Abstract
Background and Aims
The oomycete Aphanomyces euteiches causes up to 80 % crop loss in pea (Pisum sativum). Aphanomyces euteiches invades the root system leading to a complete arrest of root growth and ultimately to plant death. To date, disease control measures are limited to crop rotation and no resistant pea lines are available. The present study aims to get a deeper understanding of the early oomycete–plant interaction at the tissue and cellular levels.
Methods
Here, the process of root infection by A. euteiches on pea is investigated using flow cytometry and microscopic techniques. Dynamic changes in secondary metabolism are analysed with high-performance liquid chromatography with diode-array detection.
Key Results
Root infection is initiated in the elongation zone but not in the root cap and border cells. Border-cell production is significantly enhanced in response to root inoculation with changes in their size and morphology. The stimulatory effect of A. euteiches on border-cell production is dependent on the number of oospores inoculated. Interestingly, border cells respond to pathogen challenge by increasing the synthesis of the phytoalexin pisatin.
Conclusions
Distinctive responses to A. euteiches inoculation occur at the root tissue level. The findings suggest that root border cells in pea are involved in local defence of the root tip against A. euteiches. Root border cells constitute a convenient quantitative model to measure the molecular and cellular basis of plant–microbe interactions.
Keywords: Aphanomyces euteiches, pathogen, phenolics, Pisum sativum, pisatin, root border cells, root cap, root infection
INTRODUCTION
Aphanomyces euteiches is an oomycete plant pathogen causing devastating disease of specific leguminous crops worldwide, including alfalfa (Medicago sativa), common bean (Phaseolus vulgaris) and pea (Pisum sativum) (Kraft and Boge, 1996; Shang et al., 2000; Madoui et al., 2009). Root rot due to A. euteiches is considered to be the major destructive soil-borne disease of peas, causing up to 80 % losses per year and is widespread in North America, Europe, Japan, Australia and New Zealand (Grau et al., 1991; Wicker et al., 2003; Gaulin et al., 2007). The destructiveness of oomycetes is a major threat to agriculture and it is necessary to develop alternative methods, both to control their spread and to avoid the use of chemicals toxic to the environment.
Oomycetes are distantly related to true fungi and their particular physiology makes them insensitive to most fungicides (Baldauf et al., 2000). Oospores released from infected roots into the rhizosphere constitute the primary source of inoculum. They can remain dormant in the soil for up to 10 years thus reducing the effectiveness of crop rotation to reduce propagation of this pathogen (Papavizas and Ayers, 1974; Shang et al., 2000). Because of the economic consequences caused by diseases induced by oomycete species such as Phytophthora infestans and P. sojae, considerable efforts to elucidate the molecular basis of recognition between oomycete and their plant hosts were undertaken specifically on these species, whereas A. euteiches has received little attention (Tyler, 2002; Gaulin et al., 2006; Hardham, 2007). To date, the only effective method to control the widespread occurrence of common root rot caused by A. euteiches in peas is mainly by host crop avoidance for several years as no fully resistant pea lines are commercially available (Kjøller and Rosendahl, 1998; Sauvage et al., 2007). Cultural and prophylactic methods such as crop rotation and the development of assays to detect the presence of any inoculum in soil before sowing are essential for disease management (Vandemark et al., 2000; Gaulin et al., 2007).
The life cycle of A. euteiches includes germination of oospores in the soil and production of zoospores that encyst on the root surface (Kjøller and Rosendahl, 1998). It has been reported that zoospores are attracted to specific sites on the pea root surface immediately behind the root cap (Cunningham and Hagedorn, 1962). The 5,4'-dihydroxy-7-methoxy-isoflavone compound, also called prunetin, present in pea root and exudates was found to be strongly involved in attracting zoospores of A. euteiches (Yokosawa et al., 1986). Deacon and Saxena (1998) reported that cysts from A. euteiches are also able to germinate artificially in response to different substances. The development of mycelium occurred throughout the root cortex and oospores are produced at the latest stage of infection (Deacon and Saxena, 1998; Kjøller and Rosendahl, 1998). It has been reported that pea root tips, including the root meristem and the root cap remained free of infection and colonization upon infection by pathogens such as A. euteiches or the fungus Nectria haematococca (Cunningham and Hagedorn, 1962; Gunawardena and Hawes, 2002; Gunawardena et al., 2005). Gunawardena et al. (2005) reported that the failure of N. haematococca to infect the root tip was due to the release of signals produced by root border cells that stimulate spore germination and modulate hyphal growth. Root border cells are defined as cells that originate from the root cap meristematic cells and that disperse into suspension when root tips are placed in water (Hawes et al., 2000; 2003; Driouich et al., 2007, 2010). These atypical cells were first considered as dead ‘sloughed cells’ released passively into the rhizosphere to provide mechanical protection as the root tip grows through soil. Increasing evidence suggests that root border cells in peas are involved in root–microorganism interactions (Brigham et al., 1995; Gunawardena and Hawes, 2002; Gunawardena et al., 2005; Wen et al., 2007, 2009). Root border cells from pea secrete specific sets of proteins while they are released from the root tip as compared with the root cap and the root system (Brigham et al., 1995). Treatment with proteinase markedly increased root cap infection by the fungus N. haematococca, indicating that border cells detaching from pea roots are involved in plant defence (Wen et al., 2007). More recently the presence of extracellular DNA (exDNA) in root border-cell exudates was found to contribute to the root cap protection against N. haematococca (Wen et al., 2009).
The aim of the present study was to examine the early infection of pea roots by A. euteiches and to search for the underlying plant defensive mechanisms and the specific role played by root border cells. It was found that both the root cap and the border cells were free of mycelium at early stages of root inoculation. A significant increase in the number of border cells produced and released from the root cap occurred in response to inoculation by A. euteiches. This enhanced production of border cells was accompanied by an increase in pisatin production, suggesting a role for these cells in local resistance of the root tip against A. euteiches.
MATERIALS AND METHODS
Plant material
Pisum sativum seeds were surface-sterilized and sown onto Murashige and Skoog medium containing 1 % agarose supplemented with 3 % sucrose (Durand et al., 2009). Plants were grown in continuous light (120 µE m2 s−2, at 24 °C), as described in Vicré et al. (2005). To avoid the roots penetrating the agar and the subsequent loss of border cells, plants were grown in vertically oriented Petri dishes. Root caps and border cells were harvested from 1- to 12-d-old seedlings. To count root border cells, root tips were placed in water and gently vortexed to release root border cells. The number of root border cells was estimated on a Malassez haemocytometer. Each value represents the average of at least ten roots for each treatment.
