Salix breviserrata and one specimen of Salix herbacea. In the loose and dry ground, roots could be excavated to a depth of 80 cm. Deeper roots (in the case of two specimens of G. reptans) were cut off. The vertical extension of roots was then measured. The excavated root systems were stored in sealed plastic bags in 15 % ethanol solution at 4 °C until processing (Böhm, 1979). The root systems were gently washed to remove debris and soil particles and then scanned with a desktop optical scanner. The image analysing program WinRhizo (Regent instruments Inc., Quebec, Canada) was used to determine the total root length and the mean root diameter of each individual plant. The software also allowed us to determine the root length within five pre-defined diameter classes (0 < d ≤0·5 mm, 0·5 < d ≤2·0 mm, 2·0 <d ≤5·0 mm, 5·0 < d ≤10·0 mm, and d >10·0 mm). The mean root diameter for each plant was calculated by the program by dividing the measured projected area by the given root length. Roots were further stored as before in ethanol solution at 4 °C until the determination of plant age, and then processed for root biomass determination.
Plant age
To better interpret the re-colonization process of the individual species after the disturbance 30 years ago, annual rings were determined for replicated samples of five forbs (Arabis caerulea, Campanula scheuchzeri, G. reptans, Leucanthemopsis alpina and Trifolium badium) and two dwarf shrubs (S. breviserrata and S. herbacea). These seven species showed clear annual increments. For the remaining species, age determination was not possible for morphological reasons (e.g. the monocots, see Schweingruber and Poschlod, 2005). To better visualize ring boundaries, thin root cross-sections of 30 µm thickness were produced from the stored samples with a sledge microtome near the root collar where ring boundaries are most clearly expressed and missing rings is avoided. For tap rooting species, the tap root was analysed. For species with another root type, the thickest roots were sampled to ease the cutting and the later counting of growth rings from those roots generally small in diameter. For each plant individual, two cross-sections were taken and analysed separately to avoid the counting of false rings due to compactness and unclear ring boundaries. False rings can normally be recognized by these distinct changes in fibre forms on the latewood–earlywood boundary. Cross-sections were placed on a microscope slide and stained with phloroglycinol and hydrochloric acid, which causes a red colouration of the cell walls of the secondary xylem vessels. The annual growth can then be better recognized by the pattern formed by the alternation of latewood (end of growing season, small vessels) and earlywood (beginning of growing season, large vessels) (Schweingruber and Poschlod, 2005). Each cross-section was photographed with a digital camera (Leica DC200, Wetzlar, Germany) through the phototube of a dissecting microscope with a 10-fold magnification (Olympus BX51, Center Valley, PA, USA). Digital pictures were used for later observations. The mean number of annual rings for two cross-sections per individual was used to calculate a species ‘mean age’ (error <2 years).
Tensile strength
Additional plants were sampled for the analysis of root tensile strength in fresh roots (see section on ‘Plant sampling and root excavation’ for sampling criteria). Root systems of 4–9 plants of the 13 selected species were dug out between August and September 2007 and stored in plastic bags for a maximum of 24 h (Table 3). Roots were washed to remove debris and soil particles. Representative roots (minimum length of 7 cm) were randomly selected. Before the test, root diameters were measured under a microscope at both ends for calculation of the mean diameter (ranging from 0·16 mm in Luzula spicata to 2·50 mm in A. caerulea; Table 3). The ends of the roots were either embedded in cork or styrofoam, or glued to prevent tissue damage, and clamped between two mobile steel jaws. To hold them vertically, the upper end was fixed to a scaffold. At the lower end, a water vessel was fixed to the second jaw. That vessel was filled with water to add weight until the root failed. According to the root diameter, vessels of different sizes and weights were used. After root failure, the water supply was stopped immediately and the water volume measured. The maximum force at which root failure occurred could be calculated from all known weights (kg) and represented the peak load (N). To compensate for variations in root diameter, tensile strength was obtained by dividing the maximum force by the cross-sectional area (mm2) of the root (Smith et al., 2000). If the roots broke close to the jaws or slipped out, the test was discarded and a new root was tested.
Table 3.
