Abstract
Background and Aims
Anthropogenic depletion of stratospheric ozone in Arctic latitudes has resulted in an increase of ultraviolet-B radiation (UV-B) reaching the biosphere. UV-B exposure is known to reduce above-ground biomass and plant height, to increase DNA damage and cause accumulation of UV-absorbing compounds in polar plants. However, many studies on Arctic mosses tended to be inconclusive. The importance of different water availability in influencing UV-B impacts on lower plants in the Arctic has been poorly explored and might partially explain the observed wide variation of responses, given the importance of water in controlling bryophyte physiology. This study aimed to assess the long-term responses of three common sub-Arctic bryophytes to enhanced UV-B radiation (+UV-B) and to elucidate the influence of water supply on those responses.
Methods
Responses of three sub-Arctic bryophytes (the mosses Hylocomium splendens and Polytrichum commune and the liverwort Barbilophozia lycopodioides) to +UV-B for 15 and 13 years were studied in two field experiments using lamps for UV-B enhancement with identical design and located in neighbouring areas with contrasting water availability (naturally mesic and drier sites). Responses evaluated included bryophyte abundance, growth, sporophyte production and sclerophylly; cellular protection by accumulation of UV-absorbing compounds, β-carotene, xanthophylls and development of non-photochemical quenching (NPQ); and impacts on photosynthesis performance by maximum quantum yield (Fv /Fm) and electron transport rate (ETR) through photosystem II (PSII) and chlorophyll concentrations.
Results
Responses were species specific: H. splendens responded most to +UV-B, with reduction in both annual growth (–22 %) and sporophyte production (–44 %), together with increased β-carotene, violaxanthin, total chlorophyll and NPQ, and decreased zeaxanthin and de-epoxidation of the xanthophyll cycle pool (DES). Barbilophozia lycopodioides responded less to +UV-B, showing increased β-carotene and sclerophylly and decreased UV-absorbing compounds. Polytrichum commune only showed small morphogenetic changes. No effect of UV-B on bryophyte cover was observed. Water availability had profound effects on bryophyte ecophysiology, and plants showed, in general, lower growth and ETR, together with a higher photoprotection in the drier site. Water availability also influenced bryophyte responses to +UV-B and, in particular, responses were less detectable in the drier site.
Conclusions
Impacts of UV-B exposure on Arctic bryophytes were significant, in contrast to modest or absent UV-B effects measured in previous studies. The impacts were more easily detectable in species with high plasticity such as H. splendens and less obvious, or more subtle, under drier conditions. Species biology and water supply greatly influences the impact of UV-B on at least some Arctic bryophytes and could contribute to the wide variation of responses observed previously.
Keywords: Enhanced long-term UV-B, water availability, sub-Arctic bryophytes, xanthophylls, chlorophyll fluorescence, sporophytes
INTRODUCTION
Anthropogenic activity has depleted stratospheric ozone by about 7 % over the Arctic (Weatherhead et al., 2005) with a consequential increase in solar ultraviolet-B radiation (UV-B: 280–315 nm) reaching the biosphere. Full recovery of the Arctic ozone layer is not expected until 2050 (World Meteorological Organization, 2007). This increase in UV-B irradiance is of concern because it can reduce above-ground biomass and plant height and increase DNA damage and the accumulation of UV-absorbing compounds in polar plants (Newsham and Robinson, 2009) with possible ecosystem consequences (Caldwell et al., 1995; Paul and Gwynn-Jones, 2003; Newsham and Robinson, 2009).
Among Arctic plants, bryophytes are particularly important determinants of Arctic ecosystem functioning due to their abundance and contribution to above-ground biomass production, their importance in mediating ecosystem biogeochemical cycling, affecting water balance and regulating soil temperatures, as well as their value in creating microenvironments for vascular plants and micoorganisms (Longton, 1997; Cornelissen et al., 2007; Woodin et al., 2009). UV-B impacts on bryophyte performance may, therefore, have further consequences for the whole ecosystem.
Studies investigating the effects of enhanced UV-B on Arctic bryophytes have so far shown a diversity of responses, with UV-B either stimulating, depressing or having no effect on growth or physiology (for reviews, see Callaghan et al., 2004; Rozema et al., 2005; Boelen et al., 2006; Newsham and Robinson, 2009). This variety of responses is commonly explained by the diversity of species and different experimental approaches (e.g. filters vs. lamps) (Newsham and Robinson, 2009). Surprisingly, the influence of other environmental factors on species responses to UV-B has rarely been measured, but could play a key role in mediating responses. Sonesson et al. (2002), for instance, explained that the differing results for UV-B impacts on Sphagnum fuscum growth from similar experiments on similar mire sites were due to different weather conditions in the years when the studies were performed. Further, while Arctic moss responses to UV-B have been reported, no liverwort or hornwort has been studied.
Water availability might particularly influence UV-B impact on bryophytes because they are poikilohydric. When water is not available, their leaf cells dry out and metabolism can cease within a few minutes (Proctor, 2009). Many bryophytes have developed desiccation tolerance which is possibly assisted by the development of antioxidant and photo-protection mechanisms that can scavenge or minimize the generation of reactive oxygen species (ROS; Proctor, 2009). This cellular protection might confer an advantage during periods of UV-B exposure. Turnbull et al. (2009) found that differences in UV-B tolerance between three Antarctic species match their desiccation tolerance, with Ceratodon purpureus most tolerant of both water stress and UV-B, Bryum pseudotriquetrum intermediate and Schistidium antarctici the least tolerant to both stressors (more UV-B impact). UV-B impacts on bryophytes might therefore be related to species tolerance of moisture deficit.