Pathogen culture and inoculation of pea seedlings
Unless otherwise stated, all experiments were performed using the French reference pea isolate of A. euteiches (RB84) previously described as being highly virulent and the non-pathogenic isolate Aphanomyces cladogamus (Wicker et al., 2001). These isolates were previously characterized (Wicker et al., 2001) and all were kindly provided by B. Tivoli (INRA Rennes, France). Oospore production and root infection were performed according to Bødker et al. (2002) and Sauvage et al. (2007). Zoospores were produced according to Deacon and Saxena (1998). Oospores and mycelia of A. euteiches were produced in sterile Quaker oat medium (5 g in 1 L of distilled water) and malt liquid medium (15 g in 1 L of distilled water), respectively. Roots were inoculated 2 d after germination with a range of oospore suspensions (0, 1, 10, 100, 103, 104 and 105 oospores mL−1). The number of oospores was estimated on a Malassez haemocytometer.
Histochemical staining of root tips and border cells
Staining of A. euteiches with a fluorescein isothiocyanate–wheat germ agglutinin (FITC–WGA) conjugate was performed according to Badreddine et al. (2008). To label A. euteiches mycelia present in root tissues, infected roots were directly cut longitudinally with a razor blade and stained for 30 min in 1 mg mL−1 FITC–WGA. Surface staining of infected roots was obtained by directly incubating the root in FITC–WGA as described above. After gentle washing in deionized water, roots were observed using a microscope equipped with UV fluorescence (excitation filter, 359 nm; barrier filter, 461 nm). Vital staining with 5 µm calcein-AM (Sigma-Aldrich) was performed as described in Vicré et al. (2005). Roots were stained for 60 min, carefully washed in deionized water and observed using a microscope equipped with UV fluorescence (excitation filter, 490 nm; barrier filter, 520 nm). Images were acquired with a Leica DFC 300 FX camera.
High-performance liquid chromatography with diode-array detection (HPLC–DAD) analysis of phenolic compounds
Phenolic compounds were extracted from root tissues in a solution of methanol : water (80 : 20, v/v) as previously described by Lanoue et al. (2010). Samples were shaken at 1200 rpm for 30 min and centrifuged at 18 000 g for 5 min. The supernatant (50 µL) was analysed by DAD. The HPLC–DAD system consisted of a gradient pump (Waters 600 controller; Waters Corporation, Milford, MA, USA), a cooled autosampler (Waters 717 plus) and a UV–visible photodiode array detector set (Waters 996) to acquire data from 200 to 400 nm. Empower 2 software from Waters was used for instrument control, data acquisition and data processing. Analyses were performed on a 3-μm column (250 × 4 mm, Multospher 120 RP18HP; CS-Service, Langerwehe, Germany) at room temperature (21 °C). The mobile phase consisted of aqueous phosphoric acid (0·1 % w/v) and acetonitrile pumped at 0·5 mL min−1 into the HPLC–DAD system (Lanoue et al., 2010). The gradient started at 5 % acetonitrile and increased linearly to 72 % in 60 min, followed by washing and reconditioning of the column. Wavelength detection was set in ‘maxplot’ for tryptophan (RT = 24·05 min), the kaempferol glucoside derivate (RT = 32·24 min) and the quercetin glucoside derivate (RT = 30·77 min) and at 308 nm for pisatin (RT = 53·5 min). Compounds were identified according to their UV spectra and retention time by comparison with standards.
Extraction and quantification of pisatin from pea roots and root border-cell exudates
Root border-cell exudates were collected by holding the root tip in a drop of water. Exudates were separated from root border cells by centrifugation at 7000 rpm for 10 min (Wuyts et al., 2006). For pisatin extraction samples were incubated in hexane (10 mg mL−1) under agitation for 4 h as previously described. (Sweigard et al., 1986; Kaimoyo and Van Etten, 2008). Extracts were removed and hexane was evaporated under vacuum. The residue was dissolved in ethanol (0.l–1 mL). The presence of (+)-pisatin 8 in the extracts was determined by scanning for the characteristic UV absorption spectrum of (+)-pisatin 8 with a Beckman DU-64 UV absorbance spectrophotometer (Beckman-Coulter, Fullerton, CA, USA). The pisatin concentration was determined by measuring its absorbance at 309 nm using an extinction coefficient of 103·86 (Cruickshank and Perrin, 1960).
Liquid and culture bioassays
Some pieces of nitrocellulose containing various concentrations of pisatin were placed on corn-meal agar plates. Phenolic compounds extracted from root tissues were also tested on A. euteiches development corresponding to a concentration of 1·2 ± 0·5 µg mL−1 of pisatin. Mycelial growth and the number of oospores produced were assayed for each condition according to Sauvage et al. (2007).
Ultrastructural analysis by electron microscopy
Root tips were prepared using the high-pressure-freezing plus freeze-substitution method and embedded in Epon resin as previously described (Staehelin et al., 1990; Driouich et al., 1993). Thin sections (90 nm) were mounted on copper grids. The sections were stained with 2 % uranyl acetate for 10 min and lead stain for 30–60 s and observed with a transmission electron microscope (Tecnai 12, Bio-Twin; Philips) at 80 kV.
Flow cytometric analysis
All experiments were performed using a Coulter Epics XL cytometer (Beckman Coulter, Roissy, France) equipped with an air-cooled argon ion laser emitting at a fixed wavelength of 488 nm. The forward-scatter (FS) channel was set on linear gain and the side-scatter (SS) and fluorescence (FL1) channels were set on a logarithmic scale. Voltages were set at 500 V for FS, 400 V for SS and 1200 V for FL1. Samples were run at medium flow rate (30 µL min−1) until a minimum of 10 000 cells was analysed for each condition. Data were analysed with Expo 32 software (Beckman Coulter).
Variations of border cell size and structure from infected or control roots were assessed using forward and side-angle scatters (FS vs. SS) in isotonic PBS buffer, pH 7·4.
To assess cell viability, five root apices were immersed in a 1 mm solution of the membrane-permeable dye calcein-AM. Apices were incubated for 2 h at room temperature in the dark and immediately analysed by flow cytometry. All studies were performed at least three times, with three replicates each time.
Statistical analyses
Significant differences (P < 0·05) were calculated by using one-way analysis of variance (ANOVA) and the Newman–Keuls test (to compare all pairs of columns).
