Abstract
Background
The soil represents a reservoir that contains at least twice as much carbon as does the atmosphere, yet (apart from ‘root crops’) mainly just the above-ground plant biomass is harvested in agriculture, and plant photosynthesis represents the effective origin of the overwhelming bulk of soil carbon. However, present estimates of the carbon sequestration potential of soils are based more on what is happening now than what might be changed by active agricultural intervention, and tend to concentrate only on the first metre of soil depth.
Scope
Breeding crop plants with deeper and bushy root ecosystems could simultaneously improve both the soil structure and its steady-state carbon, water and nutrient retention, as well as sustainable plant yields. The carbon that can be sequestered in the steady state by increasing the rooting depths of crop plants and grasses from, say, 1 m to 2 m depends significantly on its lifetime(s) in different molecular forms in the soil, but calculations (http://dbkgroup.org/carbonsequestration/rootsystem.html) suggest that this breeding strategy could have a hugely beneficial effect in stabilizing atmospheric CO2. This sets an important research agenda, and the breeding of plants with improved and deep rooting habits and architectures is a goal well worth pursuing.
Keywords: Breeding, deep roots, genetics, root architecture, carbon sequestration, nutrient efficiency, drought resistance, soil structure, perenniality
INTRODUCTION
Whatever the extent and dynamics of increased levels of atmospheric CO2 [and of other greenhouse gases (GHGs), for which similar arguments apply], the greenhouse effect means that temperatures will rise monotonically with their levels. A precautionary principle seeks to stop these increases of GHGs, or even to lower them in the steady state. While this may in part be effected via lowered emissions, a major role is to be played by mechanisms that extract CO2 from the atmosphere and sequester it in the earth or oceans for a greater or lesser period. Oceans contain approx. 50 or more times the CO2 than does the atmosphere (Smith, 2004; MacKay, 2008), but increased dissolution of atmospheric CO2 in oceans (and lakes) leads to their further acidification, with many undesirable consequences (Sabine et al., 2004; Orr et al., 2005; Riebesell et al., 2007; Hall-Spencer et al., 2008; McNeil and Matear, 2008; Reid et al., 2009; Doney, 2010; Shi et al., 2010; Turley et al., 2010). Unless marine carbon storage could be effected in a recalcitrant form that sinks rapidly (Jiao et al., 2010; Stone, 2010; Jiao and Azam, 2011), this implies that CO2 should probably best be sequestered elsewhere if we are to have ecosystems in which the net ecosystem carbon budget (Chapin et al., 2006; Smith et al., 2010b) is accumulative.
Terrestrial and marine environments presently absorb about half the anthropogenic CO2 (Schimel et al., 2001), and soil contains at least twice the amount of carbon than is in the atmosphere (Batjes, 1996) (and three times that in vegetation) (Smith, 2004), with enormous if uncertain fluxes in both directions (Jackson et al., 1997; Post and Kwon, 2000; Meir et al., 2006; Reay et al., 2007; MacKay, 2008; Philippot et al., 2009; Prechtel et al., 2009; Bond-Lamberty and Thomson, 2010; Crevoisier et al., 2010; Eglin et al., 2010; Macías and Arbestain, 2010; Smith and Fang, 2010; Singh et al., 2010; Bastviken et al., 2011) (that are nevertheless quite small relative to the pools; Smith, 2004). Thus, increasing soil carbon in the steady state by just 15 % would lower atmospheric CO2 by 30 %, offering a huge environmental benefit. In addition, there are indications (Bellamy et al., 2005; Monson et al., 2006; Luo, 2007; Arnone et al., 2008; Bond-Lamberty and Thomson, 2010; Smith and Fang, 2010; Yvon-Durocher et al., 2010; Zhao and Running, 2010) of a positive feedback in which increases in global temperature lower the ability of present soils and other parts of the biosphere to absorb CO2, so clearly some kind of intervention is needed. This implies changes in agricultural practice (Robertson et al., 2000; Lal, 2004, 2008a, b, 2011; Sartori et al., 2006; Pretty, 2008; Smith et al., 2008; Burney et al., 2010; Follett and Reed, 2010; Smith and Olesen, 2010; Powlson et al., 2011), in an environment in which edible crop yields also need to increase substantially and sustainably (Beddington, 2010; Fedoroff et al., 2010; Godfray et al., 2010a, b; Lal, 2010c; Pretty et al., 2010; Tester and Langridge, 2010; Foresight, 2011), and where transport fuels and organic chemicals will need to come from modern (rather than fossil) photosynthesis (e.g. Bozell and Petersen, 2010; Somerville et al., 2010; Vispute et al., 2010; Whited et al., 2010; Demirbas, 2011). The purpose of this review, as summarized in Fig 1, is to develop the relevant arguments.
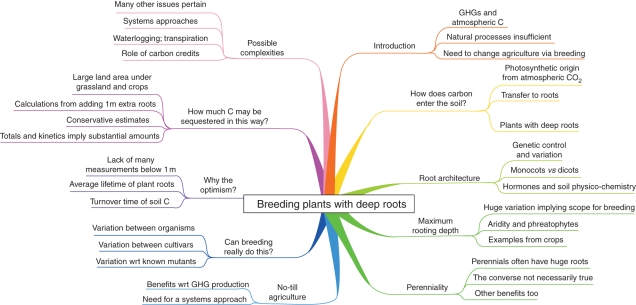
Fig 1.
A mind map (Buzan, 2002) summarizing the content of this review. To interpret this, start at the top and read clockwise.
Certainly it is recognized that the substantial (possibly 10- or even 20-fold) decreases in atmospheric CO2 over geological time, especially during the Devonian (416·0–359·2 Ma) and more gradually since the Cretaceous (145·5–65·5 Ma), have largely been effected via the production of deep-rooted trees and the rise of angiosperms, respectively (Mora et al., 1996; Berner, 1997; Berner and Kothavala, 2001; Royer et al., 2001; Taylor et al., 2009). These facts provide an important guide to what may be possible, since the kinds of decreases being needed now are rather lower (cf. Breecker et al., 2010), and the role of plants (both roots and shoots) in effecting these decreases has historically been paramount. Note too that soil production can be much slower than its erosion without intervention (Torn et al., 1997; Montgomery, 2007; Huggins and Reganold, 2008), and that roots lower erosion considerably (Gyssels et al., 2005).
The required changes in agricultural practice, plus the existence of proof that carbon was indeed once highly sequestered in plant biomass, led to the recognition that increasing the amount of below-ground biomass en route to sequestering atmospheric CO2 is a desirable goal. The purpose of this review is to point out not only that it is desirable but that it is possible, and to highlight the areas where research activities might usefully be focused.
HOW DOES CARBON ENTER THE SOIL?
Although atmospheric CO2 can of course dissolve in soil moisture, and some carbon comes from manuring (Smith et al., 2010b), these amounts are comparatively small and the chief initial method of carbon transfer to soil is via recent photosynthesis and subsequent transfer to plant roots (Jiménez and Lal, 2006; De Deyn et al., 2008; Taylor et al., 2009; Orwin et al., 2010) and thence to soil organic matter (Kögel-Knabner, 2002). The first thing to note is the huge variation in the organic (carbon) content of soils – at least 15-fold in the UK alone (Bellamy et al., 2005; Bradley et al., 2005; Ostle et al., 2009). This immediately indicates the large scope for increasing it in many places; indeed, the root content of different soils also varies at least 10-fold (Jackson et al., 1996; Schenk and Jackson, 2002a), with a large variation in the vertical distribution of carbon (Jobbágy and Jackson, 2000). The magnitude and similarity of these factors (10- and 15-fold) might be taken to imply that variation in the amounts of roots themselves (rather than their exudates and soil biota, for instance) is likely to be the major cause of the variance, but clearly all processes relevant to both incorporation and decomposition (whose difference determines net values) can contribute to this variance. Given relevant data, inferencing methods (e.g. Pearl, 2000; Rohr et al., 2008) can determine which processes drive which.
The soil ecosystem is extremely complex (e.g. Fitter et al., 2005; Nielsen et al., 2011), but a major role in sequestration of carbon secreted from roots (‘exudate’) is played by arbuscular mycorrhiza (AM) (e.g. Staddon and Fitter, 1998; Strack et al., 2003; Zhu and Miller, 2003; Peterson et al., 2004; Rillig, 2004; Parniske, 2008; Varma, 2008; Bucher et al., 2009; Lambers et al., 2009; Leigh et al., 2009; Wilson et al., 2009) that form symbioses (Helgason and Fitter, 2009) with the roots of the majority of land plants. The mycorrhizal fungi (of the genus Glomeromycota) provide nutrients, especially phosphate (Bucher, 2007), to the plants, which in turn provide up to 20 % of the carbon that they fix to the soil-dwelling fungal partners. Mycorrhiza also secrete a protein called glomalin (Gadkar and Rillig, 2006), whose extent correlates extremely well with desirable (large aggregates in) soil structure (Bedini et al., 2009; Wilson et al., 2009). The rhizosphere, as the interface between plants and soil, is clearly crucial. Roots, mycorrhiza and soil organic carbon (SOC) can all affect each other beneficially, and the interactions are complex (Feeney et al., 2006; O'Donnell et al., 2007; Gillespie et al., 2009; Hinsinger et al., 2009; Lambers et al., 2009; Luster et al., 2009). A couple of examples include the facts that the soil biota (and roots) help increase the porosity of soil (Feeney et al., 2006) and that roots both affect the physical architecture of soils and vice versa (Hinsinger et al., 2009). However, since there is no unitary explanation of which processes dominate where, for present purposes I deem it sufficient to note the role of AM in these processes, and that the breeding strategies that this article seeks to promote should take their important activities into account. Experimental approaches may need to start by studying the covariation between root architectures and mycorrhiza, en route to performing experiments in which one is changed as an independent variable.
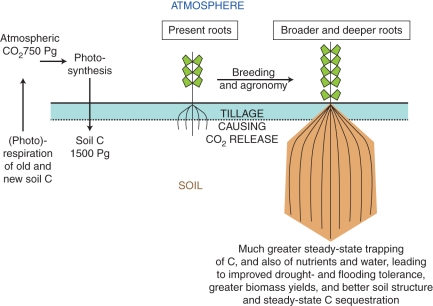
Several relevant areas of the literature are thus bound up with each other, albeit (as in most fields; e.g. Hull et al., 2008; Dobson and Kell, 2008; Kell, 2009) that they have developed independently (the balkanization of the literature into ‘silos’). Bringing them together indicates that the goal of breeding plants with extended root systems that can effect carbon, water and nutrient sequestration (Fig. 2) is not only desirable but attainable. Four particular scientific areas that pertain are root architecture and depth, perenniality and low- or no-till agriculture.