Mean values of root tensile strength and root diameter, a and b values and adjusted r2 values of the power law eqn (1), expressing the decrease in tensile strength with increasing root diameter of the 13 analysed species in the order of their a value
| Species | Mean tensile strength (N mm−2) | Mean root diameter (mm) | a | b | n | Adjusted r2 |
|---|---|---|---|---|---|---|
| Geum reptans | 7·68 (0·29) | 0·90 (0·06) | 7·00 | –0·31 | 41 | 0·32*** |
| Luzula spicata | 30·25 (2·32) | 0·16 (0·01) | 5·91 | –0·78 | 35 | 0·43*** |
| Trifolium badium | 7·62 (0·52) | 0·78 (0·06) | 5·68 | –0·60 | 41 | 0·60*** |
| Luzula alpinopilosa | 18·52 (4·03) | 0·37 (0·04) | 5·44 | –0·93 | 17 | 0·57*** |
| Salix herbacea | 6·10 (0·41) | 0·92 (0·07) | 5·39 | –0·24 | 35 | NS |
| Poa alpina | 27·91 (3·88) | 0·30 (0·03) | 4·92 | –1·17 | 21 | 0·72*** |
| Campanula scheuchzeri | 3·78 (1·24) | 2·08 (0·25) | 3·98 | –0·36 | 6 | NS |
| Leucanthemopsis alpina | 4·26 (0·25) | 0·83 (0·03) | 3·81 | –0·26 | 31 | NS |
| Ranunculus glacialis | 2·74 (0·16) | 1·53 (0·08) | 2·93 | –0·33 | 30 | 0·09* |
| Veronica alpina | 6·76 (0·90) | 0·41 (0·02) | 2·92 | –0·71 | 40 | 0·26*** |
| Trisetum spicatum | 27·49 (2·47) | 0·24 (0·02) | 2·61 | –1·38 | 50 | 0·79*** |
| Arabis caerulea | 2·01 (0·20) | 2·50 (0·23) | 2·42 | –0·28 | 11 | NS |
| Salix breviserrata | NA | NA | NA | NA | NA | NA |
n is the number of roots tested. Values are means with s.e.m. in parentheses. ***P < 0·001, **P < 0·01, *P < 0·05; NS, not significant; NA, not available.
Plant functional types
The plant species were classified into three PFTs according to their (1) life form, (2) growth form and (3) root type. Life forms are given as (a) graminoids, (b) forbs and (c) shrubs. Growth forms are, according to Schweingruber and Poschlod (2005): (a) caespitosa (caesp): multi-stemmed plants, forming tussocks; (b) scaposa (scap): single-stemmed plants, no basal leaves, leaves distributed along the stem; (c) semi-rosulata (sem): single-stemmed plants, leaves in a rosette but also distributed along the stem; and (d) reptantia (rept): plants with horizontally creeping stems. Root types are described after Marden (2005) as follows: (a) fibrous: compact root ball with many thin and obliquely or vertically descending roots; (b) lateral: long and radially spreading from the taproot, or the root bole, possibly in two or more plates or strata; (c) plate: a shallow spreading root system with abundant surface roots and no tap root; and (d) tap: the seedling radicle persists and grows into a single or branched massive root (taproot), more or less vertical; it may give rise to planes of lateral roots.
Statistical analysis
We tested for differences of all dependent variables between plant species with one-way analysis of variance (ANOVA). Geum reptans was excluded from the analysis of differences between species due to low replication. Analysis of covariance (ANCOVA) with diameter as co-variable was used to evaluate the influence of species on tensile strength (both log transformed). The PFTs (1) life form, (2) growth form and (3) root type were tested with a linear mixed-effects model with restricted maximum likelihood estimation. Plant species was specified as a random effect. The growth form ‘reptantia’ included only one species and was therefore excluded from the analysis. Assumptions of normality and homoscedasticity were fulfilled after log transformation. A posteriori contrasts were tested with Tukey HSD tests. Power law equations were fitted through the relationship of root tensile strength (Tr) and diameter (d) (Gray and Sotir, 1996):
| (1) |
where a is the exponent of the intercept from the power model expressing the strength of the roots, and b is the slope of the model which controls the rate of strength decay with diameter. All analyses were performed using R, version 2·8·1. (R Development Core Team, 2009).