RESULTS
Early infection by A. euteiches specifically occurs in the elongation zone of pea roots
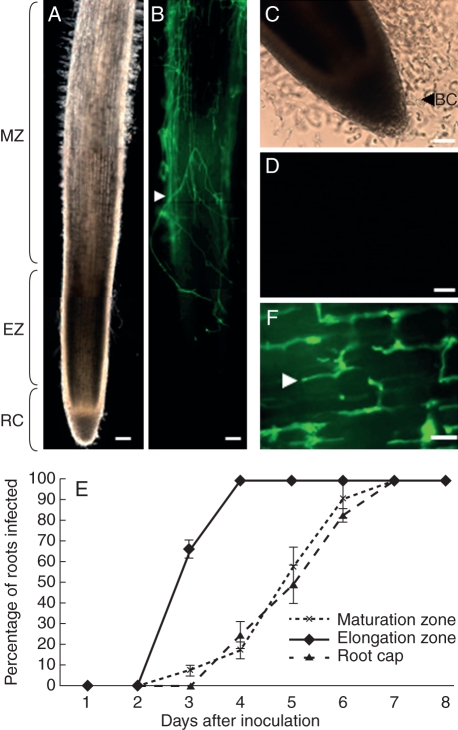
Whole roots of P. sativum were inoculated uniformly with a solution of 105 oospores of A. euteiches RB84 isolate. At day 4 post-inoculation, the presence of A. euteiches was detected on the cell surface of elongation and maturation as revealed by FITC–WGA staining of the mycelium (Fig. 1A, B). In contrast, root caps and border cells remained free of colonization (Fig. 1B–D). Control experiments performed on uninfected plants showed that the root surfaces were deprived of FITC–WGA staining (Supplementary Data Fig. S1, available online). To evaluate the infection of internal root tissues (e.g. cortical parenchyma cells), the progression of the pathogen within the tissues was investigated over time on longitudinal sections (Fig. 1E). At 3-d post-inoculation, the mycelia were extensively present in the root cortex of the elongation zone (Fig. 1F) for most roots (67 %) whereas oomycetes were rarely detected within the parenchyma cells of the maturation zone (7 %) and totally absent from the root cap tissues (0 %). While the maturation zone was abundantly colonized on the surface at 4-d post-inoculation (Fig. 1B), the cortical parenchyma in this zone remained free of infection in most roots (82 %; Fig. 1E). These observations indicated that, in P. sativum, the elongation zone is the primary site of infection by A. euteiches. By 7–8 d after inoculation, the oomycetes progressively invaded all the tissues in both the maturation zone and root cap while plants showed severe symptoms of root rot (data not shown).
Fig. 1.
Localization of A. euteiches (RB 84) in the root tip of P. sativum. (A) Bright field image of the primary root tips at 4 d post-inoculation and (B) FITC–WGA-staining showing the presence of mycelia on the root surface. (C) Bright field image showing the release of border cells from the root cap at 4-d post-inoculation. (D) Note the absence of mycelia on the root cap and border cells as revealed by FITC–WGA staining. (E) Time-course progression of A. euteiches within the root tissues in the elongation zone, maturation zone and root caps. The presence of mycelia was detected on root sections using FITC–WGA staining (n = 30 roots) Values are means ± standard deviation from three independent experiments. (F) By 3-d post-inoculation the mycelium was abundant in the root cortex of the elongtion zone. BC, Border cells; EZ, root elongation zone; RC, root cap; MZ, root maturation zone. White arrowheads indicate the presence of mycelia. Scale bars: (A, B) = 100 µm; (C, D) = 50 µm; (F) = 5 µm.
Analysis of phenolic compounds in different zones of the root tips
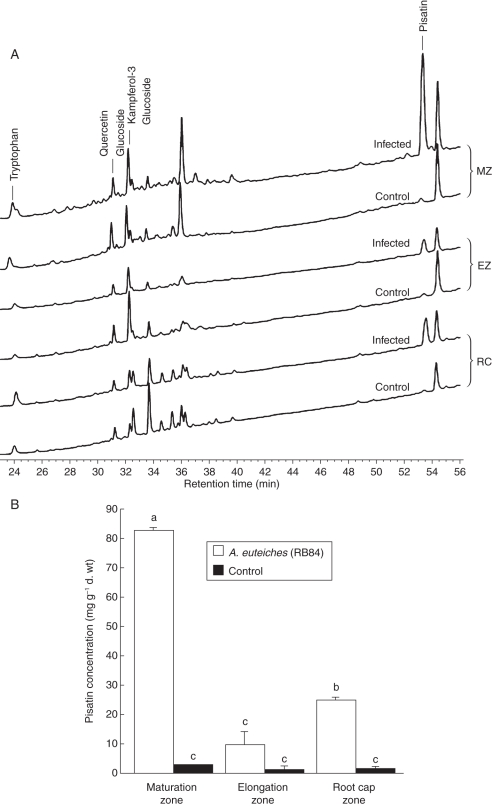
As phenolic compounds are known to be involved in root protection (Lanoue et al., 2010), the distribution of such molecules in different zones of the root, including root cap, cell elongation zone and maturation zone, were investigated. The phenolic profile appeared to be specific for the different zones of the root (Fig. 2A, B). The HPLC profiles of phenolic compounds from pea roots indicated the presence of tryptophan (RT = 24·18 min), quercetin glucoside (RT = 30·77 min) and a derivative of the kaempferol 3 glucoside intermediate (RT = 32·32 min) in the different parts of the root (Fig. 2A). The presence of unknown phenolic compounds was also detected in roots but the nature of these molecules remains undetermined as they did not correspond to any of the phenolics previously reported in P. sativum (including prunetin, genistein, genistin, naringenin, hesperetin, daidzin and daidzein). It should be noted that such molecules were specifically synthesized by root cap cells as compared with the elongation zone and the maturation zone. Interestingly, A. euteiches infection of pea roots induced a major peak (RT = 53·6 min) identified as pisatin, the major phytoalexin of pea. A significant increase in pisatin occurred in root zones that escaped early infection such as the maturation zone (82·3 ± 1 mg g−1 d. wt) and root cap cells (26·5 ± 1·1 mg g−1 d. wt; Fig. 2B). To evaluate the specific contribution of root border cells in defence against A. euteiches, pisatin was quantified specifically in root border cells (Fig. 3). Due to the very low amount of plant material, the protocol of pisatin extraction and quantification was optimized according to Sweigard et al. (1986). In healthy roots, the level of pisatin was significantly higher in root border cells (11·3 ± 2·5 mg g−1 d. wt) than in the root caps (4·0 ± 0·9 mg g−1 d. wt). Furthermore, a 54 % increase in pisatin production was found in root border cells in response to A. euteiches. In contrast, inoculation of pea root tips by the non-pathogenic A. cladogamus did not lead to any significant increase of pisatin concentration compared with healthy roots. The presence of pisatin was also found in root border-cell exudates (Supplementary Data Fig. S2). Infection of pea roots by A. euteiches resulted in a significant increase in pisatin in root zones such as the maturation zone and root cap cells that escaped early infection (Fig. 3).
Fig. 2.
Analysis of phenolic compounds in different zones of the root tip. (A) HPLC–DAD chromatogram of the phenolic compounds isolated from different root zones analysed: root elongation zone (EZ), maturation zone (MZ) and root cap (RC). (B) Quantification of pisatin (mg g−1 d. wt) in pea roots in response to A. euteiches. Values are means + s.d. from three independent experiments. Different letters indicate significant differences between mean values, whereas means with the same letter are not significantly different (Newman–Keuls test, P < 0·05).