Fig. 2.
Cartoon illustration of the potential for the improvement of agricultural and ecological traits by breeding crop plants with large root systems. The root morphologies are to be considered as illustrative only, and all details of bidirectional fluxes to and from litter and the many soil carbon pools (and including leaching and erosion) are omitted for clarity. For a summary of the various terms used to describe the most important carbon fluxes and stocks see, for example, Chapin et al. (2006) and Smith et al. (2010b).
Root architecture
A number of papers and reviews describe the genetic control of root architecture (e.g. Zhang and Forde, 1998; Casimiro et al., 2003; Hu et al., 2003; Hochholdinger et al., 2004a, Swarup et al., 2005; Chaitra et al., 2006; de Dorlodot et al., 2007; Galinha et al., 2007; Courtois et al., 2009; Hochholdinger, 2009; Hochholdinger and Tuberosa, 2009; Péret et al., 2009; Benfey et al., 2010; Bennett and Scheres, 2010; Coudert et al., 2010; Iyer-Pascuzzi et al., 2010; Paschold et al., 2010; Yang et al., 2010; Yi et al., 2010; Zimmermann et al., 2010; Lucas et al., 2011). Thus, a number of root architecture genes are known via the effects of their mutations on traits such as primary root length, root branching, root hair formation, and so on, but our present knowledge of them all, and the mechanistic details by which they affect phenotype, is comparatively limited. Important features of the genetic control differ, for example (Gregory, 2006) between monocots (such as grasses, cereals and Brachypodium distachyon; Draper et al., 2001) and dicots (such as Arabidopsis thaliana; Hochholdinger et al., 2004a; Osmont et al., 2007; Watt et al., 2009; Zimmermann et al., 2010) [and interestingly B. distachyon, unlike A. thaliana, forms associations with mycorrhiza (Bevan et al., 2010)]. The very interesting ecological and evolutionary analyses that pertain (e.g. Fitter, 1987) are outside the scope of this summary, but can clearly provide very useful pointers to the breeding of plants with the desirable rooting traits that are highlighted herein. The chief of these is of course root depth.
As well as genetic means, root architecture is also controlled by hormonal influences from both the host plant (e.g. Tanimoto, 2005; Santner et al., 2009) and soil organisms (see above), and to some degree by the physico-chemical environment (e.g. Fitter and Stickland, 1991; Cahill et al., 2010). Our focus here, however, is on the genetic control, which seems to be dominant (Kato et al., 2006).
Maximum rooting depth
There is considerable variation between both plant types and individual plant strains (cultivars) as to the maximum depth to which they are known to produce roots, but 2 m for angiosperms (and much more for trees) is not at all uncommon (Stone and Kalisz, 1991; Canadell et al., 1996; Jackson et al., 1996; Schenk and Jackson, 2002a, b; Hu et al., 2003), implying equally that there is considerable scope for increasing the depth of roots by appropriate breeding strategies. A chief point to note is that most presently cultivated agricultural crops have root depths that indeed do not extend much beyond 1 m, albeit that a number do (Kristensen and Thorup-Kristensen, 2004; Kutschera et al., 2009), such that this implies that there is indeed exceptional scope to breed this trait. (We recognize of course that many modern grains have been bred to have short stems, and with little or no attention being directed specifically to their roots.)
Root length is also typically a function of aridity (Canadell et al., 1996; Schenk and Jackson, 2002a, 2005). Some very long-rooted plants, common in arid zones, are known as phreatophytes (Pataki et al., 2008), although this term relates more to the fact that they obtain their water from deep sources. Root water dynamics in soil seem not to be as well understood as one would wish, as many mechanisms contribute, even to its sign (Burgess and Bleby, 2006 ) (i.e. whether plants add water to the soil or extract it from it), a phenomenon known as hydraulic redistribution (Burgess et al., 1998). As well as the benefits to carbon sequestration, there is evidence supporting the role of roots in improving soil structure (Gregory et al., 2010), on improving hydrology (Macleod et al., 2007) and in showing that SOC improves agronomic productivity (Lal, 2010b). Some genes [or at least quantitative trait loci (QTLs)] improve both root architecture and plant yield (Passioura, 1983, 2006; Tuberosa et al., 2002; Steele et al., 2006, 2007; Hund et al., 2007), and there are a number of examples of crops in which the below-ground biomass does contribute significantly to SOC (sequestration), including plants such as Andropogon gayunus (Fisher et al., 1994), Miscanthus × giganteus (Clifton-Brown et al., 2007; Heaton et al., 2008; Dohleman and Long, 2009; Dondini et al., 2009a, b; Hillier et al., 2009), Panicum virgatum (switchgrass) (Ma et al., 2000, 2001; Liebig et al., 2005; Al-Kaisi and Grote, 2007; Anderson-Teixeira et al., 2009; Collins et al., 2010) and vetiver (Chrysopogon zizanoides L.) (Grimshaw, 2008; Lavania and Lavania, 2009) grasses, and even sugar cane (Otto et al., 2009; Galdos et al., 2010). At least five widely cultivated crop plants can produce roots exceeding 2 m (Kutschera et al., 2009).
Perenniality
Perenniality, the use of crops that produce edible parts such as grains (seeds) without annual sowing (and ploughing), has been championed as an especially valuable idea for consideration, and this coheres significantly with the present theme. This is not least because such perennials typically develop considerably longer roots than do modern domesticated annual crops (Cox et al., 2002, 2006; DeHaan et al., 2005; Glover et al., 2007, 2010; Dohleman and Long, 2009; DuPont et al., 2010; Van Tassel et al., 2010). Such perennials are also known to exhibit hugely decreased nitrate runoff (Randall and Mulla, 2001) and, importantly, to sequester much more carbon in soil (Robertson et al., 2000; Kardol and Wardle, 2010). The extent to which perenniality and these large root architectures can be decoupled, and whether and when this is desirable for agronomic purposes, remain uncertain, though at least some flowering time genes that contribute to perenniality seem to be conserved between monocots and dicots (Wang et al., 2009; Higgins et al., 2010), in a way that root architecture is not (see above). Consequently, it would seem that perenniality – though probably helpful – is not a necessary accompaniment to crop plants with deep roots.
No-till agriculture
Ploughing releases SOC and, in a similar vein, no-till agriculture (that may also be used with perennial crops) assists carbon sequestration and decreases soil erosion (e.g. Paustian et al., 2000; West and Post, 2002; Sainju et al., 2003; Lal et al., 2004; Bernacchi et al., 2005; Amado et al., 2006; Montgomery, 2007; Huggins and Reganold, 2008; Villamil et al., 2008; López-Bellido et al., 2010), although tillage of surface layers that do not disturb deeper roots becomes at least partially a no-till process (see also Fig. 2). This said, though, it is important to analyse the entire system of GHG production to assess the detailed benefits of a more widespread no-till strategy (Robertson et al., 2000; Six et al., 2002; Grandy et al., 2006; Steinbach and Alvarez, 2006).
CAN BREEDING REALLY DO THIS?
There are, of course, many examples (e.g. Lippman and Tanksley, 2001; Hill, 2005; Edgerton, 2009; Johansson et al., 2010) that show the huge variation in phenotype achievable in agricultural breeding populations, and this is being stimulated further by techniques such as marker-assisted selection and genome-driven breeding (e.g. Moreau et al., 2000; Meuwissen et al., 2001; Eathington et al., 2007; Collard and Mackill, 2008; Utomo and Linscombe, 2009; Kean, 2010; Meuwissen and Goddard, 2010). Nonetheless, it might be argued that the role of genetics or plant breeding in increasing root depth is likely to be negligible, and that (leaving aside soils with rock strata just below the surface, where this might be true) the depth of roots is governed entirely by the physico-chemical properties of the soil, and not at all by the genetics of the host (or soil organisms). The experimental facts are against this (Doussan et al., 2003; Kato et al., 2006), and a number of simple gene-based arguments show that this is not the case.
Plant root depths vary greatly in the same soil for different organisms (e.g. Burch and Johns, 1978; Jackson et al., 1996; Jobbágy and Jackson, 2000).
Plant root depths vary substantially in the same soils or growth media for different cultivars of the same plant (e.g. O'Toole and Bland, 1987; Lilley and Fukai, 1994; Champoux et al., 1995; Fukai and Cooper, 1995; Price et al., 1997, 2002a, b; Angadi and Entz, 2002; Bonos et al., 2004; Løes and Gahoonia, 2004; Chloupek et al., 2006; Kato et al., 2006; Devaiah et al., 2007; Hund et al., 2007, 2009, 2011; Sanguineti et al., 2007; Kamoshita et al., 2008; Karcher et al., 2008; Crush et al., 2009, 2010; Gregory et al., 2009; Hargreaves et al., 2009; Kutschera et al., 2009; Trachsel et al., 2009, 2011; Ao et al., 2010; Iyer-Pascuzzi et al., 2010; Obara et al., 2010; Tuberosa et al., 2010; Bayuelo-Jiménez et al., 2011).
Plant root depths can vary substantially between different mutants (in known genes) of the same parent (e.g. Zhang and Forde, 1998; Casimiro et al., 2003; Hochholdinger et al., 2004a, b; Hochholdinger, 2009; Hochholdinger and Tuberosa, 2009; Rebouillat et al., 2009; Benfey et al., 2010; Coudert et al., 2010).
Thus any claim that it is impossible to pursue the ‘deep roots’ agenda using plant breeding methods is without merit.
Why the optimism?
It is certainly the case that a number of experts have given a slightly less optimistic view of the potential of land use changes to improve carbon sequestration (Smith, 2004; van Kessel et al., 2006; Soussana and Luscher, 2007; Ciais et al., 2010; Smith et al., 2010a) (but see Lal, 2010a). However, this seems to be based in part on the present use of comparatively shallow-rooted plants that in some regions may indeed have approached the possible saturation of carbon sequestration. A particular issue is that most studies do not make soil measurements much below a metre (Nepstad et al., 1994; Batjes, 1996; Canadell et al., 1996; Jobbágy and Jackson, 2000; Guo and Gifford, 2002; Schenk and Jackson, 2002a; Robinson, 2004; Bradley et al., 2005; Lorenz and Lal, 2005; Mokany et al., 2006; Ichii et al., 2009; Qin and Huang, 2010; Wang et al., 2010), and the kinds of root depths we are looking at here would more than double that. This doubling of root biomass from a nominal 1 m to a nominal 2 m is really the key issue, together with the longevity of the roots and carbon they secrete and sequester below-ground (a complete turnover annually, including of stover in no-till systems, obviously gives no net steady-state sequestration).