RESULTS
Differences among species
Species differences in functional traits were remarkable (Table 1). Mean shoot biomass per adult individual differed significantly between species (F11,47 = 7·96, P < 0·001), and was highest for the forb G. reptans (1·50 ± 0·46 g) and the graminoid L. spicata (1·44 ± 0·36 g) and lowest for the forbs Veronica alpina (0·16 ± 0·07 g) and L. alpina (0·12 ± 0·03 g). Root biomass was significantly different between species (F11,45 = 7·24, P < 0·001), and was also highest for G. reptans (1·72 ± 0·41 g) and the graminoid Luzula alpinopilosa (0·92 ± 0·17 g) and lowest for the forbs V. alpina (0·05 ± 0·01 g) and A. caerulea (0·04 ± 0·01 g). For horizontal root spreading, species differed significantly (F8, 33 = 116·3, P < 0·001) and reached 96·80 ± 11·41 cm for the dwarf shrub S. breviserrata, while the tap rooting A. caerulea did not spread horizontally. Total root length showed the highest values from 57 ± 34 m to 174 ± 67 m for the three graminoids Trisetum spicatum, L. alpinopilosa and L. spicata, and differed significantly from the other species, e.g. by >500 magnitudes compared with A. caerulea (0·3 ± 0·05 m) (F11,48 = 24·62, P < 0·001). Mean root diameter was highest in both tap rooting species A. caerulea (2·39 ± 0·26 mm) and G. reptans (2·33 ± 0·22 mm), and both differed significantly from all other species (F11,45 = 84·16, P < 0·001).
Root length distribution within diameter classes showed that roots of the graminoids L. spicata and Poa alpina had about 71 and 58 %, respectively, of the roots with a diameter ≤0·5 mm (Table 2), whereas the majority of the other plants had their longest roots in the second diameter class (0·5 < d ≤ 2·0 mm). Only the tap rooting forbs A. caerulea and G. reptans were characterized by a higher percentage of root length in larger diameter classes.
Table 2.
Distribution of root length (%) over five diameter classes for the 13 investigated plant species in the order of their life form
| Root length (%) per root diameter class |
||||||
|---|---|---|---|---|---|---|
| Species | Life form | 0 < d ≤ 0·5 mm | 0·5 < d ≤ 2·0 mm | 2·0 < d ≤ 5·0 mm | 5·0 < d ≤ 10·0 mm | d > 10 mm |
| Arabis caerulea | Forb | 7·3 | 28·6 | 61·0 | 2·7 | 0·3 |
| Campanula scheuchzeri | Forb | 17·1 | 70·2 | 10·7 | 1·4 | 0·6 |
| Geum reptans | Forb | 5·0 | 48·8 | 38·8 | 6·7 | 0·7 |
| Leucanthemopsis alpina | Forb | 6·8 | 89·5 | 3·2 | 0·4 | 0·01 |
| Ranunculus glacialis | Forb | 12·0 | 84·6 | 3·0 | 0·3 | 0·009 |
| Trifolium badium | Forb | 48·8 | 48·6 | 2·5 | 0·2 | 0·02 |
| Veronica alpina | Forb | 54·5 | 45·3 | 0·2 | 0·0007 | 0 |
| Luzula alpinopilosa | Graminoid | 29·9 | 68·5 | 1·7 | 0·005 | 0 |
| Luzula spicata | Graminoid | 70·6 | 28·6 | 0·7 | 0·01 | 0·0002 |
| Poa alpina | Graminoid | 57·9 | 38·1 | 3·8 | 0·2 | 0·0007 |
| Trisetum spicatum | Graminoid | 23·0 | 75·6 | 1·4 | 0·03 | 0·0006 |
| Salix breviserrata | Shrub | 15·9 | 71·7 | 11·2 | 1·1 | 0·1 |
| Salix herbacea | Shrub | 14·7 | 75·0 | 9·7 | 0·6 | 0·004 |
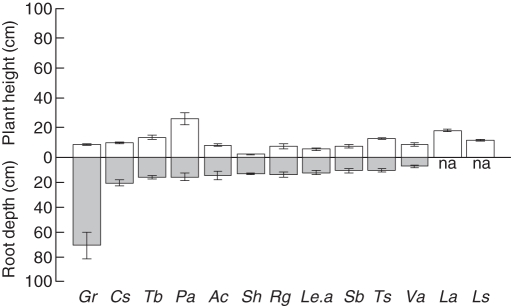
Plant height and root depth also differed among species (Fig. 1). Plant height reached 26·1 ± 4.E-02 cm for P. alpina and differed significantly from all other species (F11,47 = 44·87, P < 0·001). Root depth was significantly greater for G. reptans (70·7 ± 10·7 cm) compared with the other species, which rooted in the upper 20 cm (F9,38 = 5·32, P < 0·001).
Fig. 1.