Fig. 3.
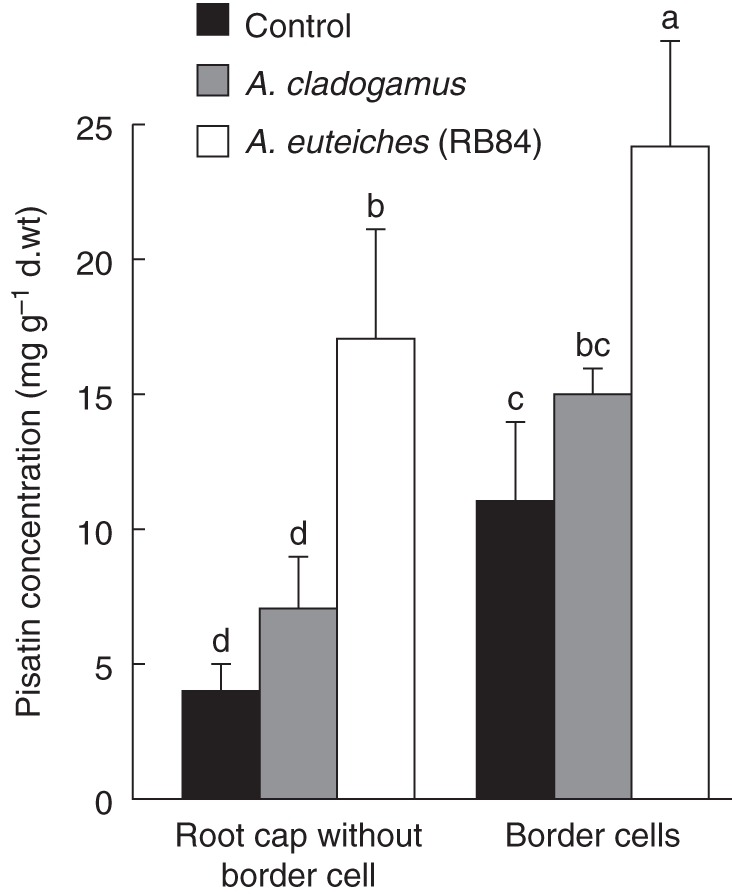
Concentration of pisatin (mg g−1 d. wt) in root caps and root border cells in healthy roots, roots inoculated with the non pathogenic isolate A. cladogamus and roots inoculated with the virulent isolate A. euteiches (RB84). To quantify pisatin in root border cells, phenolic compounds were extracted according to Sweigard et al. (1986). Data are expressed as mean values + s.d. from three independent experiments. Different letters indicate significant differences between mean values from three independent experiments (Newman–Keuls test, P < 0·05).
Phenolics from root tips inhibit A. euteiches growth
Due to the specific delay in the infection of the root cap by A. euteiches, total phenolic compounds were extracted from P. sativum root caps in order to assess their effects on mycelial growth and the number of oospores produced. It was found that phenolic compounds had inhibitory effects on A. euteiches development with a 19 % decrease in the number of oospores produced and a 41 % decrease in mycelial growth (Fig. 4A, B). The effects of exogenous commercial pisatin on mycelial growth and spore production of A. euteiches was also assessed according to Pueppke and Van Etten (1976). Spore production and hyphal growth exhibited differential sensitivity to pisatin (Fig. 4C, D). Hyphal growth showed increasing sensitivity to increasing amounts of pisatin; a slight decrease in growth was detected at 25 µg mL−1 and growth was completely inhibited at 125 µg mL−1 of pisatin (Fig. 4D). In addition, levels of spore production were significantly reduced in the presence of 50 µg mL−1 of pisatin and no spores at all were produced in 125 µg mL−1 of pisatin (Fig. 4C).
Fig. 4.
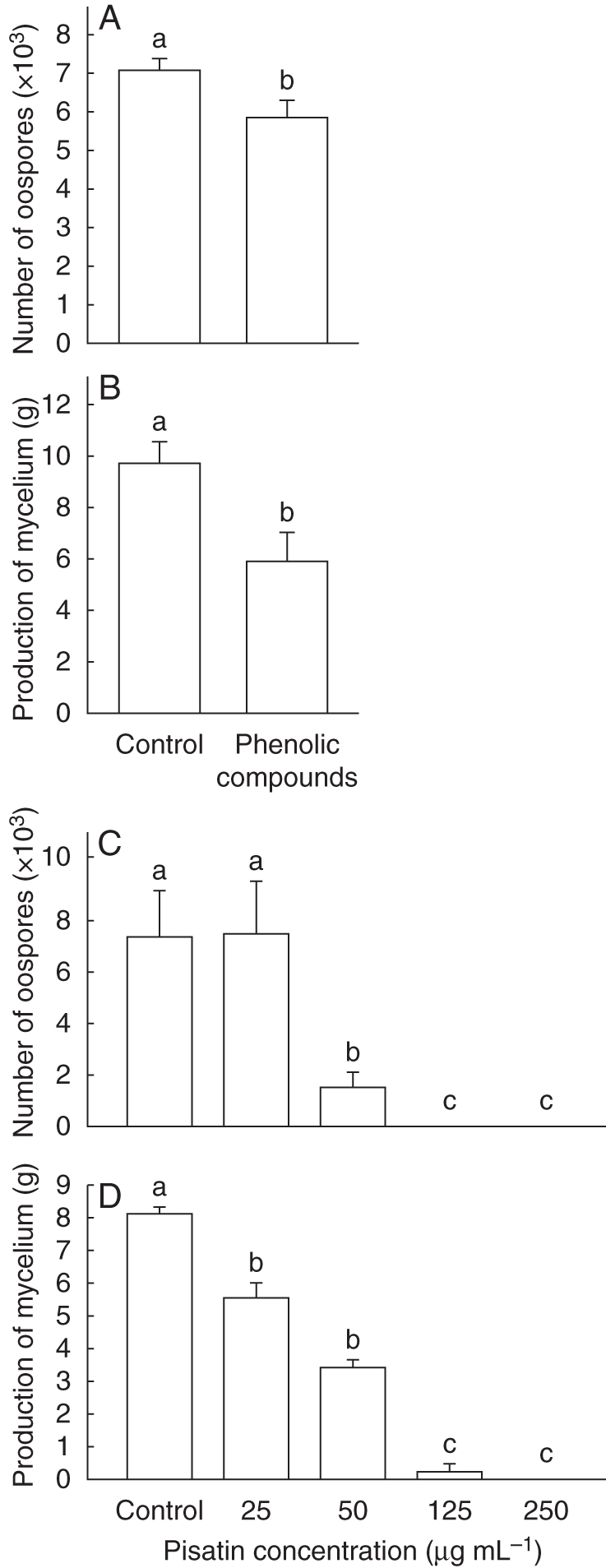
Effect of phenolic compounds isolated from healthy root border cells of pea on A. euteiches spore production (A) or mycelial growth (B). Effect of pisatin on A. euteiches spore production (C) or mycelial growth (D). Data are expressed as mean values + s.d. from seven independent experiments. Different letters refer to significant differences between mean values with Newman–Keuls test (P < 0·05).