The importance of water supply in influencing UV-B impacts in Arctic bryophytes was highlighted by Gehrke et al. (1996) and Phoenix et al. (2001), who found that negative effects of UV-B seen in Hylocomium splendens were removed by increased summer precipitation. Sonesson et al. (2002) found that increased UV-B had no significant overall effect on growth in length or chlorophyll and flavonoid contents of Sphagnum fuscum and Dicranum elongatum. However, the growth of S. fuscum – the species most sensitive to dehydration – responded negatively to increased UV-B under increased temperature at the peak of the growing season – a response considered to be driven by greater water limitation. While these studies have been very valuable, uncertainty continues, and the influence of water supply on UV-B impacts have yet to be specifically assessed in Arctic bryophytes.
In the present study, therefore, we investigated whether long-term enhanced UV-B exposure (+UV-B) changed the abundance and growth of three common bryophyte species in two sub-Arctic heathland sites of contrasting water availability (naturally mesic and drier). We assessed the development of cellular protection mechanisms from UV-B exposure and/or water stress such as the accumulation of extractable UV-absorbing compounds, antioxidant carotenoids (e.g. β-carotene and zeaxanthin) together with the development of photoprotection by de-epoxidation of the xanthophyll cycle pool (DES) and non-photochemical quenching (NPQ) (Newsham and Robinson, 2009 for UV-B exposure; Oliver, 2009 for water stress). The impact of +UV-B on bryophyte photosynthetic activity was evaluated by measuring the maximum quantum yield of photosystem II (PSII; (Fv/Fm)], the electron transport rate through PSII (ETR) and chlorophyll concentrations. In addition, the ‘sclerophylly index’ (the quotient between the shoot mass and the surface area of the fresh prostrate apex) was measured as a proxy for changes in the proportion of non-photosynthetic tissue. We also studied – apparently for the first time in bryophytes – the influence of +UV-B on sporophyte production.
The objectives of the present study were to (1) explore further the influence of water supply and species biology on the impact of UV-B on Arctic bryophytes, and (2) seek an explanation of the published variable results on UV-B effects on Arctic bryophytes. Our hypothesis was that the magnitude of UV-B impacts on the abundance or physiology (growth, reproduction, photosynthetic activity or cellular protection) of bryophytes would be related to prevailing conditions of moisture availability and their tolerance of moisture deficit. To achieve these aims we evaluated UV-B impacts in neighbouring sites with contrasting water availability (naturally mesic and drier sites) that contained two long-term UV-B-enhanced experiments established in 1991 and 1993 (Johanson et al., 1995; Gehrke et al., 1996; Phoenix et al., 2001). These sites represent a unique opportunity to test, in the long term and without the artefacts of disturbance due to the establishment of experimental treatments, the influence of species biology and water supply on the impact of UV-B on Arctic bryophytes.
MATERIALS AND METHODS
Study species
Three bryophyte species with contrasting biology were selected: the feather moss Hylocomium splendens (Hedw.) Schimp., the common haircap moss Polytrichum commune Hedw. and the foliose liverwort Barbilophozia lycopodioides (Wallr.) Loeske. The three species are widespread terrestrial bryophytes in the northern hemisphere. Hylocomium splendens is an ectohydric weft-forming pleurocarpous moss with a plagiotropic growth form, typical of dry to slightly damp heath, grasslands and forests. Polytrichum commune is an endohydric turf-forming acrocarpous moss with an orthotropic growth form, occurring in a wide range of acid habitats, usually in damp to wet situations. The biology and population ecology of H. splendens and P. commune from the study area are described in Callaghan et al. (1978). Barbilophozia lycopodioides is a circumboreal sub-arctic–sub-alpine liverwort growing in tufts on well-drained soils in forests, shrublands and grasslands (Damsholt, 2002). Differences in morphology, anatomy, habitat preference and physiology indicate varying responses to water availability that we hypothesize will, to some extent, influence the bryophytes' responses to UV-B.
Experimental design
The study was undertaken in June 2006 in a sub-Arctic heathland at the Abisko Scientific Research Station, Abisko, Sweden (68°21′N, 18°49′E, 340–370 m a.s.l.) using two long-term UV-B enhancement experiments. The first experiment was established in 1991 in a drier area of the heath (Johanson et al., 1995) and the second in 1993 in a neighbouring (<50 m away) mesic area (Gehrke et al., 1996). This natural contrast in water availability between the two experimental set-ups allowed a unique long-term comparison of the influence of the water environment (mesic vs. drier) on UV-B impacts on Arctic bryophytes. The two sites have different durations of UV-B treatment (15 and 13 years) but the difference is relatively small in comparison with the duration of the experiments and is unlikely to cause differences in UV-B response, particularly in comparison with short-term experiments in which interannual weather and UV-B variability can be significant. The heathland community consists of an open birch canopy [Betula pubescens Ehrh. ssp. czerepanovii (N. I. Orlova) Hämet-Ahti] with a dense dwarf shrub understorey dominated by Empetrum hermaphroditum Hagerup, Vaccinium vitis-idaea L. (both evergreen), V. myrtillus L. and V. uliginosum L. (both deciduous).
The experiments were established with the aim of exposing heath communities to an enhanced UV-B regime that simulated a 15 % reduction in stratospheric ozone under clear sky conditions at Abisko. This enhancement was achieved from six parallel fluorescent tube lamps (Q-PANEL UVB-313, Cleveland, OH, USA) mounted on metal frames (2·5 × 1·3 m), 1·5 m above the soil level. The middle 70 cm of the two central lamps was covered in aluminium foil to allow even irradiance throughout the plot at canopy height. Either glass or UV transparent Plexiglass (Röhm GmbH, Darmstadt, Germany) with cellulose diacetate foil (0·13 mm, Courtaulds, Derby, UK) was placed directly below each lamp to expose heath vegetation to ambient UV-B (control) and enhanced UV-B (+UV-B), respectively. UV-B lamps were switched on progressively around noon by timers which controlled three of the six lamps per frame simultaneously to gain a step-wise ‘square wave’ increase and decrease in daily UV-B enhancement. Maximum biologically effective UV-B doses, which were calculated using the generalized plant damage action spectrum from Caldwell (1971) normalized at 300 nm, corresponded to 5·8 kJ m−2 d−1 for +UV-B plots and 4·6 kJ m−2 d−1 for control plots. Further information about the experimental designs can be found in Johanson et al. (1995), Gehrke et al. (1996) and Phoenix et al. (2001).