Plant height and root depth (cm; means ± s.e.) of the 13 investigated plant species sorted according to their root depth. Abbreviated plant species are: Gr, Geum reptans; Cs, Campanula scheuchzeri; Tb, Trifolium badium; Pa, Poa alpina; Ac, Arabis caerulea; Sh, Salix herbacea; Rg, Ranunculus glacialis; Le.a, Leucanthemopsis alpina; Sb, Salix breviserrata; Ts, Trisetum spicatum; Va, Veronica alpina; La, Luzula alpinopilosa; and Ls, Luzula spicata; na, not analysed.
Plant age
The statistically analysed individuals of the six plant species differed significantly in their age (F5, 59 = 60·09, P < 0·001; Table 1). The oldest individuals were from the forb G. reptans (14·5 ± 2·4 years) and the dwarf shrub S. breviserrata (9·8 ± 0·3 years). The youngest individuals for which the age could be determined were from the two forbs T. badium (3·4 ± 0·2 years) and L. alpina (2·8 ± 0·2 years).
Root tensile strength
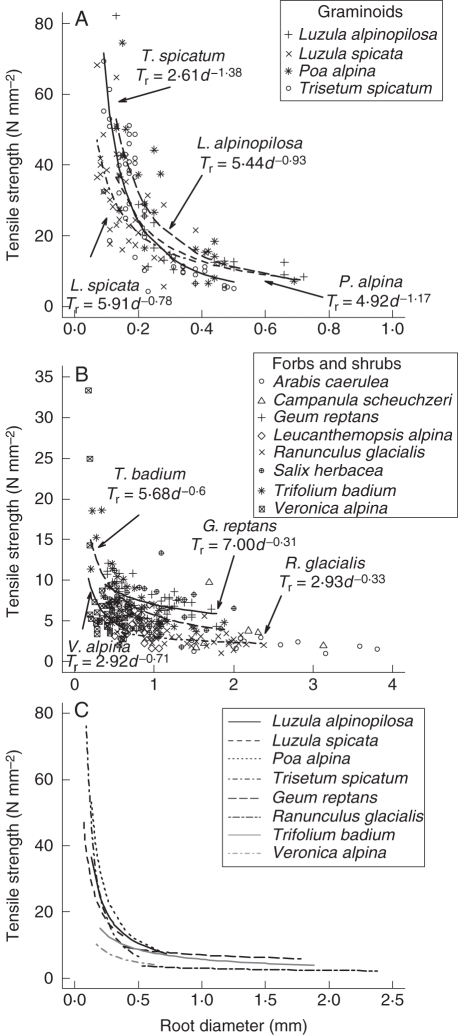
Tensile strength differed significantly between species with regard to root diameter (F11,344 = 7·29, P < 0·001), and decreased with increasing root diameter following the power law eqn (1). For eight out of 12 analysed species, tensile strength could be predicted well by root diameter (Table 3, Fig. 2A, B). The maximum values recorded for root tensile strength reached 82·4 N mm−2 for L. alpinopilosa (d = 0·13 mm) and 74·6 N mm−2 for P. alpina (d = 0·15 mm). For the same diameter range, L. spicata had a higher tensile strength as compared with L. alpinopilosa, and G. reptans was more resistant than T. badium (see high a values and low b values in Table 3).
Fig. 2.
Relationship between root tensile strength (Tr) and root diameter (d) for the analysed plant species according to life form: (A) for graminoids and (B) for forbs and shrubs. For species with a significant strength–diameter relationship, the power law eqn (1) is given. In (C) the related regression lines for species with a significant strength–diameter relationship are given. Note the different scales of the y- and x-axes in (A) and (B).
For the three forbs A. caerulea, C. scheuchzeri and L. alpina and the dwarf shrub S. herbacea no significant relationship between tensile strength and root diameter could be observed because their roots covered a relatively small range of diameters. Each regression line of the eight species covers a different range of strength–diameter relationship and this range is enlarged when all eight species are considered together (Fig. 2C).