Aphanomyces euteiches stimulates border-cell production and/or release from the root cap
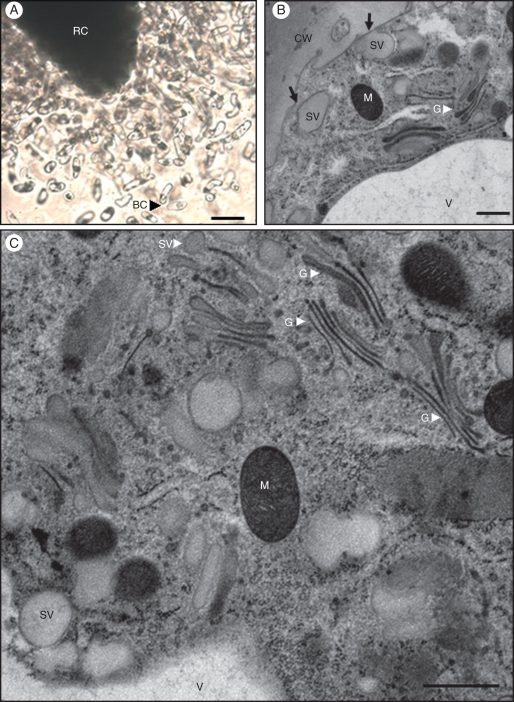
In pea, root border cells formed a large population of living cells that detached from the root cap as isolated cells (Fig. 5A). Electron microscopic examination of root border cells from control plants showed the presence of numerous Golgi stacks and mitochondria within the cytoplasm (Fig. 5B, C). Golgi stacks were characterized by well-defined cisternae with densely stained luminal content and vesicles at their margins which reflects an important activity of secretion in detached root border cells. Additionally, large vesicles were frequently seen in the vicinity of the cell wall and appeared sometimes to fuse with the plasma membrane, probably to deliver their contents into the external environment (Fig. 5B).
Fig. 5.
Microscopical characterization of pea border cells. (A) Root border cells are released from the root cap as individual cells. (B, C) Electron micrographs of isolated root border cells released from healthy root of P. sativum; in (B) note the presence of large secretory vesicles in close vicinity to the cell wall, some of which appear to fuse with the plasma membrane (indicated by black arrows) and in (C) numerous Golgi stacks and secretory vesicles in the cytoplasm. Abbreviations: BC, border cells; CW, cell wall; G, Golgi stack; M, mitochondria; RC, root cap; sv, secretory vesicles; V, vacuole. Scale bars: (A) = 20 µm; (B, C) = 0·7 µm.
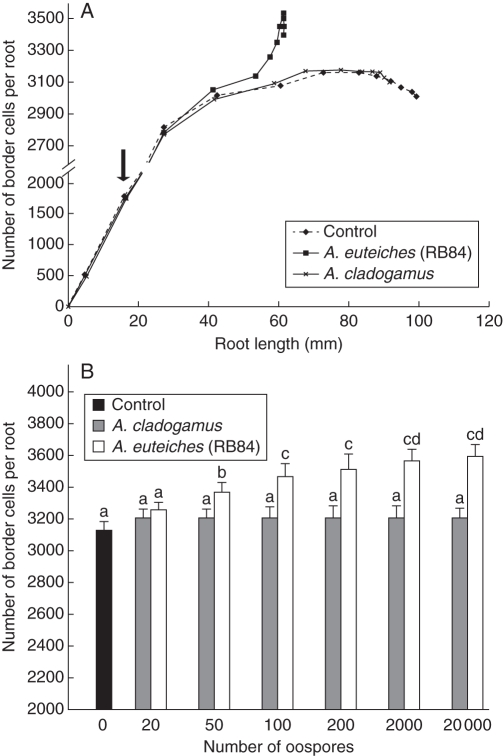
To characterize border-cell production after inoculation further, the formation of border cells over time was followed in control plants and in plants inoculated with either A. cladogamus or A. euteiches (RB84). As shown in Fig. 6A, the production of border cells (510 ± 53) occurred synchronously with primary root tip emergence. The number of border cells in control plants increased with increasing root length until a maximum of 3080 ± 82 (corresponding to a root length of 61 mm), followed by a decline in border-cell production. The number of border cells produced in response to infection by A. euteiches was significantly higher compared with the control plant with a maximum of 3530 ± 105 border cells produced. The stimulatory effect of A. euteiches on border-cell production was dependent on the number of oospores inoculated (Fig. 6B). There was no significant increase in border-cell production in response to the non-pathogenic A. cladogamus whereas a highly significant response occurred with A. euteiches (RB84). Together these data suggest that root border cells are involved in root protection against A. euteiches. To test this hypothesis, root border cells were washed off and roots were inoculated with A. euteiches oospores. However, the susceptibility of pea root to A. euteiches was not affected as root cap turnover occurred very rapidly and the production of border cells was completely renewed within 24 h (Supplementary Data Fig. S3).
Fig. 6.
Production of root border cells. (A) Average number of border cells per root tip. Root growth and border-cell production were followed for healthy P. sativum and P. sativum inoculated with the non-pathogenic A. cladogamus and the pathogenic A. euteiches isolates RB84. The arrow indicates inoculation of roots by either A. cladogamus or A. euteiches (200 oospores g−1 medium). (B) Dosage effects at 4-d post-inoculation of the number of oospores inoculated on border-cell production. Data are expressed as mean values + s.d. from three independent experiments. Different letters refer to significant differences between mean values (Newman–Keuls test, P < 0·05).