The physiognomy of the mesic and drier sites was visibly different, with the mesic site having much greater abundance of bryophytes than the drier site. Measurements of soil moisture (Delta-T probe, Cambridge, UK) during peak biomass (August) confirmed that the mean water contents of drier and mesic plots were significantly different (37·3 and 55·7 %, respectively, P < 0·01).
Each radiation regime (control and +UV-B) was replicated four times in both the mesic and the drier sites (16 plots in total).
Species abundance and physiological measurements
Plant cover and sporophyte abundance
Percentage cover of each bryophyte and sporophyte abundance were estimated using a 50 × 50 cm quadrat with 25 squares of 10 × 10 cm placed centrally within each plot. The number of sporophytes (which were found only in H. splendens) was counted in each of the 25 squares.
Annual growth measurement
Both mosses have readily visible markers of annual growth (Callaghan et al., 1978). For H. splendens, individuals with sympodial growth were selected and the length of the stem of the newest bud was measured. For P. commune, new growth was identified by changes in leaf colour (new tissue was light green compared with dark green old tissue) and leaf morphology (smaller leaves are produced at the beginning and end of the growing season: Callaghan et al., 1978), and the length of the new stem was measured. In the case of the liverwort, a clear change in colour from brown to light green was used to recognize new growth, and the length of the new stem was measured. Fifteen individuals per species per plot were measured.
Sclerophylly index (SI)
The SI was calculated as the ratio between the dry mass (oven dried at 80 °C for 24 h) and the surface area of the fresh prostrate apex (measured using an LI-3000 leaf area meter, LI-COR, Lincoln, NE, USA). In bryophytes this variable can indicate the proportion of non-photosynthetic tissue (Martínez-Abaigar et al., 1994).
In vivo chlorophyll fluorescence
Chlorophyll fluorescence measurements were made using a MINIPAM portable pulse amplitude modulation fluorometer connected to a 2060-M micro quantum/temperature sensor (Walz, Effeltrich, Germany) following Schreiber et al. (1995). Minimal and maximal fluorescence (F0 and Fm) were measured on specimens dark adapted overnight (around 15 h) and maintained fully hydrated to ensure recovery. White actinic light was then applied following a light curve (LC) with incremental PPFD (0, 30, 100, 200, 400, 600, 800, 1200, and 1600 µmol m−2 s−1) with 30 s duration, a 0·8 s saturating pulse (10 000 µmol m−2 s−1) being applied between each interval. The maximum quantum yield of PSII (Fv/Fm), the ETR and the maximal quenching due to non-photochemical dissipation of absorbed light energy (NPQmax) were recorded during the LC. LC measurements were made at a near constant temperature of 10 °C. Further information on the method can be found in Núñez-Olivera et al. (2004).
Photosynthetic pigment composition
Plant samples were collected at around 1400 h on a day with clear skies. Shoot apices (approx. 3–5 cm2 of fresh tissue per sample depending on the species) were frozen in liquid nitrogen immediately after collection and stored in the dark at –80 °C. The content of chlorophylls a and b, and the chlorophyll a/b (Chl a/b) ratio, were measured by spectrophotometry and carotenoids by high-performance liquid chromatography (HPLC), following Arróniz-Crespo et al. (2008a). The content of individual carotenoids was expressed as a percentage of total carotenoids. The pool of xanthophylls of the xanthophyll cycle (XC) was calculated as (violaxanthin + antheraxanthin + zeaxanthin), and the extent of DES as (antheraxanthin + zeaxanthin)/(violaxanthin + antheraxanthin + zeaxanthin) (Demmig-Adams and Adams, 1996). Digital pictures of each tissue sample were taken before freezing and the surface area of each sample was calculated using the program Sigma Scan Pro5 (Systat Software, Inc., Chicago, IL, USA).
Methanol-extractable UV-absorbing compounds (MEUVACs)
Plant samples were collected immediately after the lamps were switched off (around 1500 h) and were air-dried at room temperature. From 10 to 20 mg dry mass (depending on the species) was used per sample for the analysis. After extraction in methanol:water:7 m HCl (70:29:1, v/v/v), the absorbance was measured spectrophotometrically and MEUVACs were expressed in arbitrary units as the area under the absorbance curve in the wavelength interval 280–400 nm (AUC280–400) on a dry mass basis (Arróniz-Crespo et al., 2006).