Plant traits in relation to functional types (PFTs)
Plant height was better explained by the PFT life form (F2,10 = 5·77, P = 0·02) than by root type (F3,9 = 3·48, P = 0·06) or plant growth form (F2,9 = 2·21, P = 0·17). Among the life forms, plant height was greatest for graminoids and lowest for shrubs (Tukey HSD, P < 0·05; Table 4). Total root length was better described by root type (F3,9 = 25·63, P < 0·001) and life form (F2,10 = 11·07, P = 0·003) than by growth form (F2,9 = 7·72, P = 0·01). Among the root types, the total root length was greatest for the fibrous rooting plants and lowest for tap rooting plants (Tukey HSD, P < 0·05). Mean root diameter could be explained by the two PFTs root type (F3,9 = 7·05, P = 0·01) and growth form (F2,9 = 7·30, P = 0·01), but not by life form (F2,10 = 0·42, P = 0·67). Root tensile strength could be explained by the three PFTs growth form (F2,319 = 14·09, P < 0·001), life form (F2,9 = 17·24, P < 0·001) and root type (F3,8 = 10·28, P < 0·01). Mean tensile strength was greatest for graminoids and lowest for forbs (Tukey HSD, P < 0·05). For the other measured variables, no significant differences were observed.
Table 4.
Above- and below-ground characteristics and tensile strength for three PFTs: life form, growth form and root type (for details see Materials and Methods)
| PFT | Plant height (cm) | Shoot biomass (g) | Root biomass (g) | Root depth (cm) | Root expansion (cm) | Total root length (cm) | Root diameter (mm) | Tensile strength (N mm−2) |
|---|---|---|---|---|---|---|---|---|
| Life form | ||||||||
| Graminoids | 17·08 (1·64)a | 0·89 (0·14) | 0·49 (0·10) | 12·72 (1·23) | 38·80 (1·66) | 106·63 (25·06)a | 0·87 (0·03) | 27·09 (1·49)a |
| Forbs | 8·89 (0·59)b | 0·47 (0·09) | 0·27 (0·08) | 17·66 (2·71) | 40·17 (5·74) | 5·63 (0·84)b | 1·23 (0·13) | 5·78 (0·41)b |
| Shrubs | 4·85 (1·05)b | 0·38 (0·04) | 0·53 (0·13) | 11·90 (1·07) | 80·20 (10·26) | 7·33 (1·28)b | 1·23 (0·02) | 6·10 (0·26)b |
| Growth form | ||||||||
| Caespitosa | 15·16 (1·54) | 0·80 (0·12) | 0·55 (0·09) | 11·96 (1·01) | 67·80 (7·07) | 87·33 (21·44)a | 0·95 (0·04)ab | 26·91 (1·49)a |
| Reptantia | 2·20 (0·37) | 0·30 (0·06) | 0·24 (0·06) | 13·20 (0·73) | 63·60 (14·34) | 4·51 (1·10) | 1·19 (0·03) | 6·10 (0·41) |
| Scaposa | 11·10 (1·17) | 0·56 (0·18) | 0·15 (0·05) | 11·70 (1·70) | 52·89 (7·36) | 6·78 (1·36)ab | 0·58 (0·03)a | 7·21 (0·50)b |
| Semi-rosulata | 7·89 (0·58) | 0·43 (0·10) | 0·34 (0·11) | 20·36 (3·75) | 34·45 (7·44) | 5·11 (1·05)b | 1·52 (1·14)b | 4·79 (0·24)b |
| Root type | ||||||||
| Fibrous | 17·08 (1·64)a | 0·89 (0·14) | 0·49 (0·10) | 12·72 (1·23) | 38·80 (1·66) | 106·63 (25·06)a | 0·87 (0·03)a | 27·09 (1·49)a |
| Lateral | 9·06 (0·74)ab | 0·38 (0·08) | 0·19 (0·04) | 14·06 (1·16) | 50·23 (6·03) | 7·02 (0·89)b | 0·91 (0·06)a | 5·54 (0·33)b |
| Plate | 4·85 (1·05)b | 0·38 (0·04) | 0·53 (0·13) | 11·90 (1·07) | 80·20 (10·26) | 7·33 (1·28)b | 1·23 (0·02)ab | 6·10 (0·41)ab |
| Tap | 8·29 (0·65)ab | 0·77 (0·24) | 0·60 (0·34) | 30·50 (10·90) | 8·57 (5·53) | 0·68 (0·24)c | 2·38 (0·19)b | 6·49 (0·40)b |
Values are means with s.e.m. in parentheses. Within a column and PFT, values that share a common letter do not differ significantly according to Tukey HSD (P < 0·05); other variables did not differ significantly among a PFT classification.