The turnover rate or time is an especially important measure here. However, data on the longevity of soil roots and the (other) pools of carbon that are obtained therefrom in the soil (Zimmermann et al., 2007; Smith et al., 2010b) (let alone their variation with depth, soil type, vegetation type, etc.) are both uncertain and not very easy to come by (Baggs, 2006; Gregory, 2006; Kuzyakov, 2006; Koerber et al., 2010; Sanderman and Baldock, 2010), but Gill and Jackson (2000) indicate a loss of 40 % per year in temperate grasslands (i.e. a ‘linear’ lifetime of 2·5 years), with a greater decay as temperature increases, while Högberg and Read (2006) summarize some of the evidence for the increasing recognition that roots in soil are more long-lived than previously credited (see also Collins et al., 2010), and there is increasing evidence for the role of physical protection (occlusion/aggregation) in improving carbon retention (e.g. Krull et al., 2003; von Lützow et al., 2006; Jastrow et al., 2007; McCarthy et al., 2008; Virto et al., 2008, 2010; Moni et al., 2010). The residence time of more refractory forms of SOC, albeit derived originally from manure or photosynthate, may be considerably longer (Bull et al., 2000; Paustian et al., 2000), and isotopic methods (e.g. Dungait et al., 2008, 2009, 2010; Rubino et al., 2010; Smith et al., 2010b) have an important role to play in the analysis of the turnover of carbon-containing soil components and their biomarkers. Biochars (e.g. Lehmann, 2007; Atkinson et al., 2010; Sohi et al., 2010) are seen as especially recalcitrant. Clearly the rate of degradation is controlled by at least two classes of factor, the rate of biochemical alteration and the extent of physico-chemical protection (Jastrow et al., 2007), and these vary among different substances. The rate of biochemical alteration of a molecule (related to its recalcitrance), and the eventual loss of carbon as CO2, also depends on what enzymes and organisms are present that are able to degrade it under the relevant conditions (e.g. of pH, oxygen tension, etc.) (Jastrow et al., 2007). Without going into the specific chemical details, there are obvious relationships between all of these and the overall ability to sequester carbon in various forms. How to ensure that deep root carbon is more recalcitrant when we know which molecules are the most recalcitrant, or have other properties desirable for building soil structure, is another goal of the breeding process.
HOW MUCH CARBON MAY BE SEQUESTERED IN THIS WAY?
It would be desirable to give a precise, quantitative answer to this question, but it is affected by so many variables that the possible range is quite large; these variables include the baseline carbon content, photosynthetic yields, microbial and other respiratory activity, root turnover, soil biophysics and aridity, soil aggregate water stability and repellency, and so on, and so we suffice with an approximation. (No attempt is made to discriminate the many known pools of soil carbon.) The key issues are the amount that can be sequestered (whether as roots or as other forms of SOC) per year, and the lifetime of the carbon so sequestered before it is eventually re-respired to the atmosphere. Most of the estimates for the carbon sequestration potential range from about 0·3 to 0·8 tC ha−1 year−1 (Smith, 2004), but some estimates are well outside (especially above) this range. The point, though, is that what matters is not so much what is happening now as what might be achieved with suitable breeding of plants with deep (and reasonably long-lived) roots. Increasing root mass by an extra 1 m depth with a very modest carbon content of just 1 % carbon by volume of overall soil mass equates (assuming a relative density of 1) to 10 kg m−2 (100 t ha−1), or on average 5 kg m−2 (50 t ha−1) if it turns over every 2 years. Lal (2004) indicates that some cultivated soils have lost one-half to two-thirds of their original SOC pool, with a cumulative loss of 30–40 Mg C ha−1 (i.e. 30–40 t ha−1), implying that these levels are a minimum that can be sequestered (since they once were), so the 50 t ha−1 number seems both conservative and reasonable. Some analyses of existing grasslands and energy crops imply that at least 100 t ha−1 of steady-state carbon sequestration in roots is routinely attainable (Dondini et al., 2009a; Silver et al., 2010) [forests typically sequester even more (Malhi et al., 1999)], with gross global primary production exceeding 100 Pg year−1 (Beer et al., 2010).
The carbon being produced from fossil fuel burning is some 8·4 Gt year−1 (MacKay, 2008), and so to mop this up at the rate of 50 t ha−1 some 1·6 × 108 ha or 1·6 × 106 km2 would be required. This compares with some 41·4 × 106 km2 (Bot et al., 2000) of just rainfed arable land, and 130·56. 106 km2 of total land area excluding polar regions (http://www.worldmapper.org/). Thus, one thing is clear: doubling the steady-state depth of roots from approx. 1 m to 2 m can have a significantly beneficial impact on lowering the levels of atmospheric CO2. Since the calculations at this level of granularity are straightforward (without spatial analysis or details of economics, infrastructure issues, transitional arrangements, the time required to breed the appropriate crops and to bulk them up and to disseminate the necessary germplasm, and so on), we have made them available at http://dbkgroup.org/carbonsequestration/rootsystem.html. Default values include the facts that there are 2300 Mha of cropland and a similar amount of grassland (rangeland) [and note the comparatively recent loss of an additional approx. 20 % of agricultural land (Campbell et al., 2008; Somerville et al., 2010)], the carbon in the atmosphere (essentially as CO2) is some 750–821 Pg (approx. 385 ppm by volume) while that in the soil is approx. 1500 Pg, and that the relative density of soil is that of water, i.e. 1. Calculations based on this imply that an extra 2 % of carbon occupying an extra 1 m depth over these areas = 20 kg m−2 = 200 t ha−1 (or, simplistically ignoring any feedbacks, 100 t ha−1 fixed on average in the steady state if the lifetime of the average ‘carbon’ held in different molecules is 2 years).
POSSIBLE COMPLEXITIES
This short overview has concentrated on breeding plants with deep (and bushy) roots per se, but I recognize that it is necessary to take a full systems approach. For instance, I have not discussed in any detail the interactions of roots with soil micro-organisms and other invertebrates. In addition, one would wish to check details of the consequences of the biochemical turnover of the deeper roots of plants (especially if waterlogged and anaerobic), lest they produce methane or nitrous oxide (Philippot et al., 2009), GHGs far more potent than CO2 (Soussana et al., 2007). Other aspects of plant breeding for carbon sequestration may interact positively or negatively with, or may be decoupled from, agricultural outputs such as useful (i.e. agriculturally productive) yield. Thus an improved opening of stomata, that might assist CO2 uptake, may also lead to greater transpirational losses. The economics of agriculture-based carbon sequestration will also be affected significantly by any carbon credits that may be applied (Smith et al., 2008; MacLeod et al., 2010; Smith and Olesen, 2010; Lal, 2011).
CONCLUDING REMARKS
In this brief commentary, I have sought to draw attention to the potentially substantial benefits that are to be had from breeding and growing crops with very extensive root systems. The analysis differs explicitly from the more common analysis of what pertains now as it seeks to understand what might be done by explicit human breeding of the necessary crops. In addition to the simple carbon sequestration that these imply – possibly double that of common annual grain crops – such crops seem to mobilize and retain nutrients and water very effectively over extended periods, thus providing resistance to drought (e.g. Burch and Johns, 1978; Passioura, 1983, 2006; Ekanayake et al., 1985; Champoux et al., 1995; Price et al., 2002a; Kato et al., 2006; Kirkegaard et al., 2007; Bernier et al., 2008; Kamoshita et al., 2008; Karcher et al., 2008; Cairns et al., 2009; Hund et al., 2009; McKenzie et al., 2009), flooding and other consequences of climate change, as well as to fertilizer runoff. In addition, the development of plants with deep roots may in fact stimulate photosynthetic yields as these are considered to be more controlled by the carbon sinks of plants (e.g. Zhu et al., 2010) [demand typically being considerably more controlling than supply when one is seeking to increase biotechnological fluxes (Cornish-Bowden et al., 1995; Hofmeyr and Cornish-Bowden, 2000)]. The production (by breeding) of deep roots in some cultivars will undoubtedly be at the expense of above-ground biomass yields, but there is no evidence that it has to be so (e.g. Fisher et al., 1994). Thus, the research agenda is clear: we need to learn much more about those genes that control root development as part of whole plant development, the interactions of various roots with soil and soil organisms, and the actual benefits of net carbon, nutrient and water sequestration that can be effected by such crops under various agronomic conditions. This is likely to include the requirement to develop novel instrumentation (and algorithms) to measure root and other phenotypes (e.g. Nadezhdina and Čermák, 2003; Granier et al., 2006; French et al., 2009; Gregory et al., 2009; Iyer-Pascuzzi et al., 2010; Wielopolski et al., 2010), as well as the informatics necessary (e.g. Jenkins et al., 2004) for storing and making available such data, including the anticipated flood of genomics data. While there is a way to go before such crops might have, for example, the grain yields of present day cereals, their breeding and deployment seems a very promising avenue for sustainable agriculture.
ACKNOWLEDGEMENTS
I thank a considerable number of individuals (there are too many to name you all) for guiding my thinking here, and Steve O'Hagan for setting up the web page at http://dbkgroup.org/carbonsequestration/rootsystem.html. I thank referees for some useful suggestions.