Statistical analysis
Overall effects of UV-B (control vs. long-term enhanced UV-B) and site (water regime: mesic vs. drier) were determined separately for each species with two-way analysis of variance (ANOVA). To identify if water availability can affect the magnitude of UV-B impacts, a Student's t-test between radiation regime was run at each site separately within the ANOVA when an overall UV-B effect or interaction between both factors was detected. Bonferroni's correction was used to determine the level of significance of the t-test (Rice, 1989). For all variables, measurements were taken from several plants (6–15 depending on the variable) in each plot; hence mean values per plot were used in the analyses. All statistical procedures were performed with SPSS 17·0·1 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Bryophyte percentage cover and sporophyte abundance
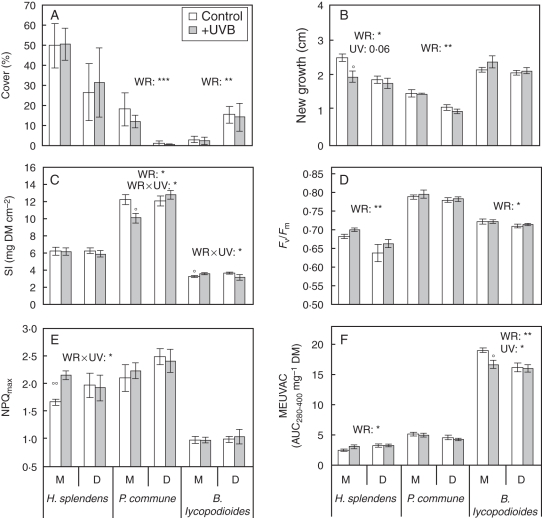
Hylocomium splendens was the most abundant bryophyte at both sites, with P. commune and B. lycopodioides being the least abundant in the drier and mesic sites, respectively. Percentage cover of the individual species was unaffected by +UV-B at both the mesic and drier sites (Fig. 1A). Cover of H. splendens was not significantly different between mesic and drier sites, whereas P. commune and B. lycopodioides showed significantly more and less abundance, respectively, in the mesic compared with the drier site (P < 0·001 and P < 0·01, respectively; Fig. 1A). Sporophytes were only observed on H. splendens at the mesic site where +UV-B almost halved their abundance (83 ± 3·5 and 46 ± 8·0 % in control and +UV-B plots, respectively; P < 0·025 Bonferroni correction).
Fig. 1.
(A) Percentage cover, (B) new growth, (C) sclerophylly index (SI; dry matter per surface area), (D) maximum quantum yield of PSII (Fv/Fm), (E) maximal non-photochemical quenching (NPQmax) and (F) methanol-extractable UV-absorbing compounds (MEUVACs) of the three bryophytes (the mosses Hylocomium splendens and Polytrichum commune, and the foliose liverwort Barbilophozia lycopodioides), studied in plots exposed to two radiation regimes (control or enhanced UV-B, as indicated) under two different water regimes (M, mesic plots; D, drier plots). Means ± s.e. are shown (n = 4). The main factor effects (two-way ANOVA) of water regime (WR) and UV-B radiation (UV), and the interactions between both (WR×UV), are indicated for each species when significant (***P < 0·001; **P < 0·01; *P < 0·05). Differences between control and +UV-B plots for each species and water regime separately are shown over the corresponding bars (Student́s t-test adjusted with Bonferroni; °P <0·025; °°P < 0·005).
Annual growth and sclerophylly index
Enhanced UV-B did not have an overall significant effect on growth or SI for any of the three species (Fig. 1B, C), although a near significant UV-B effect on growth was observed in H. splendens (ANOVA; P = 0·06). Growth of H. splendens decreased by 22 % under +UV-B (t-test, P < 0·025 Bonferroni correction) in the mesic site (Fig. 1B). Significant UV-B × site interactions were observed in P. commune and B. lycopodioides SI (ANOVA; P < 0·05). At the mesic site, P. commune SI was reduced (t-test, P < 0·025 Bonferroni correction) and B. lycopodioides SI increased under +UV-B (t-test, P < 0·025 Bonferroni correction), whereas no UV-B effect was detected in any species at the drier site (Fig. 1C).
Site effects (water regime) generally showed a greater overall impact than UV-B enhancement on the bryophytes. Both H. splendens and P. commune showed significantly lower growth (–18 and –35 %, respectively) in the drier compared with the mesic site (P < 0·05 and P < 0·01, respectively; Fig. 1B), while SI of P. commune was greater in the drier site compared with the mesic site (P < 0·05; Fig. 1C).
Chlorophyll concentration
Enhanced UV-B increased total chlorophyll concentrations overall in H. splendens (ANOVA; P < 0·05), but a significant UV-B × site interaction showed the increase to be greater at the drier site (Table 1; t-test, P < 0·025 Bonferroni correction). The mesic site had significantly lower total chlorophylls in P. commune and higher total chlorophylls in B. lycopodioides compared with the drier site (ANOVA: P < 0·001 and P < 0·01, respectively; Table 1).