DISCUSSION
Understanding species differences in root characteristics is an important step towards assessing which species and species mixtures may provide erosion control. In our study, the 13 investigated alpine plant species showed remarkable differences in their morphology, and three species had very contrasting plant traits. Geum reptans, although a forb of small height, had the highest biomass of shoots and roots and the deepest roots (and the oldest age; see below). The long tap root grew down to 1 m below the ground and could therefore anchor into soil layers deeper than any other species could reach. The total root length of G. reptans, on the other hand, was the second lowest of the investigated species. The rush species L. spicata had the highest total root length. Luzula spicata had a similar root biomass to G. reptans but a small mean root diameter, which is reflected in the enormous amount of fine roots (<0·5 mm, 71 %). The dwarf willow S. breviserrata was most remarkable in its horizontal root spreading of up to 1 m, with roots not exceeding a soil depth of 20 cm. Root biomass, especially of fine roots, may be highest in summer and lowest in winter. As surface erosion, on the other hand, may be high during snowmelt after winter, future research should also investigate changes in root characteristics during the course of the year.
For erosion control, it is highly likely that no single plant species can provide the desired positive effect in these conditions and that a combination of different plant species and growth forms may be needed. We did not measure soil stability or surface erosion in this study, but a likely mechanism is the coexistence of species with contrasting root architecture and mechanical characteristics. In our example, the tap rooting forb G. reptans can anchor into deeper soil layers, while the woody S. breviserrata can fix the topsoil superficially and laterally with its long horizontally spreading roots. Many dwarf shrubs can reach enormous extensions of up to 40 m in undisturbed conditions. Such an extensive horizontally spreading root system provides efficient water absorption under the soil surface during the short growing season (Polomski and Kuhn, 1998). The graminoids, in our case L. spicata, but to some extent also L. alpinopilosa and P. alpina, have large amounts of fine roots. This amount is not unusual for alpine graminoids, and also Körner et al. (1987) report a fine root allocation of 56 % (percentage of total dry matter of fine roots) for Poa laxa. Graminoids therefore increase the topsoil aggregate stability (Pohl et al., 2009), resist concentrated surface flow to a large extent (De Baets et al., 2007) and stabilize the plant through a large number of anchor points in the ground (Jonasson and Callaghan, 1992).
Root tensile strength decreased with increasing root diameter, as found by many other authors, e.g. Mattia et al. (2005) and De Baets et al. (2008). These authors also suggested that small roots provide proportionally greater cohesive strength than larger roots. The strength–diameter relationship across our species looks very similar: those species with very fine roots (e.g. graminoids L. alpinopilosa, L. spicata and P. alpina) showed the highest values of root tensile strength (see also De Baets et al., 2008 for Mediterranean grass species). The tensile strength of plants with larger root diameters, such as tap rooting forbs and shrubs, was considerably lower, as was also found in arctic species (Jonasson and Callaghan, 1992). Probably the most interesting finding about root tensile strength in our study was the differing strength between some of our investigated alpine plant species of similar root diameter. For instance, the mean tensile strength of the three similar graminoids L. alpinopilosa, L. spicata and P. alpina differed greatly, although their root diameters were similar. These differences in tensile strength between species of similar diameter and the same life form may be attributed to different root structures. Genet et al. (2005) reported higher cellulose contents per weight of dry matter for fine roots and observed an increase in tensile strength with decreasing root diameter and increasing cellulose content in the tree species Pinus pinaster and Castanea sativa. Variations in site conditions such as soil texture, soil moisture content and nutrients can also be responsible for the differences in tensile strength.
The re-colonization process of the individual species after the disturbance 30 years ago was reflected by age analysis. Geum reptans was, with a maximum age of 20 years, the oldest plant of the study site, whereas the youngest plant, for which the age could be determined, was L. alpina, a plant generally <3 years old. A long-lived pioneer species such as G. reptans may be needed to facilitate re-colonization of a disturbed site by other species. Geum reptans has been found to tolerate surface movements and to be well adapted to withstanding mechanical disturbance through elongation and regeneration (Cannone and Gerdol, 2003). Furthermore, individuals of the clonal species have a relatively high colonization range and therefore ensure a rapid re-colonization after disturbance (Stöcklin and Bäumler, 1996). The woody plant S. breviserrata also reached an age of 9·8 years, which is, however, not a very high age for an alpine willow, but is in our case related to the age of the last perturbation.