LITERATURE CITED
- Al-Kaisi MM, Grote JB. Cropping systems effects on improving soil carbon stocks of exposed subsoil. Soil Science Society of America Journal. 2007;71:1381–1388. [Google Scholar]
- Amado TJ, Bayer C, Conceicao PC, Spagnollo E, de Campos BH, da Veiga M. Potential of carbon accumulation in no-till soils with intensive use and cover crops in southern Brazil. Journal of Environmental Quality. 2006;35:1599–1607. doi: 10.2134/jeq2005.0233. [DOI] [PubMed] [Google Scholar]
- Anderson-Teixeira KJ, Davis SC, Masters MD, Delucia EH. Changes in soil organic carbon under biofuel crops. Global Change Biology Bioenergy. 2009;1:75–96. [Google Scholar]
- Angadi SV, Entz MH. Root system and water use patterns of different height sunflower cultivars. Agronomy Journal. 2002;94:136–145. [Google Scholar]
- Ao JH, Fu JB, Tian J, Yan XL, Liao H. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Functional Plant Biology. 2010;37:304–312. [Google Scholar]
- Arnone JA, 3rd, Verburg PS, Johnson DW, et al. Prolonged suppression of ecosystem carbon dioxide uptake after an anomalously warm year. Nature. 2008;455:383–386. doi: 10.1038/nature07296. [DOI] [PubMed] [Google Scholar]
- Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant and Soil. 2010;337:1–18. [Google Scholar]
- Baggs EM. Partitioning the components of soil respiration: a research challenge. Plant and Soil. 2006;284:1–5. [Google Scholar]
- Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. Freshwater methane emissions offset the continental carbon sink. Science. 2011;331:50. doi: 10.1126/science.1196808. [DOI] [PubMed] [Google Scholar]
- Batjes NH. Total carbon and nitrogen in the soils of the world. European Journal of Soil Science. 1996;47:151–163. [Google Scholar]
- Bayuelo-Jiménez JS, Gallardo-Valdéz M, Pérez-Decelis VA, Magdaleno-Armas L, Ochoa I, Lynch JP. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Research. 2011;121:350–362. [Google Scholar]
- Beddington J. Food security: contributions from science to a new and greener revolution. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:61–71. doi: 10.1098/rstb.2009.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedini S, Pellegrino E, Avio L, et al. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biology and Biochemistry. 2009;41:1491–1496. [Google Scholar]
- Beer C, Reichstein M, Tomelleri E, et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science. 2010;329:834–838. doi: 10.1126/science.1184984. [DOI] [PubMed] [Google Scholar]
- Bellamy PH, Loveland PJ, Bradley RI, Lark RM, Kirk GJ. Carbon losses from all soils across England and Wales 1978–2003. Nature. 2005;437:245–248. doi: 10.1038/nature04038. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Bennett M, Schiefelbein J. Getting to the root of plant biology: impact of the Arabidopsis genome sequence on root research. The Plant Journal. 2010;61:992–1000. doi: 10.1111/j.1365-313X.2010.04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Scheres B. Root development – two meristems for the price of one? Plant Development. 2010;91:67–102. doi: 10.1016/S0070-2153(10)91003-X. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Hollinger SE, Meyers T. The conversion of the corn/soybean ecosystem to no-till agriculture may result in a carbon sink. Global Change Biology. 2005;11:1867–1872. [Google Scholar]
- Berner RA. Paleoclimate – the rise of plants and their effect on weathering and atmospheric CO2. Science. 1997;276:544–546. [Google Scholar]
- Berner RA, Kothavala Z. GEOCARB III: a revised model of atmospheric CO2 over phanerozoic time. American Journal of Science. 2001;301:182–204. [Google Scholar]
- Bernier J, Atlin GN, Serraj R, Kumar A, Spaner D. Breeding upland rice for drought resistance. Journal of the Science of Food and Agriculture. 2008;88:927–939. [Google Scholar]
- Bevan MW, Garvin DF, Vogel JP. Brachypodium distachyon genomics for sustainable food and fuel production. Current Opinion in Biotechnology. 2010;21:211–217. doi: 10.1016/j.copbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Bond-Lamberty B, Thomson A. Temperature-associated increases in the global soil respiration record. Nature. 2010;464:579–582. doi: 10.1038/nature08930. [DOI] [PubMed] [Google Scholar]
- Bonos SA, Rush D, Hignight K, Meyer WA. Selection for deep root production in tall fescue and perennial ryegrass. Crop Science. 2004;44:1770–1775. [Google Scholar]
- Bot AJ, Nachtergaele FO, Young A. Land resource potential and constraints at regional and country levels. 2000 Rome, FAO. ftp://ftp.fao.org/agl/agll/docs/wsr.pdf. [Google Scholar]
- Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates – the US Department of Energy's ‘Top 10’ revisited. Green Chemistry. 2010;12:539–554. [Google Scholar]
- Bradley RI, Milne R, Bell J, Lilly A, Jordan C, Higgins A. A soil carbon and land use database for the United Kingdom. Soil Use and Management. 2005;21:363–369. [Google Scholar]
- Breecker DO, Sharp ZD, McFadden LD. Atmospheric CO2 concentrations during ancient greenhouse climates were similar to those predicted for A.D. 2100. Proceedings of the National Academy of Sciences, USA. 2010;107:576–580. doi: 10.1073/pnas.0902323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytologist. 2007;173:11–26. doi: 10.1111/j.1469-8137.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- Bucher M, Wegmüller S, Drissner D. Chasing the structures of small molecules in arbuscular mycorrhizal signaling. Current Opinion in Plant Biology. 2009;12:500–507. doi: 10.1016/j.pbi.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Bull ID, van Bergen PF, Nott CJ, Poulton PR, Evershed RP. Organic geochemical studies of soils from the Rothamsted classical experiments – V. The fate of lipids in different long-term experiments. Organic Geochemistry. 2000;31:389–408. [Google Scholar]
- Burch GJ, Johns GG. Root absorption of water and physiological responses to water deficits by Festuca arundinacea Schreb. and Trifolim repens L. Australian Journal of Plant Physiology. 1978;5:859–871. [Google Scholar]
- Burgess SSO, Bleby TM. Redistribution of soil water by lateral roots mediated by stem tissues. Journal of Experimental Botany. 2006;57:3283–3291. doi: 10.1093/jxb/erl085. [DOI] [PubMed] [Google Scholar]
- Burgess SSO, Adams MA, Turner NC, Ong CK. The redistribution of soil water by tree root systems. Oecologia. 1998;115:306–311. doi: 10.1007/s004420050521. [DOI] [PubMed] [Google Scholar]
- Burney JA, Davis SJ, Lobell DB. Greenhouse gas mitigation by agricultural intensification. Proceedings of the National Academy of Sciences, USA. 2010;107:12052–12057. doi: 10.1073/pnas.0914216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzan T. How to mind map. London: Thorsons; 2002. [Google Scholar]
- Cahill JF, Jr, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St Clair CC. Plants integrate information about nutrients and neighbors. Science. 2010;328:1657. doi: 10.1126/science.1189736. [DOI] [PubMed] [Google Scholar]
- Cairns JE, Audebert A, Mullins CE, Price AH. Mapping quantitative trait loci associated with root growth in upland rice (Oryza sativa L.) exposed to soil water-deficit in fields with contrasting soil properties. Field Crops Research. 2009;114:108–118. [Google Scholar]
- Campbell JE, Lobell DB, Genova RC, Field CB. The global potential of bioenergy on abandoned agriculture lands. Environmental Science and Technology. 2008;42:5791–5794. doi: 10.1021/es800052w. [DOI] [PubMed] [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, et al. Dissecting Arabidopsis lateral root development. Trends in Plant Science. 2003;8:165–171. doi: 10.1016/S1360-1385(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Chaitra J, Vinod MS, Sharma N, Hittalmani S, Shashidhar HE. Validation of markers linked to maximum root length in rice (Oryza sativa L.) Current Science. 2006;90:835–838. [Google Scholar]
- Champoux MC, Wang G, Sarkarung S, et al. Locating genes associated with root morphology and drought avoidance in rice via linkage to molecular markers. Theoretical and Applied Genetics. 1995;90:969–981. doi: 10.1007/BF00222910. [DOI] [PubMed] [Google Scholar]
- Chapin FS, Woodwell GM, Randerson JT, et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems. 2006;9:1041–1050. [Google Scholar]
- Chloupek O, Forster BP, Thomas WTB. The effect of semi-dwarf genes on root system size in field-grown barley. Theoretical and Applied Genetics. 2006;112:779–786. doi: 10.1007/s00122-005-0147-4. [DOI] [PubMed] [Google Scholar]
- Ciais P, Wattenbach M, Vuichard N, et al. The European carbon balance. Part 2: croplands. Global Change Biology. 2010;16:1409–1428. [Google Scholar]
- Clifton-Brown JC, Breuer J, Jones MB. Carbon mitigation by the energy crop. Miscanthus. Global Change Biology. 2007;13:2296–2307. [Google Scholar]
- Collard BC, Mackill DJ. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:557–572. doi: 10.1098/rstb.2007.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins HP, Smith JL, Fransen S, Alva AK, Kruger CE, Granatstein DM. Carbon sequestration under irrigated switchgrass (Panicum virgatum L.) production. Soil Science Society of America Journal. 2010;74:2049–2058. [Google Scholar]
- Cornish-Bowden A, Hofmeyr J-HS, Cárdenas ML. Strategies for manipulating metabolic fluxes in biotechnology. Bioorganic Chemistry. 1995;23:439–449. [Google Scholar]
- Coudert Y, Perin C, Courtois B, Khong NG, Gantet P. Genetic control of root development in rice, the model cereal. Trends in Plant Science. 2010;15:219–226. doi: 10.1016/j.tplants.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Courtois B, Ahmadi N, Khowaja F, et al. Rice root genetic architecture: meta-analysis from a drought QTL database. Rice. 2009;2:115–128. [Google Scholar]
- Cox TS, Bender M, Picone C, et al. Breeding perennial grain crops. Critical Reviews in Plant Sciences. 2002;21:59–91. [Google Scholar]
- Cox TS, Glover JD, Van Tassel DL, Cox CM, DeHaan LR. Prospects for developing perennial-grain crops. Bioscience. 2006;56:649–659. [Google Scholar]
- Crevoisier C, Sweeney C, Gloor M, Sarmiento JL, Tans PP. Regional US carbon sinks from three-dimensional atmospheric CO2 sampling. Proceedings of the National Academy of Sciences, USA. 2010;107:18348–18353. doi: 10.1073/pnas.0900062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crush JR, Nichols SN, Easton HS, Ouyang L, Hume DE. Comparisons between wild populations and bred perennial ryegrasses for root growth and root/shoot partitioning. New Zealand Journal of Agricultural Research. 2009;52:161–169. [Google Scholar]
- Crush JR, Nichols SN, Ouyang L. Adventitious root mass distribution in progeny of four perennial ryegrass (Lolium perenne L.) groups selected for root shape. New Zealand Journal of Agricultural Research. 2010;53:193–200. [Google Scholar]