Table 1.
Total chlorophyll concentration (Total Chl, mg m−2), chlorophyll a/b ratio (Chl a/b), neoxanthin (Neo), lutein (Lut), β-carotene (β-car), violaxanthin (Vio), antheraxanthin (Ant), zeaxanthin (Zea), together with the sum of the xanthophylls involved in the xanthophyll cycle (XC, Vio + Ant + Zea) and the extent of de-epoxidation of the xanthophyll cycle pool (DES), species and treatment details as for Fig. 1
|
Hylocomium splendens |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water regime | Radiation regime | Total Chl (mg m−2) | Chl a/b | Neo | Lut | β-car | Vio | Ant | Zea | XC | DES |
| M | Control | 33·1 ± 1·9 | 2·15 ± 0·07 | 6·7 ± 0·3 | 57·9 ± 0·8 | 18·0 ± 0·6 | 1·5 ± 0·3 | 2·2 ± 0·1 | 13·7 ± 0·5 | 17·4 ± 0·3 | 89·5 ± 0·8 |
| +UVB | 34·9 ± 0·9 | 2·22 ± 0·04 | 6·2 ± 0·1 | 55·3 ± 1·5 | 22·0 ± 0·9 ° | 1·8 ± 0·2 | 2·2 ± 0·2 | 12·4 ± 0·6 | 16·5 ± 0·7 | 88·3 ± 1·6 | |
| D | Control | 26·3 ± 0·6 | 2·61 ± 0·01 | 5·6 ± 0·5 | 48·7 ± 0·9 | 24·7 ± 0·9 | 3·2 ± 0·3 | 2·9 ± 0·1 | 14·8 ± 1·0 | 21·0 ± 0·8 | 84·5 ± 2·5 |
| +UVB | 37·7 ± 3·8 ° | 2·50 ± 0·05 | 7·6 ± 0·3 ° | 51·0 ± 0·6 | 22·3 ± 0·5 | 4·0 ± 0·2 | 2·7 ± 0·2 | 12·2 ± 0·8 | 19·0 ± 0·7 | 77·2 ± 1·6 | |
| UV*, WR × UV * | WR*** | WR*, WR × UV** | WR***, WR × UV* | WR***, WR × UV*** | WR***, UV* | WR** | UV* | WR***, UV * | WR**, UV* | ||
|
Polytrichum commune |
|||||||||||
| Water regime | Radiation regime | Total Chl (mg m−2) | Chl a/b | Neo | Lut | β-car | Vio | Ant | Zea | XC | DES |
| M | Control | 84·4 ± 6·1 | 2·50 ± 0·08 | 9·3 ± 0·2 | 54·7 ± 0·4 | 23·1 ± 0·3 | 6·3 ± 0·2 | 1·2 ± 0·1 | 5·3 ± 0·3 | 12·8 ± 0·7 | 50·2 ± 0·8 |
| +UVB | 65·1 ± 7·1 | 2·53 ± 0·04 | 8·8 ± 0·3 | 55·1 ± 0·9 | 23·6 ± 0·7 | 7·0 ± 0·1 | 1·3 ± 0·1 | 4·0 ± 0·6 | 12·3 ± 0·5 | 42·5 ± 3·3 | |
| D | Control | 151·4 ± 11·7 | 2·56 ± 0·05 | 8·0 ± 0·4 | 51·0 ± 1·0 | 25·2 ± 0·5 | 7·4 ± 0·9 | 2·5 ± 0·3 | 5·7 ± 1·4 | 15·7 ± 0·9 | 51·2 ± 8·1 |
| +UVB | 143·1 ± 9·7 | 2·47 ± 0·04 | 8·9 ± 0·1 | 53·7 ± 0·9 | 22·1 ± 2·4 | 8·5 ± 0·3 | 2·2 ± 0·4 | 4·5 ± 1·2 | 15·1 ± 1·6 | 42·7 ± 5·6 | |
| WR*** | WR × UV* | WR* | WR* | WR*** | WR* | ||||||
|
Barbilophozia lycopodioides |
|||||||||||
| Water regime | Radiation regime | Total Chl (mg m−2) | Chl a/b | Neo | Lut | β-car | Vio | Ant | Zea | XC | DES |
| M | Control | 69·1 ± 3·4 | 2·03 ± 0·02 | 10·1 ± 0·2 | 59·9 ± 0·5 | 19·8 ± 0·2 | 3·7 ± 0·3 | 1·7 ± 0·3 | 4·8 ± 0·7 | 10·2 ± 0·6 | 61·5 ± 5·7 |
| +UVB | 60·1 ± 5·8 | 2·05 ± 0·05 | 10·4 ± 0·4 | 60·1 ± 0·2 | 19·5 ± 0·3 | 3·9 ± 0·5 | 1·8 ± 0·2 | 4·2 ± 0·6 | 9·9 ± 0·4 | 59·4 ± 6·2 | |
| D | Control | 48·2 ± 11·7 | 2·19 ± 0·07 | 10·0 ± 0·9 | 56·6 ± 0·5 | 18·4 ± 0·2 | 8·1 ± 0·5 | 2·2 ± 0·4 | 4·6 ± 1·4 | 15·0 ± 1·4 | 43·4 ± 7·1 |
| +UVB | 42·3 ± 9·7 | 2·26 ± 0·03 | 10·6 ± 0·3 | 55·7 ± 0·2 | 19·5 ± 0·3 ° | 8·1 ± 0·3 | 1·9 ± 0·1 | 4·1 ± 0·4 | 14·0 ± 0·3 | 41·2 ± 2·2 | |
| WR** | WR** | WR*** | WR × UV* | WR*** | WR*** | WR** | |||||
The content of individual carotenoids is expressed as a percentage of total carotenoids. Means ± s.e. are shown (n = 4). The overall effects (two-way ANOVA) of the water regime (WR) and the radiation regime (UV, UV-B effect), and the interactions between both factors (WR × UV), are indicated for each species when significant (***P < 0·001; **P < 0·01; *P < 0·05). Differences between control and +UV-B plots for each species and environment separately are shown beside the corresponding values (Student's t-test adjusted with Bonferroni; °P < 0·025).
The Chl a/b ratios remained unaffected by +UV-B in all species (Table 1). In contrast, both H. splendens and B. lycopodioides had significantly higher Chl a/b ratios in the drier site compared with the mesic site (ANOVA: P < 0·001 and P < 0·01, respectively; Table 1).
Impacts on PSII: Fv/Fm and ETR
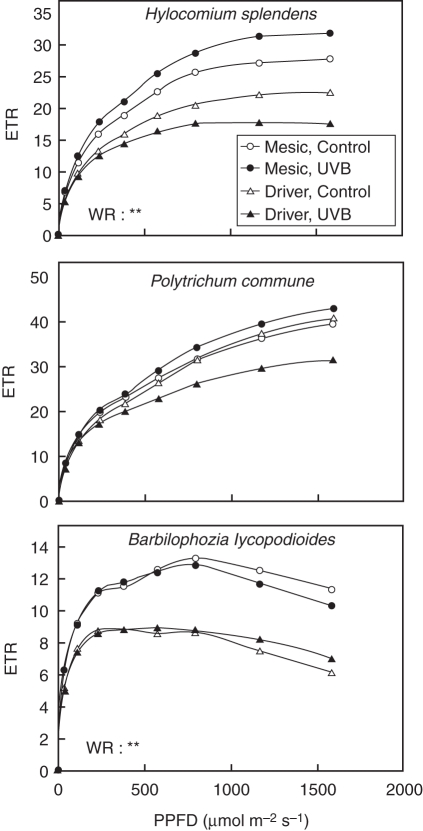
Both Fv/Fm and ETR of all species were unaffected by long-term +UV-B exposure (Figs 1D and 2). In contrast, the water regime significantly impacted these parameters, with both H. splendens and B. lycopodioides having higher Fv/Fm and ETR values overall in the mesic site compared with the drier site (ANOVA: Fv/Fm P < 0·01 and P < 0·05, respectively, for each species; ETR P < 0·01 for both species; Figs 1D and 2).