Our analysis of PFTs showed that many plant characteristics were highly species specific. Nevertheless, our different approaches to aggregate species in functional groups were useful for some variables: plant height, total root length and root tensile strength could be explained significantly by life forms of graminoids, shrubs and forbs (see also Jonasson and Callaghan, 1992 for arctic species). The categorizations into growth forms and root types did explain below-ground parameters such as total root length and tensile strength. Grouping species into PFTs can help in simplifying and categorizing plant traits (Jonasson and Callaghan, 1992; Lavorel et al., 1997; Cornelissen et al., 1999). However, in many cases patterns are better explained at the species level than at the PFT level (Körner, 1993; Martin et al., 2010b). Nevertheless, where soil stability is concerned, our study suggests that functional types other than graminoids, forbs and shrubs, such as root types, can be useful providing they are well selected and easily measurable (Guerrero-Campo and Fitter, 2001; Guerrero-Campo et al., 2006).
Conclusions
Our study illustrates remarkable differences in the morphology of 13 alpine plant species that have grown without the influence of neighbours on a disturbed site at 2625 m a.s.l. Species differences occurred even if plants were from the same life form (graminoids, forbs and shrubs). Most remarkably, roots of some species (G. reptans and L. spicata) were more than twice as strong as those of other species with the same root diameter. Further, species with different root types provided functions that are expected to be relevant for erosion control, e.g. total root length, root depth and horizontal root spreading. Our suggested PFT classification based on root types was shown to be useful to categorize plant traits; however, some functional differences were better described at the individual species level. Knowledge of plant characteristics and their differences in root traits is, for instance, necessary to include in databases in order to help to better select species and species mixtures for erosion control.
ACKNOWLEDGEMENTS
The study was financed by the Swiss MAVA-Foundation. We thank Martin Meier for assistance in the field, and Maria Kindermann for her valuable laboratory work. Christian Körner and Melissa Martin helped to improve a previous version of this manuscript.
LITERATURE CITED
- Amezketa E. Soil aggregate stability: a review. Journal of Sustainable Agriculture. 1999;14:83–151. [Google Scholar]
- Bautista S, Mayor AG, Bourakhouadar J, Bellot J. Plant spatial pattern predicts hillslope semiarid runoff and erosion in a Mediterranean landscape. Ecosystems. 2007;10:987–998. [Google Scholar]
- Böhm W. Methods of studying root systems. Berlin: Springer-Verlag; 1979. [Google Scholar]
- Cannone N, Gerdol R. Vegetation as an ecological indicator of surface instability in rock glaciers. Arctic, Antarctic and Alpine Research. 2003;35:384–390. [Google Scholar]
- Cornelissen JHC, Perez-Harguindeguy N, Diaz S, et al. Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytologist. 1999;143:191–200. [Google Scholar]
- Daubenmire RF. Some ecologic features of the subterranean organs of alpine plants. Ecology. 1941;22:370–378. [Google Scholar]
- De Baets S, Poesen J, Knapen A, Galindo P. Impact of root architecture on the erosion-reducing potential of roots during concentrated flow. Earth Surface Processes and Landforms. 2007;32:1323–1345. [Google Scholar]
- De Baets S, Poesen J, Reubens B, Wemans K, De Baerdemaeker J, Muys B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant and Soil. 2008;305:207–226. [Google Scholar]
- Delgado R, Sanchez-Maranon M, Martin-Garcia JM, Aranda V, Serrano-Bernardo F, Rosua JL. Impact of ski pistes on soil properties: a case study from a mountainous area in the Mediterranean region. Soil Use and Management. 2007;23:269–277. [Google Scholar]
- Ennos ER, Pellerin S. Plant anchorage. In: Smith AL, editor. Root methods: a handbook. Berlin: Springer-Verlag; 2000. pp. 545–566. [Google Scholar]
- Genet M, Stokes A, Salin F, et al. The influence of cellulose content on tensile strength in tree roots. Plant and Soil. 2005;278:1–9. [Google Scholar]
- Gray DH, Sotir RB. Biotechnical and soil bioengineering slope stabilization: a practical guide for erosion control. New York: Wiley; 1996. [Google Scholar]
- Guerrero-Campo J, Fitter AH. Relationships between root characteristics and seed size in two contrasting floras. Acta Oecologica-International Journal of Ecology. 2001;22:77–85. [Google Scholar]