- De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- DeHaan LR, Van Tassel DL, Cox TS. Perennial grain crops: a synthesis of ecology and plant breeding. Renewable Agriculture and Food Systems. 2005;20:5–14. [Google Scholar]
- Demirbas A. Competitive liquid biofuels from biomass. Applied Energy. 2011;88:17–28. [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG. Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiology. 2007;145:147–159. doi: 10.1104/pp.107.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nature Reviews Drug Discovery. 2008;7:205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- Dohleman FG, Long SP. More productive than maize in the Midwest: how does Miscanthus do it? Plant Physiology. 2009;150:2104–2115. doi: 10.1104/pp.109.139162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondini M, Hastings A, Saiz G, Jones MB, Smith P. The potential of Miscanthus to sequester carbon in soils: comparing field measurements in Carlow, Ireland to model predictions. Global Change Biology Bioenergy. 2009a;1:413–425. [Google Scholar]
- Dondini M, Van Groenigen KJ, Del Galdo I, Jones MB. Carbon sequestration under Miscanthus: a study of C-13 distribution in soil aggregates. Global Change Biology Bioenergy. 2009b;1:321–330. [Google Scholar]
- Doney SC. The growing human footprint on coastal and open-ocean biogeochemistry. Science. 2010;328:1512–1516. doi: 10.1126/science.1185198. [DOI] [PubMed] [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science. 2007;12:474–481. doi: 10.1016/j.tplants.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Doussan C, Pages L, Pierret A. Soil exploration and resource acquisition by plant roots: an architectural and modelling point of view. Agronomie. 2003;23:419–431. [Google Scholar]
- Draper J, Mur LA, Jenkins G, et al. Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiology. 2001;127:1539–1555. [PMC free article] [PubMed] [Google Scholar]
- Dungait JAJ, Stear NA, van Dongen BE, Bol R, Evershed RP. Off-line pyrolysis and compound-specific stable carbon isotope analysis of lignin moieties: a new method for determining the fate of lignin residues in soil. Rapid Communications in Mass Spectrometry. 2008;22:1631–1639. doi: 10.1002/rcm.3454. [DOI] [PubMed] [Google Scholar]
- Dungait JAJ, Bol R, Bull ID, Evershed RP. Tracking the fate of dung-derived carbohydrates in a temperate grassland soil using compound-specific stable isotope analysis. Organic Geochemistry. 2009;40:1210–1218. [Google Scholar]
- Dungait JAJ, Bol R, Lopez-Capel E, et al. Applications of stable isotope ratio mass spectrometry in cattle dung carbon cycling studies. Rapid Communications in Mass Spectrometry. 2010;24:495–500. doi: 10.1002/rcm.4332. [DOI] [PubMed] [Google Scholar]
- DuPont ST, Culman SW, Ferris H, Buckley DH, Glover JD. No-tillage conversion of harvested perennial grassland to annual cropland reduces root biomass, decreases active carbon stocks, and impacts soil biota. Agriculture, Ecosystems and Environment. 2010;137:25–32. [Google Scholar]
- Eathington SR, Crosbie TM, Edwards MD, Reiter RS, Bull JK. Molecular markers in a commercial breeding program. Crop Science. 2007;47:S154–S163. [Google Scholar]
- Edgerton MD. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiology. 2009;149:7–13. doi: 10.1104/pp.108.130195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglin T, Ciais P, Piao SL, et al. Historical and future perspectives of global soil carbon response to climate and land-use changes. Tellus Series B-Chemical and Physical Meteorology. 2010;62:700–718. [Google Scholar]
- Ekanayake IJ, Otoole JC, Garrity DP, Masajo TM. Inheritance of root characters and their relations to drought resistance in rice. Crop Science. 1985;25:927–933. [Google Scholar]
- Fedoroff NV, Battisti DS, Beachy RN, et al. Radically rethinking agriculture for the 21st century. Science. 2010;327:833–834. doi: 10.1126/science.1186834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney DS, Crawford JW, Daniell T, et al. Three-dimensional microorganization of the soil–root–microbe system. Microbial Ecology. 2006;52:151–158. doi: 10.1007/s00248-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Fisher MJ, Rao IM, Ayarza MA, et al. Carbon storage by introduced deep-rooted grasses in the South American savannas. Nature. 1994;371:236–238. [Google Scholar]
- Fitter AH. An architectural approach to the comparative ecology of plant root systems. New Phytologist. 1987;106:61–77. [Google Scholar]
- Fitter AH, Stickland TR. Architectural analysis of plant root systems. 2. Influence of nutrient supply on architecture in contrasting plant species. New Phytologist. 1991;118:383–389. [Google Scholar]
- Fitter AH, Gilligan CA, Hollingworth K, Kleczkowski A, Twyman RM, Pitchford JW NERC Soil Biodiversity Programme. Biodiversity and ecosystem function in soil. Functional Ecology. 2005;19:369–377. [Google Scholar]
- Follett RF, Reed DA. Soil carbon sequestration in grazing lands: societal benefits and policy implications. Rangeland Ecology and Management. 2010;63:4–15. [Google Scholar]
- Foresight. The future of food and farming: final project report. London: Government Office for Science; 2011. [Google Scholar]
- French A, Ubeda-Tomas S, Holman TJ, Bennett MJ, Pridmore T. High-throughput quantification of root growth using a novel image-analysis tool. Plant Physiology. 2009;150:1784–1795. doi: 10.1104/pp.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai S, Cooper M. Development of drought-resistant cultivars using physio-morphological traits in rice. Field Crops Research. 1995;40:67–86. [Google Scholar]
- Gadkar V, Rillig MC. The arbuscular mycorrhizal fungal protein glomalin is a putative homolog of heat shock protein 60. FEMS Microbiology Letters. 2006;263:93–101. doi: 10.1111/j.1574-6968.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Galdos MV, Cerri CC, Lal R, Bernoux M, Feigl B, Cerri CEP. Net greenhouse gas fluxes in Brazilian ethanol production systems. Global Change Biology Bioenergy. 2010;2:37–44. [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- Gill RA, Jackson RB. Global patterns of root turnover for terrestrial ecosystems. New Phytologist. 2000;147:13–31. [Google Scholar]
- Gillespie AW, Walley FL, Farrell RE, et al. Profiling rhizosphere chemistry: evidence from carbon and nitrogen K-edge XANES and pyrolysis-FIMS. Soil Science Society of America Journal. 2009;73:2002–2012. [Google Scholar]
- Glover JD, Cox CM, Reganold JP. Future farming: a return to roots? Scientific American. 2007;297:82–89. doi: 10.1038/scientificamerican0807-82. [DOI] [PubMed] [Google Scholar]
- Glover JD, Reganold JP, Bell LW, et al. Increased food and ecosystem security via perennial grains. Science. 2010;328:1638–1639. doi: 10.1126/science.1188761. [DOI] [PubMed] [Google Scholar]
- Godfray HC, Beddington JR, Crute IR, et al. Food security: the challenge of feeding 9 billion people. Science. 2010a;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Godfray HCJ, Crute IR, Haddad L, et al. The future of the global food system. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010b;365:2769–2777. doi: 10.1098/rstb.2010.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy AS, Loecke TD, Parr S, Robertson GP. Long-term trends in nitrous oxide emissions, soil nitrogen, and crop yields of till and no-till cropping systems. Journal of Environmental Quality. 2006;35:1487–1495. doi: 10.2134/jeq2005.0166. [DOI] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, et al. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytologist. 2006;169:623–635. doi: 10.1111/j.1469-8137.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- Gregory AS, Webster CP, Watts CW, et al. Soil management and grass species effects on the hydraulic properties of shrinking soils. Soil Science Society of America Journal. 2010;74:753–761. [Google Scholar]
- Gregory PJ. Plant roots: growth, activity and interaction with soils. Oxford: Blackwell; 2006. [Google Scholar]
- Gregory PJ, Bengough AG, Grinev D, et al. Root phenomics of crops: opportunities and challenges. Functional Plant Biology. 2009;36:922–929. doi: 10.1071/FP09150. [DOI] [PubMed] [Google Scholar]
- Grimshaw RG. Introduction and review: introducing the vetiver system, vetiver networking, agricultural applications, and future uses for energy/fuel and carbon sequestration. In: Truong P, editor. Proceedings of the 1st Indian National Vetiver Workshop. San Antonio, TX: The Vetiver Network International; 2008. pp. 4–22. [Google Scholar]
- Guo LB, Gifford RM. Soil carbon stocks and land use change: a meta analysis. Global Change Biology. 2002;8:345–360. [Google Scholar]
- Gyssels G, Poesen J, Bochet E, Li Y. Impact of plant roots on the resistance of soils to erosion by water: a review. Progress in Physical Geography. 2005;29:189–217. [Google Scholar]
- Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 2008;454:96–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- Hargreaves CE, Gregory PJ, Bengough AG. Measuring root traits in barley (Hordeum vulgare ssp vulgare and ssp spontaneum) seedlings using gel chambers, soil sacs and X-ray microtomography. Plant and Soil. 2009;316:285–297. [Google Scholar]
- Heaton EA, Dohleman FG, Long SP. Meeting US biofuel goals with less land: the potential of Miscanthus. Global Change Biology. 2008;14:2000–2014. [Google Scholar]
- Helgason T, Fitter AH. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota) Journal of Experimental Botany. 2009;60:2465–2480. doi: 10.1093/jxb/erp144. [DOI] [PubMed] [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA. Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS One. 2010;5:e10065. doi: 10.1371/journal.pone.0010065. doi:10.1371/journal.pone.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WG. A century of corn selection. Science. 2005;307:683–684. doi: 10.1126/science.1105459. [DOI] [PubMed] [Google Scholar]
- Hillier J, Whittaker C, Dailey G, et al. Greenhouse gas emissions from four bioenergy crops in England and Wales: integrating spatial estimates of yield and soil carbon balance in life cycle analyses. GCB Bioenergy. 2009;1:267–281. [Google Scholar]
- Hinsinger P, Bengough AG, Vetterlein D, Young IM. Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant and Soil. 2009;321:117–152. [Google Scholar]
- Hochholdinger F. The maize root system: morphology, anatomy, and genetics. In: Bennetzen JL, Hake SC, editors. Handbook of maize: its biology. New York: Springer; 2009. pp. 145–160. [Google Scholar]
- Hochholdinger F, Tuberosa R. Genetic and genomic dissection of maize root development and architecture. Current Opinion in Plant Biology. 2009;12:172–177. doi: 10.1016/j.pbi.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. From weeds to crops: genetic analysis of root development in cereals. Trends in Plant Science. 2004a;9:42–48. doi: 10.1016/j.tplants.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany. 2004b;93:359–368. doi: 10.1093/aob/mch056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyr JS, Cornish-Bowden A. Regulating the cellular economy of supply and demand. FEBS Letters. 2000;476:47–51. doi: 10.1016/s0014-5793(00)01668-9. [DOI] [PubMed] [Google Scholar]