Border cell characterization using flow cytometric analysis
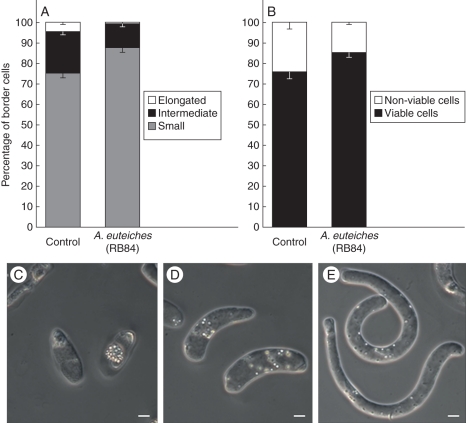
Flow cytometry was used to analyse whether A. euteiches inoculation influenced border cell size and/or viability. Measurement of FS provides an estimation of cell size (high FS corresponds to larger cells) and SS provides a measure of granularity of the cell (high SS corresponds to greater granularity) (Weir et al., 2005). Three subpopulations of root border cells were identified on the FS vs. SS cytograms (Supplementary data Fig. S4). The border cell population with higher FS corresponded to 5 %, intermediate FS corresponds to 20 % and the lower population to 75 % of the total population of border cells in control plants (Fig. 7A). Differences in FS vs. SS indicating populations of border cells with various sizes were further confirmed by microscopy. Border cells were shown to be present as small and cytoplasmically dense cells, intermediate cells and more elongated and vacuolated cells (Fig. 7C–E). Upon inoculation with A. euteiches, the presence of a new border cell population was not detected on the FS vs. SS cytogram. However, a significant increase in the proportion of smaller cells (characterized by low FS) was reported corresponding to 85 % of the whole population (Fig. 7A). This subpopulation appeared to be more homogeneous as compared with control plants (Supplementary Data Fig. S4). Furthermore calcein-AM staining revealed that the percentage of living border cells (at 5 d after inoculation) slightly increased in response to inoculation with A. euteiches (Fig. 7B). The percentage of living cells correlates with the percentage of small root border cells and we speculate that these specific root border cells are particularly active and involved in root defence.
Fig. 7.
Effect of A. euteiches infection on both (A) border cell morphology and (B) viability analysed by flow cytometry. (C–E) Micrographs showing the three types of border cells released by the root tip of P. sativum: small spherical cells (C), intermediate-size cells (D) and elongated cells (E). Scale bars = 5 µm.
DISCUSSION
Root tips of susceptible species have been shown to be more resistant than other root tissues to pathogen infection under diverse conditions, but the underlying basis for this phenomenon remains unclear (Rovira and Campbell, 1974; Foster et al., 1983; Bruehl, 1986; Turlier et al., 1994; Baluska et al., 1996; Olivain and Alabouvette, 1999; Lagopodi et al., 2002). In the present study, it was found that root caps and border cells from P. sativum remain uninvaded at early stages of infection by the pathogenic oomycete A. euteiches. The present major findings were that border cells respond to pathogen challenge by changing their morphology and producing a significant amount of pisatin. Furthermore, stimulation of border-cell production upon inoculation was found to occur proportionally with the number of oospores inoculated.
Roots are known to secrete a great diversity of chemicals with antimicrobial activity into the rhizosphere, pisatin being the major isoflavonoid phytoalexin synthesized by pea (Wu and Van Etten, 2004). It was found that the phenolic profile was specific for the maturation zone, the root elongation zone and root cap cells. In P. sativum root tips, high levels of pisatin were detected in the root maturation zone (showing the presence of mycelium over the surface but no infection of internal tissues), and root border cells and root cap cells (deprived of pathogen) at an early stage of infection. It was shown that the elongation zone produced only a low amount of this isoflavonoid in response to infection by A. euteiches. One plausible explanation is that pisatin is not produced by the root elongation zone or is produced in such low levels that it is not sufficient to protect the root against invasion by A. euteiches. In contrast, root border cells responded to A. euteiches inoculation with a significant increase in the production of pisatin. As far as is known this finding has not been reported previously. An increase in the number of border cells in the presence of A. euteiches was also observed in the present study. Interestingly, Curlango-Rivera et al. (2010) showed that treatment of root tips with exogenous pisatin enhanced border-cell production in vitro. Whether pisatin could play a role in vivo in both border-cell production and defence remains to be established. The root cap turnover and subsequent border-cell formation is self-regulated by a signal called ‘factor B’ produced by root border cells and released in root exudates (Brigham et al., 1998). As root border cells accumulate on the root cap, factor B accumulates in the root exudate until it reaches a level that inhibits the root-cap turnover and border-cell separation from the root cap. Removal of this factor by washing off root border cells induces renewed production of border cells by the root cap. It could be speculated that, upon infection by pathogens, other factors such as pisatin produced by border cells would in turn stimulate the production of root border cells, possibly enhancing the root cap protection. The impact of pisatin has been previously questioned in the interaction A. euteiches–P. sativum (Pueppke and Van Etten, 1976). Although pisatin was shown to inhibit in vitro growth of the pathogen, A. euteiches was insensitive to pisatin in vivo in pea epicotyls. The authors proposed several hypotheses to explain this apparent discrepancy. One of these was that the distribution of pisatin was tissue or cell specific allowing the pathogen to successfully avoid the phytoallexin in vivo. The present studies revealed that pisatin differentially accumulates in root tissues. One can envisage that the relative susceptibility of the different parts of the pea root could be correlated with different abilities to produce and/or accumulate pisatin even though pisatin by itself is not sufficient to completely prevent the occurrence of root infection.
What then would be the mechanism responsible for the specific exclusion of the pathogen from root border cells? Border cells synthesize and secrete >100 proteins into the extracellular environment including defence-related proteins such as proteases and peroxidases (Brigham et al., 1995; Wen et al., 2007; Hawes et al., 2011). When pea roots are inoculated with the pathogenic fungi N. haematococca, lesions are present in the region just behind the root tip whereas the root caps remain uninfected. The protection of the root cap appears to be correlated with the presence of root border cells (Gunawardena et al., 2002, 2005). Treatment with protease increases the root-cap infection by N. haematococca showing that proteins synthesized by root border cells are crucial in root-cap defence (Wen et al., 2007). Furthermore, the presence of exDNA in root border-cell exudate of P. sativum was reported in a recent paper (Wen et al., 2009). In mammals, it is now well established that exDNA from blood cells such as neutrophils, eosinophils and mast cells plays a major role in defence against microbial pathogen invasion. Upon infection, exDNA with extracellular peptides and proteins forms a complex neutrophil extracellular trap called ‘NET’ that aggregates and kills the pathogens (Wartha et al., 2007; Medina, 2009). In peas, the digestion of exDNA from root border-cell exudate using enzymes such as DNase I results in loss of root tip resistance to N. haematococca infection (Wen et al., 2009). Hawes et al. (2011) proposed that root tips produce extracellular root slime ‘traps’ involving exDNA and proteins that modulate adhesion and aggregation of the pathogens and contribute to root cap protection (Wen et al., 2009; Hawes et al., 2011). Therefore, it is tempting to speculate that such a mechanism of defence involving exDNA, together with antimicrobial metabolites including pisatin, could be responsible for border-cell resistance to A. euteiches.
Another major finding of this study is that upon infection root border cells change their size and morphology, being released as small, spherical and viable cells. Furthermore border-cell production was significantly enhanced in response to A. euteiches infection. Increasing the release of border cells in response to A. euteiches infection could prevent anchorage of encysted zoospores at the root cap surface and/or increase the release of antimicrobial compounds such as pisatin in the close vicinity of the root cap. It is clear that protection by border cells is not sufficient to protect roots from A. euteiches infection but such a mechanism would provide early and transitory defence to the root cap and root meristem. These results support the hypothesis that root border cells in pea are involved in local root defence against pathogen attack both mechanically and chemically. We suggest that small spherical cells are more active in root defence than elongated ones and further investigation is required to identify the metabolites specifically produced by this category of root border cells.