- Guerrero-Campo J, Palacio S, Perez-Rontome C, Montserrat-Marti G. Effect of root system morphology on root-sprouting and shoot-rooting abilities in 123 plant species from eroded lands in north-east Spain. Annals of Botany. 2006;98:439–447. doi: 10.1093/aob/mcl122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyssels G, Poesen J. The importance of plant root characteristics in controlling concentrated flow erosion rates. Earth Surface Processes and Landforms. 2003;28:371–384. [Google Scholar]
- Gyssels G, Poesen J, Bochet E, Li Y. Impact of plant roots on the resistance of soils to erosion by water: a review. Progress in Physical Geography. 2005;29:189–217. [Google Scholar]
- Isselin-Nondedeu F, Rey F, Bedecarrats A. Contributions of vegetation cover and cattle hoof prints towards seed runoff control on ski pistes. Ecological Engineering. 2006;27:193–201. [Google Scholar]
- Jonasson S, Callaghan T. Root mechanical properties related to disturbed and stressed habitats in the Arctic. New Phytologist. 1992;122:179–186. doi: 10.1111/j.1469-8137.1992.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Körner C. Scaling from species to vegetation: the usefulness of functional groups. In: Schulze ED, Mooney HA, editors. Biodiversity and ecosystem function. Berlin: Springer; 1993. pp. 117–140. [Google Scholar]
- Körner C. Alpine plant life – functional plant ecology of high mountain ecosystems. 2nd edn. Heidelberg: Springer; 2003. [Google Scholar]
- Körner C, Renhardt U. Dry-matter partitioning and root lenght leaf-area ratios in herbaceous perennial plants with diverse altitudinal distribution. Oecologia. 1987;74:411–418. doi: 10.1007/BF00378938. [DOI] [PubMed] [Google Scholar]
- Krautzer B, Wittmann H, Peratoner G, et al. Standortgerechte Hochlagenbegrünung im Alpenraum – der aktuelle Stand der Technik. Raumberg-Gumpenstein, Irdning: Federal Research and Education Centre (HBLFA); 2006. [Google Scholar]
- Kutschera L, Sobotik M. Bewurzelung von Pflanzen in den verschiedenen Lebensräumen. Linz: Landesmuseum; 1997. [Google Scholar]
- Lavorel S, McIntyre S, Landsberg J, Forbes TDA. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends in Ecology and Evolution. 1997;12:474–478. doi: 10.1016/s0169-5347(97)01219-6. [DOI] [PubMed] [Google Scholar]
- Marden M, Rowan D, Phillips C. Stabilising characteristics of New Zealand indigenous riparian colonising plants. Plant and Soil. 2005;278:95–105. [Google Scholar]
- Martin C, Pohl M, Alewell C, Körner C, Rixen C. Interrill erosion at disturbed alpine sites: effects of plant functional diversity and vegetation cover. Basic and Applied Ecology. 2010a;11:619–626. [Google Scholar]
- Martin M, Gavazov K, Körner C, Hättenschwiler S, Rixen C. Reduced early growing season freezing resistance in alpine treeline plants under elevated atmospheric CO2. Global Change Biology. 2010b;16:1057–1070. [Google Scholar]
- Mattia C, Bischetti G, Gentile F. Biotechnical characteristics of root systems of typical Mediterranean species. Plant and Soil. 2005;278:23–32. [Google Scholar]
- Pohl M, Alig D, Körner C, Rixen C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant and Soil. 2009;324:91–102. [Google Scholar]
- Polomski J, Kuhn N. Wurzelsysteme. Bern: Haupt; 1998. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. http://www.r-project.org/ [Google Scholar]
- Reubens B, Poesen J, Danjon F, Geudens G, Muys B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: a review. Trees-Structure and Function. 2007;21:385–402. [Google Scholar]
- Schweingruber FH, Poschlod P. Growth rings in herbs and shrubs: life span, age determination and stem anatomy. Forest Snow and Landscape Research. 2005;79:195–415. [Google Scholar]
- Smith AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, Van De Geijn SC. Root methods: a handbook. Berlin: Springer-Verlag; 2000. [Google Scholar]
- Stöcklin J, Bäumler E. Seed rain, seedling establishment and clonal growth strategies on a glacier foreland. Journal of Vegetation Science. 1996;7:45–56. [Google Scholar]
- Stokes A, Atger C, Bengough AG, Fourcaud T, Sidle RC. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant and Soil. 2009;324:1–30. [Google Scholar]
- Wipf S, Rixen C, Fischer M, Schmid B, Stoeckli V. Effects of ski piste preparation on alpine vegetation. Journal of Applied Ecology. 2005;42:306–316. [Google Scholar]
- Zuazo VHD, Pleguezuelo CRR. Soil-erosion and runoff prevention by plant covers. A review. Agronomy for Sustainable Development. 2008;28:65–86. [Google Scholar]