- Högberg P, Read DJ. Towards a more plant physiological perspective on soil ecology. Trends in Ecology and Evolution. 2006;21:548–554. doi: 10.1016/j.tree.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hu FY, Tao DY, Sacks E, et al. Convergent evolution of perenniality in rice and sorghum. Proceedings of the National Academy of Sciences, USA. 2003;100:4050–4054. doi: 10.1073/pnas.0630531100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins DR, Reganold JP. No-till: the quiet revolution. Scientific American. 2008;299:70–77. doi: 10.1038/scientificamerican0708-70. [DOI] [PubMed] [Google Scholar]
- Hull D, Pettifer SR, Kell DB. Defrosting the digital library: bibliographic tools for the next generation web. PLoS Computational Biology. 2008;4:e1000204. doi: 10.1371/journal.pcbi.1000204. doi:10.1371/journal.pcbi.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund A, Richner W, Soldati A, van Fracheboud Y, Stamp P. Root morphology and photosynthetic performance of maize inbred lines at low temperature. European Journal of Agronomy. 2007;27:52–61. [Google Scholar]
- Hund A, Ruta N, Liedgens M. Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant and Soil. 2009;318:311–325. [Google Scholar]
- Hund A, Reimer R, Messmer R. A consensus map of QTLs controlling the root length of maize. Plant and Soil. 2011 (in press). doi:10.1007/s11104-011-0735-9. [Google Scholar]
- Ichii K, Wang WL, Hashimoto H, et al. Refinement of rooting depths using satellite-based evapotranspiration seasonality for ecosystem modeling in California. Agricultural and Forest Meteorology. 2009;149:1907–1918. [Google Scholar]
- Iyer-Pascuzzi AS, Symonova O, Mileyko Y, et al. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiology. 2010;152:1148–1157. doi: 10.1104/pp.109.150748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. A global analysis of root distributions for terrestrial biomes. Oecologia. 1996;108:389–411. doi: 10.1007/BF00333714. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Mooney HA, Schulze ED. A global budget for fine root biomass, surface area, and nutrient contents. Proceedings of the National Academy of Sciences, USA. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrow JD, Amonette JE, Bailey VL. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Climatic Change. 2007;80:5–23. [Google Scholar]
- Jenkins H, Hardy N, Beckmann M, et al. A proposed framework for the description of plant metabolomics experiments and their results. Nature Biotechnology. 2004;22:1601–1606. doi: 10.1038/nbt1041. [DOI] [PubMed] [Google Scholar]
- Jiao N, Azam F. Microbial carbon pump and its significance for carbon sequestration in the ocean. In: Jiao N, Azam F, Sanders S, editors. Microbial carbon pump in the ocean. Washington, DC: Science/AAAS; 2011. [Google Scholar]
- Jiao N, Herndl GJ, Hansell DA, et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nature Reviews Microbiology. 2010;8:593–599. doi: 10.1038/nrmicro2386. [DOI] [PubMed] [Google Scholar]
- Jiménez JJ, Lal R. Mechanisms of C sequestration in soils of Latin America. Critical Reviews in Plant Sciences. 2006;25:337–365. [Google Scholar]
- Jobbágy EG, Jackson RB. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications. 2000;10:423–436. [Google Scholar]
- Johansson AM, Pettersson ME, Siegel PB, Carlborg Ö. Genome-wide effects of long-term divergent selection. PLoS Genetics. 2010;6:e1001188. doi: 10.1371/journal.pgen.1001188. doi:10.1371/journal.pgen.1001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshita A, Babu RC, Boopathi NM, Fukai S. Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. Field Crops Research. 2008;109:1–23. [Google Scholar]
- Karcher DE, Richardson MD, Hignight K, Rush D. Drought tolerance of tall fescue populations selected for high root/shoot ratios and summer survival. Crop Science. 2008;48:771–777. [Google Scholar]
- Kardol P, Wardle DA. How understanding aboveground–belowground linkages can assist restoration ecology. Trends in Ecology and Evolution. 2010;25:670–679. doi: 10.1016/j.tree.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Kato Y, Abe J, Kamoshita A, Yamagishi J. Genotypic variation in root growth angle in rice (Oryza sativa L.) and its association with deep root development in upland fields with different water regimes. Plant and Soil. 2006;287:117–129. [Google Scholar]
- Kean S. Besting Johnny Appleseed. Science. 2010;328:301–303. doi: 10.1126/science.328.5976.301. [DOI] [PubMed] [Google Scholar]
- Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Medical Genomics. 2009;2(2) doi: 10.1186/1755-8794-2-2. doi:10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel C, Boots B, de Graaff MA, Harris D, Blum H, Six J. Total soil C and N sequestration in a grassland following 10 years of free air CO2 enrichment. Global Change Biology. 2006;12:2187–2199. [Google Scholar]
- Kirkegaard JA, Lilley JM, Howe GN, Graham JM. Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research. 2007;58:303–315. [Google Scholar]
- Koerber GR, Hill PW, Edwards-Jones G, Jones DL. Estimating the component of soil respiration not dependent on living plant roots: comparison of the indirect y-intercept regression approach and direct bare plot approach. Soil Biology and Biochemistry. 2010;42:1835–1841. [Google Scholar]
- Kögel-Knabner I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry. 2002;34:139–162. [Google Scholar]
- Kristensen HL, Thorup-Kristensen K. Root growth and nitrate uptake of three different catch crops in deep soil layers. Soil Science Society of America Journal. 2004;68:529–537. [Google Scholar]
- Krull ES, Baldock JA, Skjemstad JO. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Functional Plant Biology. 2003;30:207–222. doi: 10.1071/FP02085. [DOI] [PubMed] [Google Scholar]
- Kutschera L, Lichtenegger E, Sobotik M. Wurzelatlas der Kulturpflanzen gemäßigter Gebiete mit Arten des Feldgemüsebaues. 2009 Frankfurt/Main: DLG Verlag. [Google Scholar]
- Kuzyakov Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biology and Biochemistry. 2006;38:425–448. [Google Scholar]
- Lal R. Soil carbon sequestration to mitigate climate change. Geoderma. 2004;123:1–22. [Google Scholar]
- Lal R. Carbon sequestration. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008a;363:815–830. doi: 10.1098/rstb.2007.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal R. Sequestration of atmospheric CO2 in global carbon pools. Energy and Environmental Science. 2008b;1:86–100. [Google Scholar]
- Lal R. Beyond Copenhagen: mitigating climate change and achieving food security through soil carbon sequestration. Food Security. 2010a;2:169–177. [Google Scholar]
- Lal R. Enhancing eco-efficiency in agro-ecosystems through soil carbon sequestration. Crop Science. 2010b;50:S120–S131. [Google Scholar]
- Lal R. Managing soils for a warming earth in a food-insecure and energy-starved world. Journal of Plant Nutrition and Soil Science. 2010c;173:4–15. [Google Scholar]
- Lal R. Sequestering carbon in soils of agro-ecosystems. Food Policy. 2011;36:S33–S39. [Google Scholar]
- Lal R, Griffin M, Apt J, Lave L, Morgan MG. Ecology. Managing soil carbon. Science. 2004;304:393. doi: 10.1126/science.1093079. [DOI] [PubMed] [Google Scholar]
- Lambers H, Mougel C, Jaillard B, Hinsinger P. Plant–microbe–soil interactions in the rhizosphere: an evolutionary perspective. Plant and Soil. 2009;321:83–115. [Google Scholar]
- Lavania UC, Lavania S. Sequestration of atmospheric carbon into subsoil horizons through deep-rooted grasses – vetiver grass model. Current Science. 2009;97:618–619. [Google Scholar]
- Lehmann J. Bio-energy in the black. Frontiers in Ecology and the Environment. 2007;5:381–387. [Google Scholar]
- Leigh J, Hodge A, Fitter AH. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist. 2009;181:199–207. doi: 10.1111/j.1469-8137.2008.02630.x. [DOI] [PubMed] [Google Scholar]
- Liebig MA, Johnson HA, Hanson JD, Frank AB. Soil carbon under switchgrass stands and cultivated cropland. Biomass and Bioenergy. 2005;28:347–354. [Google Scholar]
- Lilley JM, Fukai S. Effect of timing and severity of water-deficit on 4 diverse rice cultivars. 1. Rooting pattern and soil-water extraction. Field Crops Research. 1994;37:205–213. [Google Scholar]
- Lippman Z, Tanksley SD. Dissecting the genetic pathway to extreme fruit size in tomato using a cross between the small-fruited wild species Lycopersicon pimpinellifolium and L. esculentum var. giant heirloom. Genetics. 2001;158:413–422. doi: 10.1093/genetics/158.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løes AK, Gahoonia TS. Genetic variation in specific root length in Scandinavian wheat and barley accessions. Euphytica. 2004;137:243–249. [Google Scholar]
- López-Bellido RJ, Lal R, Owens LB, López-Bellido L. Does North Appalachian agriculture contribute to soil carbon sequestration? Agriculture, Ecosystems and Environment. 2010;137:373–376. [Google Scholar]
- Lorenz K, Lal R. The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Advances in Agronomy. 2005;88:35–66. [Google Scholar]
- Lucas M, Swarup R, Paponov IA, et al. Short-Root regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiology. 2011;155:384–398. doi: 10.1104/pp.110.165126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YQ. Terrestrial carbon-cycle feedback to climate warming. Annual Review of Ecology, Evolution, and Systematics. 2007;38:683–712. [Google Scholar]
- Luster J, Gottlein A, Nowack B, Sarret G. Sampling, defining, characterising and modeling the rhizosphere – the soil science tool box. Plant and Soil. 2009;321:457–482. [Google Scholar]
- von Lützow M, Kögel-Knabner I, Ekschmitt K, et al. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review. European Journal of Soil Science. 2006;57:426–445. [Google Scholar]
- Ma Z, Wood CW, Bransby DI. Impacts of soil management on root characteristics of switchgrass. Biomass and Bioenergy. 2000;18:105–112. [Google Scholar]
- Ma Z, Wood CW, Bransby DI. Impact of row spacing, nitrogen rate, and time on carbon partitioning of switchgrass. Biomass and Bioenergy. 2001;20:413–419. [Google Scholar]
- Macías F, Arbestain MC. Soil carbon sequestration in a changing global environment. Mitigation and Adaptation Strategies for Global Change. 2010;15:511–529. [Google Scholar]
- MacKay DJC. Sustainable energy – without the hot air. Cambridge: UIT Cambridge; 2008. Available free online at http://www.withouthotair.com/ [Google Scholar]
- Macleod CJA, Binley A, Hawkins SL, et al. Genetically modified hydrographs: what can grass genetics do for temperate catchment hydrology? Hydrological Processes. 2007;21:2217–2221. [Google Scholar]