To conclude, the present findings offer new perspectives to promote natural root protection against disease. One strategy would be to stimulate the production of root border cells by natural treatments of the plant (e.g. elicitors from pathogens such as cellulose-binding elicitor lectin from Phytophhtora parasitica, or cell wall fragments from A. euteiches) to increase the synthesis and release of defence molecules in the rhizosphere. Such a new technology would be particularly promising to improve P. sativum resistance towards pathogenic fungi and oomycetes.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The authors thank Lisa Castel (Laboratoire Biosol Esitpa – Rouen, France) for excellent technical assistance with the flow cytometric analyses, Prof. Jean Claude Mollet (Université de Rouen, France) for discussions and Prof. Hans Van Etten (University of Arizona) for help and advice concerning the pisatin quantification and for critical reading of the manuscript. Anne Moussart and Prof. Bernard Tivoli (INRA Rennes, France) are also acknowledged for providing isolates of A. euteiches. This work was supported by l'Université de Rouen and le Grand Réseau de Recherche « Végétal, Agronomie et Tranformation des Agroressources », Conseil Régional de Haute Normandie, and was co-supervised by A.D. and M.V.-G.
This paper is dedicated to the memory of our colleague Professor Sylvie Barray, a respected member of the GGR VATA, who passed away on 8 October 2010.
LITERATURE CITED
- Badreddine I, Lafitte C, Heux L, et al. Cell wall chitosaccharides are essential components and exposed patterns of the phytopathogenic oomycete Aphanomyces euteiches. Eukaryotic Cell. 2008;7:1980–1993. doi: 10.1128/EC.00091-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;290:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- Baluska F, Volkmann D, Barlow PW. Specialized zones of development in roots: view from the cellular level. Plant Physiology. 1996;112:3–4. doi: 10.1104/pp.112.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bødker L, Kjøller R, Kristensen K, Rosendahl S. Interactions between indigenous arbuscular mycorrhizal fungi and Aphanomyces euteiches in field-grown pea. Mycorrrhiza. 2002;12:7–12. doi: 10.1007/s00572-001-0139-4. [DOI] [PubMed] [Google Scholar]
- Brigham LA, Woo HH, Nicoll SM, Hawes MC. Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiology. 1995;109:457–463. doi: 10.1104/pp.109.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham LA, Woo HH, Wen F, Hawes MC. Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea by endogenous signals. Plant Physiology. 1998;118:1223–1231. doi: 10.1104/pp.118.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl GW. Soilborne plant pathogens. New York, NY: Macmillan Publishing Company; 1986. [Google Scholar]
- Cruickshank IAM, Perrin DR. Isolation of a phytoalexin from Pisum sativum L. Nature. 1960;187:799–800. doi: 10.1038/187799b0. [DOI] [PubMed] [Google Scholar]
- Cunningham JL, Hagedorn DJ. Attraction of Aphanomyces euteiches zoospores to pea and other plant roots. Phytopathology. 1962;52:616–618. [Google Scholar]
- Curlango-Rivera G, Duclos DV, Ebolo JJ, Hawes M. Transient exposure of root tips to primary and secondary metabolites: impact on root growth and production of border cells. Plant and Soil. 2010;332:267–275. [Google Scholar]
- Deacon JW, Saxena G. Germination triggers of zoospore cysts of Aphanomyces euteiches and Phytophthora parasitica. Mycological Research. 1998;102:33–41. [Google Scholar]
- Driouich A, Zhang GF, Staehelin LA. Effect of brefeldin A on the structure of the Golgi apparatus and on the synthesis and secretion of proteins and polysaccharides in sycamore maple (Acer pseudoplatanus) suspension-cultured cells. Plant Physiology. 1993;101:1363–1373. doi: 10.1104/pp.101.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A, Durand C, Vicré-Gibouin M. Formation and separation of root border cells. Trends in Plant Science. 2007;12:14–19. doi: 10.1016/j.tplants.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Driouich A, Durand C, Cannesan MA, Percoco G, Vicré-Gibouin M. Border cells versus border-like cells: are they alike? Journal of Experimental Botany. 2010;61:3827–3831. doi: 10.1093/jxb/erq216. [DOI] [PubMed] [Google Scholar]
- Durand C, Vicré-Gibouin M, Follet-Gueye ML, et al. The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiology. 2009;150:1411–1421. doi: 10.1104/pp.109.136382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RC, Rovira AD, Cock TW. Ultrastructure of the root–soil interface. St Paul, MN: American Phytopathological Society; 1983. [Google Scholar]
- Gaulin E, Dramé N, Lafitte C, et al. Cellulose binding domains of Phytophtora cell wall protein are novel pathogen-associated molecular patterns. The Plant Cell. 2006;18:1766–1777. doi: 10.1105/tpc.105.038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin E, Jacquet C, Bottin A, Dumas B. Root rot disease of legumes caused by Aphanomyces euteiches. Molecular Plant Pathology. 2007;8:539–548. doi: 10.1111/j.1364-3703.2007.00413.x. [DOI] [PubMed] [Google Scholar]
- Grau CR, Muehlchen AM, Tofte JE. Variability in virulence of Aphanomyces euteiches. Plant Disease. 1991;75:1153–1156. [Google Scholar]
- Gunawardena U, Hawes MC. Tissue specific localization of root infection by fungal pathogens: role of root border cells. Molecular Plant–Microbe Interactions. 2002;15:1128–1136. doi: 10.1094/MPMI.2002.15.11.1128. [DOI] [PubMed] [Google Scholar]
- Gunawardena U, Rodriguez M, Straney D, Romeo JT, Van Etten HD, Hawes MC. Tissue-specific localization of pea root infection by Nectria haematococca: mechanisms and consequences. Plant Physiology. 2005;137:1363–1374. doi: 10.1104/pp.104.056366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham AR. Cell biology of plant–oomycete interactions. Cellular Microbiology. 2007;9:31–39. doi: 10.1111/j.1462-5822.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Gunawardena U, Miyasaka S, Zhao X. The role of root border cells in plant defense. Trends in Plant Science. 2000;5:128–133. doi: 10.1016/s1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Bengough G, Cassab G, Ponce G. Root caps and rhizosphere. Journal of Plant Growth Regulation. 2003;21:352–367. [Google Scholar]