Fig. 2.
Curves of the electron transport rate through PSII (ETR) against photosynthetic photon flux density (PPFD); species and treatment details as for Fig. 1. The s.e. are not shown for clarity. The overall effect (two-way ANOVA) of the water regime (WR) is indicated when significant (*** P <0·001; **P < 0·01; *P < 0·05). The effect of the radiation regime (UV, UV-B effect) was not significant in any species.
Photoprotection mechanisms: carotenoids, NPQmax and MEUVACs
Few overall effects of +UV-B on carotenoids were observed and only in H. splendens (Table 1). Overall across both sites, +UV-B increased violaxanthin and decreased zeaxanthin, XC and DES in H. splendens (ANOVA: P < 0·05 in each case). Significant UV-B × site interactions (P < 0·01) revealed that in H. splendens, neoxanthin increased under +UV-B only in the drier site (t-test, P < 0·025 Bonferroni correction). UV-B × site interactions were also observed for β-carotene in H. splendens (P < 0·001) and B. lycopodioides (P < 0·05) that showed that β-carotene increased in H. splendens under +UV-B in the mesic site (Table 1; t-test, P < 0·025 Bonferroni correction) and in B. lycopodioides in the drier site (Table 1; t-test, P < 0·025 Bonferroni correction). Neoxanthin in P. commune and lutein in H. splendens increased under +UV-B in the drier site but decreased under +UV-B in the mesic site, again showing interaction between both factors (ANOVA interaction: P < 0·05 for both).
In comparison with +UV-B effects, many more and clearer differences were observed between the sites (i.e. the effect of contrasting water regime; Table 1). Site significantly affected most carotenoid concentrations in the three species (Table 1). There were significantly greater XC and violaxanthin concentrations in all bryophyte species in the drier site compared with the mesic site (ANOVA: P < 0·001 in all cases except P < 0·05 for both parameters in P. commune) and greater antheraxanthin concentrations in P. commune (P < 0·001) and H. splendens (P < 0·01). β-Carotene was also higher in H. splendens plants growing in the drier site (P < 0·001). In contrast, the relative concentration of lutein was significantly lower in all species growing at the drier site (P < 0·05 for P. commune and P < 0·001 for H. splendens and B. lycopodioides). Similarly, DES was significantly lower at the drier site in H. splendens and B. lycopodioides (P < 0·01 for both species).
The two-way ANOVA did not show any overall significant effect of +UV-B or site on NPQmax, although a significant UV-B × site interaction (P < 0·05) revealed a considerable increase in H. splendens under +UV-B (t-test, P < 0·005 Bonferroni correction) at the mesic site (Fig. 1E).
At the mesic site, +UV-B reduced MEUVACs by 12 % in B. lycopodioides (t-test, P < 0·025 Bonferroni correction) and slightly, although not significantly, increased it in H. splendens (Fig. 1F). Site also had significant effects on MEUVACs, with a 14 % increase in H. splendens (P < 0·05) and a 10 % decrease in B. lycopodioides (P < 0·01) in the drier site (Fig. 1F).
DISCUSSION
This study, using essentially identical long-term +UV-B experiments located in neighbouring areas with contrasting water availability (mesic vs. drier), shows that species biology and water supply influenced UV-B impacts on Arctic bryophytes. In particular, H. splendens was the most sensitive species to +UV-B whereas UV-B impacts on P. commune and B. lycopodioides were significantly more subtle. Site differences in water availability, although relatively small, had a more profound effect on bryophyte ecophysiology than +UV-B. Water availability also influenced bryophyte responses to +UV-B as responses were less detectable in the drier site. The large influence of relatively small differences in water availability probably contributes considerably to the wide variation of UV-B responses observed in other studies previously.
Bryophyte percentage cover and sporophyte abundance
Enhanced UV-B had no effect on the cover of the individual species in either the mesic or drier sites despite the considerable long-term +UV-B treatment. This agrees with other studies on sub-Arctic bryophytes (H. splendens, Polytrichum hyperboreum and Sanionia uncinata: Phoenix et al., 2001; Rozema et al., 2006). In contrast, bryophyte cover differed between sites (mesic vs. drier), reflecting their individual hydrological preferences. Polytrichum commune was less abundant in the drier site and B. lycopodioides in the wetter site. Hylocomium splendens showed no difference between sites (Callaghan et al., 1978; Damsholt, 2002; see also species description).
There was a very large impact of UV-B on sporophyte production in H. splendens (44 % reduction), but a much greater difference between sites as sporophytes were only produced at the mesic sites. Sporophyte production in this species is apparently very demanding of resources (Rydgren and Ökland, 2003) and the reduction may indicate that +UV-B causes some stress to this moss.
Annual growth and sclerophylly index
As found for sporophyte production, H. splendens also showed a considerable reduction in length growth under +UV-B (–22 %) but again only at the mesic site. Nevertheless, this species appears to be the most sensitive to +UV-B as growth of the other two bryophytes was unaffected. Greater responsiveness of H. splendens has also been observed by Gehrke (1999) and might be related to the strong flexibility in life strategy shown by eurioic species such as H. splendens (Grime, 1979). UV-B exposure caused subtle morphogenetic changes in P. commune and B. lycopodioides, but again only in the mesic site. The SI was lower for P. commune under +UV-B – the shoots were visibly less dense – but was higher for the liverwort.