- MacLeod M, Moran D, Eory V, et al. Developing greenhouse gas marginal abatement cost curves for agricultural emissions from crops and soils in the UK. Agricultural Systems. 2010;103:198–209. [Google Scholar]
- Malhi Y, Baldocchi DD, Jarvis PG. The carbon balance of tropical, temperate and boreal forests. Plant, Cell and Environment. 1999;22:715–740. [Google Scholar]
- McCarthy JF, Ilavsky J, Jastrow JD, Mayer LM, Perfect E, Zhuang J. Protection of organic carbon in soil microaggregates via restructuring of aggregate porosity and filling of pores with accumulating organic matter. Geochimica Cosmochimica Acta. 2008;72:4725–4744. [Google Scholar]
- McKenzie BM, Bengough AG, Hallett PD, Thomas WTB, Forster B, McNicol JW. Deep rooting and drought screening of cereal crops: a novel field-based method and its application. Field Crops Research. 2009;112:165–171. [Google Scholar]
- McNeil BI, Matear RJ. Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. Proceedings of the National Academy of Sciences, USA. 2008;105:18860–18864. doi: 10.1073/pnas.0806318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir P, Cox P, Grace J. The influence of terrestrial ecosystems on climate. Trends in Ecology and Evolution. 2006;21:254–260. doi: 10.1016/j.tree.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Meuwissen T, Goddard M. Accurate prediction of genetic values for complex traits by whole-genome resequencing. Genetics. 2010;185:623–631. doi: 10.1534/genetics.110.116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen TH, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokany K, Raison RJ, Prokushkin AS. Critical analysis of root: shoot ratios in terrestrial biomes. Global Change Biology. 2006;12:84–96. [Google Scholar]
- Moni C, Rumpel C, Virto I, Chabbi A, Chenu C. Relative importance of sorption versus aggregation for organic matter storage in subsoil horizons of two contrasting soils. European Journal of Soil Science. 2010;61:958–969. [Google Scholar]
- Monson RK, Lipson DL, Burns SP, et al. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- Montgomery DR. Soil erosion and agricultural sustainability. Proceedings of the National Academy of Sciences, USA. 2007;104:13268–13272. doi: 10.1073/pnas.0611508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora CI, Driese SG, Colarusso LA. Middle to late Paleozoic atmospheric CO2 levels from soil carbonate and organic matter. Science. 1996;271:1105–1107. [Google Scholar]
- Moreau L, Lemarie S, Charcosset A, Gallais A. Economic efficiency of one cycle of marker-assisted selection. Crop Science. 2000;40:329–337. [Google Scholar]
- Nadezhdina N, Čermák J. Instrumental methods for studies of structure and function of root systems of large trees. Journal of Experimental Botany. 2003;54:1511–1521. doi: 10.1093/jxb/erg154. [DOI] [PubMed] [Google Scholar]
- Nepstad DC, Decarvalho CR, Davidson EA, et al. The role of deep roots in the hydrological and carbon cycles of amazonian forests and pastures. Nature. 1994;372:666–669. [Google Scholar]
- Nielsen UN, Ayres E, Wall DH, Bardgett RD. Soil biodiversity and carbon cycling: a review and synthesis of studies examining diversity–function relationships. European Journal of Soil Science. 2011;62:105–116. [Google Scholar]
- Obara M, Tamura W, Ebitani T, Yano M, Sato T, Yamaya T. Fine-mapping of qRL6·1, a major QTL for root length of rice seedlings grown under a wide range of NH4+ concentrations in hydroponic conditions. Theoretical and Applied Genetics. 2010;121:535–547. doi: 10.1007/s00122-010-1328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell AG, Young IM, Rushton SP, Shirley MD, Crawford JW. Visualization, modelling and prediction in soil microbiology. Nature Reviews Microbiology. 2007;5:689–699. doi: 10.1038/nrmicro1714. [DOI] [PubMed] [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- Orwin KH, Buckland SM, Johnson D, et al. Linkages of plant traits to soil properties and the functioning of temperate grassland. Journal of Ecology. 2010;98:1074–1083. [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. Hidden branches: developments in root system architecture. Annual Review of Plant Biology. 2007;58:93–113. doi: 10.1146/annurev.arplant.58.032806.104006. [DOI] [PubMed] [Google Scholar]
- Ostle NJ, Levy PE, Evans CD, Smith P. UK land use and soil carbon sequestration. Land Use Policy. 2009;26:S274–S283. [Google Scholar]
- O'Toole JC, Bland WL. Genotypic variation in crop plant root systems. Advances in Agronomy. 1987;41:91–145. [Google Scholar]
- Otto R, Trivelin PCO, Franco HCJ, Faroni CE, Vitti AC. Root system distribution of sugar cane as related to nitrogen fertilization, evaluated by two methods: monolith and probes. Revista Brasileira De Ciencia Do Solo. 2009;33:601–611. [Google Scholar]
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews Microbiology. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- Paschold A, Marcon C, Hoecker N, Hochholdinger F. Molecular dissection of heterosis manifestation during early maize root development. Theoretical and Applied Genetics. 2010;120:383–388. doi: 10.1007/s00122-009-1082-6. [DOI] [PubMed] [Google Scholar]
- Passioura J. Increasing crop productivity when water is scarce – from breeding to field management. Agricultural Water Management. 2006;80:176–196. [Google Scholar]
- Passioura JB. Roots and drought resistance. Agricultural Water Management. 1983;7:265–280. [Google Scholar]
- Pataki DE, Billings SA, Naumburg E, Goedhart CM. Water sources and nitrogen relations of grasses and shrubs in phreatophytic communities of the Great Basin Desert. Journal of Arid Environments. 2008;72:1581–1593. [Google Scholar]
- Paustian K, Six J, Elliott ET, Hunt HW. Management options for reducing CO2 emissions from agricultural soils. Biogeochemistry. 2000;48:147–163. [Google Scholar]
- Pearl J. Causality: models, reasoning and inference. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Péret B, De Rybel B, Casimiro I, et al. Arabidopsis lateral root development: an emerging story. Trends in Plant Science. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Massicotte HB, Melville LH. Mycorrhizas: anatomy and cell biology. London: CABI Publishing; 2004. [Google Scholar]
- Philippot L, Hallin S, Börjesson G, Baggs EM. Biochemical cycling in the rhizosphere having an impact on global change. Plant and Soil. 2009;321:61–81. [Google Scholar]
- Post WM, Kwon KC. Soil carbon sequestration and land-use change: processes and potential. Global Change Biology. 2000;6:317–327. [Google Scholar]
- Powlson DS, Whitmore AP, Goulding KWT. Soil carbon sequestration to mitigate climate change: a critical re-examination to identify the true and the false. European Journal of Soil Science. 2011;62:42–55. [Google Scholar]
- Prechtel A, von Lützow M, Schneider BU, et al. Organic carbon in soils of Germany: status quo and the need for new data to evaluate potentials and trends of soil carbon sequestration. Journal of Plant Nutrition and Soil Science. 2009;172:601–614. [Google Scholar]
- Pretty J. Agricultural sustainability: concepts, principles and evidence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:447–465. doi: 10.1098/rstb.2007.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretty J, Sutherland WJ, Ashby J, et al. The top 100 questions of importance to the future of global agriculture. International Journal of Agricultural Sustainability. 2010;8:219–236. [Google Scholar]
- Price AH, Tomos AD, Virk DS. Genetic dissection of root growth in rice (Oryza sativa L).1. a hydroponic screen. Theoretical and Applied Genetics. 1997;95:132–142. [Google Scholar]
- Price AH, Steele KA, Gorham J, et al. Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes I. Root distribution, water use and plant water status. Field Crops Research. 2002a;76:11–24. [Google Scholar]
- Price AH, Steele KA, Moore BJ, Jones RGW. Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes II. Mapping quantitative trait loci for root morphology and distribution. Field Crops Research. 2002b;76:25–43. [Google Scholar]
- Qin Z, Huang Y. Quantification of soil organic carbon sequestration potential in cropland: a model approach. Science China-Life Sciences. 2010;53:868–884. doi: 10.1007/s11427-010-4023-3. [DOI] [PubMed] [Google Scholar]
- Randall GW, Mulla DJ. Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural practices. Journal of Environmental Quality. 2001;30:337–344. doi: 10.2134/jeq2001.302337x. [DOI] [PubMed] [Google Scholar]
- Reay DS, Smith P, Hymus G, Sabine C. New directions: the changing role of the terrestrial carbon sink in determining atmospheric CO2 concentrations. Atmospheric Environment. 2007;41:5813–5815. [Google Scholar]
- Rebouillat J, Dievart A, Verdeil JL, et al. Molecular genetics of rice root development. Rice. 2009;2:15–34. [Google Scholar]
- Reid PC, Fischer AC, Lewis-Brown E, et al. Impacts of the oceans on climate change. Advances in Marine Biology. 2009;56:1–150. doi: 10.1016/S0065-2881(09)56001-4. [DOI] [PubMed] [Google Scholar]
- Riebesell U, Schulz KG, Bellerby RG, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature. 2007;450:545–548. doi: 10.1038/nature06267. [DOI] [PubMed] [Google Scholar]
- Rillig MC. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters. 2004;7:740–754. [Google Scholar]
- Robertson GP, Paul EA, Harwood RR. Greenhouse gases in intensive agriculture: contributions of individual gases to the radiative forcing of the atmosphere. Science. 2000;289:1922–1925. doi: 10.1126/science.289.5486.1922. [DOI] [PubMed] [Google Scholar]
- Robinson D. Scaling the depths: below-ground allocation in plants, forests and biomes. Functional Ecology. 2004;18:290–295. [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- Royer DL, Berner RA, Beerling DJ. Phanerozoic atmospheric CO2 change: evaluating geochemical and paleobiological approaches. Earth-Science Reviews. 2001;54:349–392. [Google Scholar]
- Rubino M, Dungait JAJ, Evershed RP, et al. Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a C-13 labelled-leaf litter experiment. Soil Biology and Biochemistry. 2010;42:1009–1016. [Google Scholar]
- Sabine CL, Feely RA, Gruber N, et al. The oceanic sink for anthropogenic CO2. Science. 2004;305:367–371. doi: 10.1126/science.1097403. [DOI] [PubMed] [Google Scholar]
- Sainju UM, Whitehead WF, Singh BP. Agricultural management practices to sustain crop yields and improve soil and environmental qualities. Scientific World Journal. 2003;3:768–789. doi: 10.1100/tsw.2003.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderman J, Baldock JA. Accounting for soil carbon sequestration in national inventories: a soil scientist's perspective. Environmental Research Letters. 2010;5:1–6. [Google Scholar]
- Sanguineti MC, Li S, Maccaferri M, et al. Genetic dissection of seminal root architecture in elite durum wheat germplasm. Annals of Applied Biology. 2007;151:291–305. [Google Scholar]