- Hawes MC, Curlango-Rivera G, Wen F, White GJ, Van Etten HD, Xiong Z. Extracelluar DNA: the tip of root defenses. Plant Science. 2011;180:741–745. doi: 10.1016/j.plantsci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Kaimoyo E, Van Etten HD. Inactivation of pea genes by RNAi supports the involvement of two similar O-methyltransferases in the biosynthesis of (+)-pisatin and of chiral intermediates with a configuration opposite that found in (+)-pisatin. Phytochemistry. 2008;69:76–87. doi: 10.1016/j.phytochem.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Kjøller R, Rosendahl S. Enzymatic activity of the mycelium compared with oospore development during infection of pea roots by Aphanomyces euteiches. Phytopathology. 1998;88:992–996. doi: 10.1094/PHYTO.1998.88.9.992. [DOI] [PubMed] [Google Scholar]
- Kraft JM, Boge WL. Identification of characteristics associated with resistance to root rot disease caused by Aphanomyces euteiches in pea. Plant Disease. 1996;80:1383–1386. [Google Scholar]
- Lagopodi AL, Ram AFJ, Lamers GEM, et al. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Molecular Plant–Microbe Interactions. 2002;15:172–179. doi: 10.1094/MPMI.2002.15.2.172. [DOI] [PubMed] [Google Scholar]
- Lanoue A, Burlat V, Henkes GJ, Koch I, Schurr U, Röse USR. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytologist. 2010;185:577–588. doi: 10.1111/j.1469-8137.2009.03066.x. [DOI] [PubMed] [Google Scholar]
- Madoui MA, Bertrand-Michel J, Gaulin E, Dumas B. Sterol metabolism in the oomycete Aphanomyces euteiches, a legume root pathogen. New Phytologist. 2009;183:291–300. doi: 10.1111/j.1469-8137.2009.02895.x. [DOI] [PubMed] [Google Scholar]
- Medina E. Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. Journal of Innate Immunity. 2009;1:176–180. doi: 10.1159/000203699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivain C, Alabouvette C. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Phytologist. 1999;141:497–510. doi: 10.1046/j.1469-8137.1997.00855.x. [DOI] [PubMed] [Google Scholar]
- Papavizas GC, Ayers WA. Aphanomyces species and their root diseases on pea and sugarbeet: a review. 1974 USDA Technical Bulletin No. 1485, 1–158. [Google Scholar]
- Pueppke SG, Van Etten HD. The relation between pisatin and the development of Aphanomyces euteiches in diseased Pisum sativum. Phytopathology. 1976;66:1174–1185. [Google Scholar]
- Rovira AD, Campbell R. Scanning electron microscopy of microorganisms on roots of wheat. Microbial Ecology. 1974;1:15–23. doi: 10.1007/BF02512376. [DOI] [PubMed] [Google Scholar]
- Sauvage H, Moussart A, Bois F, Tivoli B, Barray S, Laval K. Development of a molecular method to detect and quantify Aphanomyces euteiches in soil. FEMS Microbiology Letters. 2007;273:64–69. doi: 10.1111/j.1574-6968.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- Shang H, Grau CR, Peters RD. Oospore germination of Aphanomyces euteiches in root exudates and on the rhizoplanes of crop plants. Plant Disease. 2000;84:994–998. doi: 10.1094/PDIS.2000.84.9.994. [DOI] [PubMed] [Google Scholar]
- Staehelin LA, Giddings TH, Kiss JZ, Sack FD. Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze substituted samples. Protoplasma. 1990;157:75–91. doi: 10.1007/BF01322640. [DOI] [PubMed] [Google Scholar]
- Sweigard JA, Matthews DE, Van Etten HD. Synthesis of the phytoalexin pisatin by a methyltransferase from pea. Plant Physiology. 1986;80:277–279. doi: 10.1104/pp.80.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlier M, Eparvier A, Alabouvette C. Early dynamic interactions between Fusarium oxysporum f. sp. lini and the roots of Linum usilatissimum as revealed by transgenic GUS-marked hyphae. Canadian Journal of Botany. 1994;72:1605–1612. [Google Scholar]
- Tyler BM. Molecular basis of recognition between Phytophtora pathogens and their hosts. Annual Review of Phytopathology. 2002;40:137–167. doi: 10.1146/annurev.phyto.40.120601.125310. [DOI] [PubMed] [Google Scholar]
- Vandemark G, Kraft JM, Larsen R, Gritsenko M, Boge W. A PCR based assay by sequence-characterized DNA markers for the identification and detection of Aphanomyces euteiches. Phytopathology. 2000;90:1137–1144. doi: 10.1094/PHYTO.2000.90.10.1137. [DOI] [PubMed] [Google Scholar]
- Vicré M, Santaella C, Blanchet S, Gateau A, Driouich A. Root border-like cells of Arabidopsis: microscopical characterization and role in the interaction with rhizobacteria. Plant Physiology. 2005;138:998–1008. doi: 10.1104/pp.104.051813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartha F, Beiter K, Normark S, Henriques-Normark B. Neutrophil extracellular traps: casting the NET over pathogenesis. Current Opinion in Microbiology. 2007;10:52–56. doi: 10.1016/j.mib.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Weir IE, Maddumage R, Allan AC, Ferguson IB. Flow cytometric analysis of tracheary element differentiation in Zinnia elegans cells. Cytometry. 2005;68:81–91. doi: 10.1002/cyto.a.20194. [DOI] [PubMed] [Google Scholar]
- Wen F, Van Etten HD, Tsaprailis G, Hawes MC. Extracellular proteins in pea root tip and border-cell exudates. Plant Physiology. 2007;143:773–783. doi: 10.1104/pp.106.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, White GJ, Van Etten HD, Xiong Z, Hawes MC. Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiology. 2009;151:820–829. doi: 10.1104/pp.109.142067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker E, Hullé M, Rouxel F. Pathogenic characteristics of isolates of Aphanomyces euteiches recovered from pea in France. Plant Pathology. 2001;50:433–442. [Google Scholar]
- Wicker E, Moussart A, Duparque M, Rouxel F. Further contributions to the development of a differential set of pea cultivars (Pea sativum) to investigate the virulence of isolates of Aphanomyces euteiches. European Journal of Plant Pathology. 2003;109:47–60. [Google Scholar]
- Wu Q, Van Etten HD. Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their responses to a fungal pathogen. Molecular Plant–Microbe Interactions. 2004;17:798–804. doi: 10.1094/MPMI.2004.17.7.798. [DOI] [PubMed] [Google Scholar]
- Wuyts N, Maung ZTZ, Swennen R, Waele DD. Banana rhizodeposition: characterization of root border cell production and effects on chemotaxis and motility of the parasitic nematode Radopholus similis. Plant and Soil. 2006;283:217–228. [Google Scholar]
- Yokosava R, Kuninaga S, Sekizaki H. Aphanomyces euteiches zoospore attractant isolated from pea root: prunetin. Annals of Phytopathological Society of Japan. 1986;52:809–816. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.