Overall, the results highlight that the impacts of +UV-B on bryophyte growth and sclerophylly are species specific and less likely to be detected under drier conditions. This might well explain the variability in other studies of UV-B impacts on bryophyte growth where reductions (Gehrke 1998, 1999; Rozema et al., 2006), no effect (Björn et al., 1998; Sonesson et al., 2002; Lappalainen et al., 2008) or increases (Phoenix et al., 2001) have been reported. Similarly, for sclerophylly, Lappalainen et al. (2008) reported a decrease in Pleurozium schreberi while Gehrke (1999) found the opposite for P. commune.
Chlorophyll concentration
The impact of +UV-B on chlorophyll concentration was again species specific. There was no effect on P. commune and B. lycopodioides, a common result for polar bryophytes under field conditions (Boelen et al., 2006; Newsham and Robinson, 2009). However, chlorophyll concentration increased in H. splendens and, surprisingly, the increment was stronger in the drier site. Increased chlorophyll content under +UV-B was also previously observed in Sphagnum (Gehrke, 1998; Niemi et al., 2002a). The Chl a/b ratio was not affected by +UV-B in any species, in line with previous field studies (Gehrke, 1998, 1999).
The influence of water availability on chlorophyll concentration depended on the species considered and did not show an overall trend. However, the Chl a/b ratio was higher in H. splendens and B. lycopodioides at the drier site, possibly indicating a smaller antenna size to reduce the rate of light absorption and photodamage (Baroli and Melis, 1998).
Impacts on PSII: Fv/Fm and ETR
The Fv/Fm and ETR were not affected by +UV-B in any of the species, and this is typical for polar bryophytes (Boelen et al., 2006; Newsham and Robinson, 2009), with rapid adaptation to increased UV radiation being reported (Green et al., 2005). These results, taken together with other previous studies, suggest that photosynthetic performance is unlikely to be affected by +UV-B in Arctic bryophytes.
In contrast to this unresponsiveness to +UV-B, moderate photoinhibition (Fv/Fm decrease) was observed in H. splendens and B. lycopodioides growing in the drier site. Full recovery of hydrated plants was possibly achieved overnight and thus the reduction in Fv/Fm might represent an active photoprotection mechanism by increased protective energy dissipation (Horton et al., 1996). In this respect, the ETR was also significantly lower in the drier site in these two species, but no signs of full PSII closure (full reduction of PSII) at high light were detected, again suggesting that efficient dissipation mechanisms existed to avoid photodamage. In relation to these results, Proctor and Smirnoff (2011) recently showed that desiccation-tolerant moss species can maintain high rates of photosynthetic electron transport (high quantum efficiency of PSII) at high irradiance by oxygen photoreduction that is associated with a high capacity to dissipate excess energy (photoprotection) and ROS tolerance.
Photoprotection mechanisms: carotenoids, NPQmax and MEUVACs
Enhanced UV-B caused different changes in the carotenoid concentrations, especially in the most UV-B-sensitive species (H. splendens), with zeaxanthin tending to decrease and violaxanthin increasing, which is consistent with the results obtained in Antarctic bryophytes during periods of ozone depletion (Newsham et al., 2002). This may be related to the inhibition of violaxanthin de-epoxidation by UV-B radiation (Pfundel et al., 1992). Enhanced UV-B also increased the concentration of β-carotene in H. splendens as previously observed in Antarctic bryophytes (Robinson et al., 2005) but only at the mesic site, whereas there was a general increase in the drier site – possibly reflecting the importance of its antioxidant capacity to counteract stress in general in this moss.
Site (water regime) had a stronger effect on carotenoids than +UV-B. Species in the drier site had higher concentrations of violaxanthin and the XC pool, and reduced lutein, suggesting that these are important changes for tolerance of water stress in these bryophytes. This might be linked to xanthophylls, especially violaxanthin, being the major abscisic acid (ABA) precursors in water-stressed plants (Li and Walton, 1987), and ABA is an important molecule for desiccation tolerance in some bryophytes (Proctor et al., 2007). In addition, zeaxanthin and antheraxanthin are also required for constitutive thermal energy dissipation in plants under long-term environmental stress (Demmig-Adams and Adams, 2002).
NPQmax was unresponsive to both +UV-B and water availability, except for H. splendens in the mesic site which continued the trend that UV-B impacts were more common in H. splendens and less likely under drier conditions.
There were no clear effects of +UV-B on MEUVAC concentration. Published results are highly variable, with concentration increases, decreases or no change reported (Newsham et al., 2002; Niemi et al., 2002b; Newsham, 2003; Arróniz-Crespo et al., 2008b). These diverse responses may reflect not only interspecific differences and interaction with other environmental factors as shown in our study but also the lack of extraction of cell wall-bound compounds (Clarke and Robinson, 2008). Overall, measurements of MEUVACs do not necessarily provide a good indicator of long-term UV-B tolerance in the species studied here.
In conclusion, our study shows that impacts of UV-B exposure on Arctic bryophytes were significant, compared with previously published modest or absent UV-B effects, and were more readily detectable in species with high plasticity such as H. splendens and less likely, or more subtle, under drier conditions. Overall, species biology and even small differences in water supply can greatly influence the impact of UV-B on Arctic bryophytes.
ACKNOWLEDGEMENTS
This work was funded by the Royal Society UK (Research Grant 2006/R1). Long-term funding for the experiments has been provided by the Swedish Environmental Protection Agency, the Royal Swedish Academy of Sciences and the EU (UVECOS: contract EV5V-CT910032). M.A.-C. benefited from a postdoctoral grant from the Ministerio de Educación y Ciencia of Spain and an ATANS grant from the Abisko Scientific Research Station. G.K.P. was funded by an RCUK academic fellowship. E.N.O., J.M.A. and M.A.-C. are grateful to the Ministerio de Ciencia e Innovación of Spain (Project CGL2008-04450) for financial support. T. G. A. Green provided helpful comments on the manuscript. We are indebted to L. O. Björn and M. Sonesson who, together with T. V. Callaghan, were PIs on the 1991 experiment, and also to J. A. Lee, PI on the 1993 experiment. We thank staff at the Abisko Scientific Research Station for help and support.