- Santner A, Calderon-Villalobos LI, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nature Chemical Biology. 2009;5:301–307. doi: 10.1038/nchembio.165. [DOI] [PubMed] [Google Scholar]
- Sartori F, Lal R, Ebinger MH, Parrish DJ. Potential soil carbon sequestration and CO2 offset by dedicated energy crops in the USA. Critical Reviews in Plant Sciences. 2006;25:441–472. [Google Scholar]
- Schenk HJ, Jackson RB. The global biogeography of roots. Ecological Monographs. 2002a;72:311–328. [Google Scholar]
- Schenk HJ, Jackson RB. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. Journal of Ecology. 2002b;90:480–494. [Google Scholar]
- Schenk HJ, Jackson RB. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma. 2005;126:129–140. [Google Scholar]
- Schimel DS, House JI, Hibbard KA, et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature. 2001;414:169–172. doi: 10.1038/35102500. [DOI] [PubMed] [Google Scholar]
- Shi D, Xu Y, Hopkinson BM, Morel FM. Effect of ocean acidification on iron availability to marine phytoplankton. Science. 2010;327:676–679. doi: 10.1126/science.1183517. [DOI] [PubMed] [Google Scholar]
- Silver WL, Ryals R, Eviner V. Soil carbon pools in California's annual grassland ecosystems. Rangeland Ecology and Management. 2010;63:128–136. [Google Scholar]
- Singh BK, Bardgett RD, Smith P, Reay DS. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nature Reviews Microbiology. 2010;8:779–790. doi: 10.1038/nrmicro2439. [DOI] [PubMed] [Google Scholar]
- Six J, Feller C, Denef K, Ogle SM, de Moraes Sa JC, Albrecht A. Soil organic matter, biota and aggregation in temperate and tropical soils: effects of no-tillage. Agronomie. 2002;22:755–775. [Google Scholar]
- Smith P. Soils as carbon sinks: the global context. Soil Use and Management. 2004;20:212–218. [Google Scholar]
- Smith P, Fang C. A warm response by soils. Nature. 2010;464:499–500. doi: 10.1038/464499a. [DOI] [PubMed] [Google Scholar]
- Smith P, Olesen JE. Synergies between the mitigation of, and adaptation to, climate change in agriculture. Journal of Agricultural Science. 2010;148:543–552. [Google Scholar]
- Smith P, Martino D, Cai Z, et al. Greenhouse gas mitigation in agriculture. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:789–813. doi: 10.1098/rstb.2007.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Bhogal A, Edgington P, et al. Consequences of feasible future agricultural land-use change on soil organic carbon stocks and greenhouse gas emissions in Great Britain. Soil Use and Management. 2010a;26:381–398. [Google Scholar]
- Smith P, Lanigan G, Kutsch WL, et al. Measurements necessary for assessing the net ecosystem carbon budget of croplands. Agriculture, Ecosystems and Environment. 2010b;139:302–315. [Google Scholar]
- Sohi SP, Krull E, Lopez-Capel E, Bol R. A review of biochar and its use and function in soil. Advances in Agronomy. 2010;105:47–82. [Google Scholar]
- Somerville C, Youngs H, Taylor C, Davis SC, Long SP. Feedstocks for lignocellulosic biofuels. Science. 2010;329:790–792. doi: 10.1126/science.1189268. [DOI] [PubMed] [Google Scholar]
- Soussana JF, Luscher A. Temperate grasslands and global atmospheric change: a review. Grass and Forage Science. 2007;62:127–134. [Google Scholar]
- Soussana JF, Fuhrer J, Jones M, Van Amstel A. The greenhouse gas balance of grasslands in Europe. Agriculture, Ecosystems and Environment. 2007;121:1–4. [Google Scholar]
- Staddon PL, Fitter AH. Does elevated atmospheric carbon dioxide affect arbuscular mycorrhizas? Trends in Ecology and Evolution. 1998;13:455–458. doi: 10.1016/s0169-5347(98)01493-1. [DOI] [PubMed] [Google Scholar]
- Steele KA, Price AH, Shashidhar HE, Witcombe JR. Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theoretical and Applied Genetics. 2006;112:208–221. doi: 10.1007/s00122-005-0110-4. [DOI] [PubMed] [Google Scholar]
- Steele KA, Virk DS, Kumar R, Prasad SC, Witcombe JR. Field evaluation of upland rice lines selected for QTLs controlling root traits. Field Crops Research. 2007;101:180–186. [Google Scholar]
- Steinbach HS, Alvarez R. Changes in soil organic carbon contents and nitrous oxide emissions after introduction of no-till in Pampean agroecosystems. Journal of Environmental Quality. 2006;35:3–13. doi: 10.2134/jeq2005.0050. [DOI] [PubMed] [Google Scholar]
- Stone EL, Kalisz PJ. On the maximum extent of tree roots. Forest Ecology and Management. 1991;46:59–102. [Google Scholar]
- Stone R. The invisible hand behind a vast carbon reservoir. Science. 2010;328:1476–1477. doi: 10.1126/science.328.5985.1476. [DOI] [PubMed] [Google Scholar]
- Strack D, Fester T, Hause B, Schliemann W, Walter MH. Arbuscular mycorrhiza: biological, chemical, and molecular aspects. Journal of Chemical Ecology. 2003;29:1955–1979. doi: 10.1023/a:1025695032113. [DOI] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nature Cell Biology. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- Tanimoto E. Regulation of root growth by plant hormones – roles for auxin and gibberellin. Critical Reviews in Plant Sciences. 2005;24:249–265. [Google Scholar]
- Taylor LL, Leake JR, Quirk J, Hardy K, Banwart SA, Beerling DJ. Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology. 2009;7:171–191. doi: 10.1111/j.1472-4669.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- Tester M, Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2010;327:818–822. doi: 10.1126/science.1183700. [DOI] [PubMed] [Google Scholar]
- Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM. Mineral control of soil organic carbon storage and turnover. Nature. 1997;389:170–173. [Google Scholar]
- Trachsel S, Messmer R, Stamp P, Hund A. Mapping of QTLs for lateral and axile root growth of tropical maize. Theoretical and Applied Genetics. 2009;119:1413–1424. doi: 10.1007/s00122-009-1144-9. [DOI] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP. Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant and Soil. 2011;341:75–87. [Google Scholar]
- Tuberosa R, Salvi S, Sanguineti MC, Landi P, Maccaferri M, Conti S. Mapping QTLs regulating morpho-physiological traits and yield: case studies, shortcomings and perspectives in drought-stressed maize. Annals of Botany. 2002;89:941–963. doi: 10.1093/aob/mcf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuberosa R, Salvi S, Giuliani S, et al. Genomics of root architecture and functions in maize. In: Costa de Oliveira A, Varshney RK, editors. Root genomics. Heidelberg: Springer; 2010. pp. 179–204. [Google Scholar]
- Turley C, Eby M, Ridgwell AJ, et al. The societal challenge of ocean acidification. Marine Pollution Bulletin. 2010;60:787–792. doi: 10.1016/j.marpolbul.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Utomo HS, Linscombe SD. Current patents and future development underlying marker-assisted breeding in major grain crops. Recent Patents on DNA and Gene Sequencing. 2009;3:53–62. doi: 10.2174/187221509787236174. [DOI] [PubMed] [Google Scholar]
- Van Tassel DL, DeHaan LR, Cox TS. Missing domesticated plant forms: can artificial selection fill the gap? Evolutionary Applications. 2010;3:434–452. doi: 10.1111/j.1752-4571.2010.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A. Mycorrhiza: genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics. New York: Springer; 2008. [Google Scholar]
- Villamil MB, Miguez FE, Bollero GA. Multivariate analysis and visualization of soil quality data for no-till systems. Journal of Environmental Quality. 2008;37:2063–2069. doi: 10.2134/jeq2007.0349. [DOI] [PubMed] [Google Scholar]
- Virto I, Barre P, Chenu C. Microaggregation and organic matter storage at the silt-size scale. Geoderma. 2008;146:326–335. [Google Scholar]
- Virto I, Moni C, Swanston C, Chenu C. Turnover of intra- and extra-aggregate organic matter at the silt-size scale. Geoderma. 2010;156:1–10. [Google Scholar]
- Vispute TP, Zhang H, Sanna A, Xiao R, Huber GW. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science. 2010;330:1222–1227. doi: 10.1126/science.1194218. [DOI] [PubMed] [Google Scholar]
- Wang R, Farrona S, Vincent C, et al. PEP1 regulates perennial flowering in Arabis alpina. Nature. 2009;459:423–427. doi: 10.1038/nature07988. [DOI] [PubMed] [Google Scholar]
- Wang YG, Li Y, Ye XH, Chu Y, Wang XP. Profile storage of organic/inorganic carbon in soil: from forest to desert. Science of the Total Environment. 2010;408:1925–1931. doi: 10.1016/j.scitotenv.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Watt M, Schneebeli K, Dong P, Wilson IW. The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops. Functional Plant Biology. 2009;36:960–969. doi: 10.1071/FP09214. [DOI] [PubMed] [Google Scholar]
- West TO, Post WM. Soil organic carbon sequestration rates by tillage and crop rotation: a global data analysis. Soil Science Society of America Journal. 2002;66:1930–1946. [Google Scholar]
- Whited GM, Feher FJ, Benko DA, et al. Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Industrial Biotechnology. 2010;6:152–163. [Google Scholar]
- Wielopolski L, Yanai RD, Levine CR, Mitra S, Vadeboncoeur MA. Rapid, non-destructive carbon analysis of forest soils using neutron-induced gamma-ray spectroscopy. Forest Ecology and Management. 2010;260:1132–1137. [Google Scholar]
- Wilson GW, Rice CW, Rillig MC, Springer A, Hartnett DC. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecology Letters. 2009;12:452–461. doi: 10.1111/j.1461-0248.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Ding G, Shi L, Feng J, Xu F, Meng J. Quantitative trait loci for root morphology in response to low phosphorus stress in Brassica napus. Theoretical and Applied Genetics. 2010;121:181–193. doi: 10.1007/s00122-010-1301-1. [DOI] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L. A basic helix–loop–helix transcription factor controls cell growth and size in root hairs. Nature Genetics. 2010;42:264–267. doi: 10.1038/ng.529. [DOI] [PubMed] [Google Scholar]
- Yvon-Durocher G, Jones JI, Trimmer M, Woodward G, Montoya JM. Warming alters the metabolic balance of ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:2117–2126. doi: 10.1098/rstb.2010.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhao M, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science. 2010;329:940–943. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biololgy. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Miller RM. Carbon cycling by arbuscular mycorrhizal fungi in soil–plant systems. Trends in Plant Science. 2003;8:407–409. doi: 10.1016/s1360-1385(03)00184-5. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Leifeld J, Schmidt MWI, Smith P, Fuhrer J. Measured soil organic matter fractions can be related to pools in the RothC model. European Journal of Soil Science. 2007;58:658–667. [Google Scholar]
- Zimmermann R, Sakai H, Hochholdinger F. The Gibberellic Acid Stimulated-Like gene family in maize and its role in lateral root development. Plant Physiology. 2010;152:356–365. doi: 10.1104/pp.109.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]