LITERATURE CITED
- Arróniz-Crespo M, Núñez-Olivera E, Martínez-Abaigar J, et al. Physiological changes and UV protection in the aquatic liverwort Jungermannia exsertifolia subsp. cordifolia along an altitudinal gradient of UV-B radiation. Functional Plant Biology. 2006;33:1025–1036. doi: 10.1071/FP06096. [DOI] [PubMed] [Google Scholar]
- Arróniz-Crespo M, Leake JR, Horton P, Phoenix GK. Bryophyte physiological responses to, and recovery from, long-term nitrogen deposition and phosphorus fertilisation in acidic grassland. New Phytologist. 2008a;180:864–874. doi: 10.1111/j.1469-8137.2008.02617.x. [DOI] [PubMed] [Google Scholar]
- Arróniz-Crespo M, Núñez-Olivera E, Martínez-Abaigar J. Hydroxycinnamic acid derivatives in an aquatic liverwort as possible bioindicators of enhanced UV radiation. Environmental Pollution. 2008b;151:8–16. doi: 10.1016/j.envpol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Baroli I, Melis A. Photoinhibitory damage is modulated by the rate of photosynthesis and by the photosystem II light-harvesting chlorophyll antenna size. Planta. 1998;205:288–296. doi: 10.1007/s004250050323. [DOI] [PubMed] [Google Scholar]
- Björn LO, Callaghan TV, Gehrke C, Johanson U, Sonesson M, Gwynn-Jones D. The problem of ozone depletion in northern Europe. Ambio. 1998;27:275–279. [Google Scholar]
- Boelen P, De Boer MK, De Bakker NVJ, Rozema J. Outdoor studies on the effects of solar UV-B on bryophytes: overview and methodology. Plant Ecology. 2006;182:137–152. [Google Scholar]
- Caldwell MM. Solar UV irradiation and the growth and development of higher plants. In: Giese AC, editor. Photophysiology: current topics in photobiology and photochemistry. Vol. 6. New York: Academic Press; 1971. pp. 131–177. [Google Scholar]
- Caldwell MM, Teramura AH, Tevini M, Bornman JF, Björn LO, Kulandaivelu G. Effects of increased solar ultraviolet radiation on terrestrial plants. Ambio. 1995;24:166–173. [Google Scholar]
- Callaghan TV, Collins NJ, Callaghan CH. Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish Lapland. Oikos. 1978;31:73–88. [Google Scholar]
- Callaghan TV, Bjorn LO, Chernov Y, et al. Responses to projected changes in climate and UV-B at the species level. Ambio. 2004;33:418–435. doi: 10.1579/0044-7447-33.7.418. [DOI] [PubMed] [Google Scholar]
- Clarke LJ, Robinson SA. Cell wall-bound ultraviolet-screening compounds explain the high ultraviolet tolerance of the Antarctic moss, Ceratodon purpureus. New Phytologist. 2008;179:776–783. doi: 10.1111/j.1469-8137.2008.02499.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Lang S, Soudzilovskaia N, During HJ. Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Annals of Botany. 2007;99:987–1001. doi: 10.1093/aob/mcm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsholt K. Illustrated flora of nordic liverworts and hornworts. Lund, Sweden: Nordic Bryological Society; 2002. [Google Scholar]
- Demmig-Adams B, III, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Science. 1996;1:21–26. [Google Scholar]
- Demmig-Adams B, III, Adams WW., III Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2149–2153. doi: 10.1126/science.1078002. [DOI] [PubMed] [Google Scholar]
- Gehrke C, Johanson U, Gwynn-Jones D, Björn LO, Callaghan TV, Lee JA. Effects of enhanced ultraviolet-B radiation on terrestrial subarctic ecosystems and implications for interactions with increased atmospheric CO2. Ecological Bulletin. 1996;45:192–203. [Google Scholar]
- Gehrke C. Effects of enhanced UV-B radiation on production related properties of a Sphagnum fuscum dominated subarctic bog. Functional Ecology. 1998;12:940–947. [Google Scholar]
- Gehrke C. Impacts of enhanced ultraviolet-B radiation on mosses in a subarctic heath ecosystem. Ecology. 1999;80:1844–1851. [Google Scholar]
- Green TGA, Kulle D, Pannewitz S, Sancho LG, Schroeter B. UV-A protection in mosses growing in continental Antarctica. Polar Biology. 2005;28:822–827. [Google Scholar]
- Grime JP. Plant strategies and vegetation processes. Chichester, UK: Wiley; 1979. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Johanson U, Gehrke C, Björn LO, Callaghan TV, Sonesson M. The effects of enhanced UV-B radiation on a subarctic heath ecosystem. Ambio. 1995;24:106–111. [Google Scholar]
- Lappalainen NM, Huttunen S, Suokanerva H. Acclimation of a pleurocarpous moss Pleurozium schreberi (Britt.) Mitt. to enhanced ultraviolet radiation in situ. Global Change Biology. 2008;14:321–333. [Google Scholar]
- Li Y, Walton DC. Xanthophylls and abscisic acid biosynthesis in water-stressed bean leaves. Plant Physiology. 1987;85:910–915. doi: 10.1104/pp.85.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longton RE. The role of bryophytes and lichens in polar ecosystems. In: Woodin SJ, Marquiss M, editors. Ecology of Arctic environments. Oxford: Blackwell Science Ltd; 1997. pp. 69–96. [Google Scholar]
- Martínez-Abaigar J, Núñez-Olivera E, Sánchez-Díaz M. Seasonal changes in photosynthetic pigment composition of aquatic bryophytes. Journal of Bryology. 1994;18:97–113. [Google Scholar]
- Newsham KK. UV-B radiation arising from stratospheric ozone depletion influences the pigmentation of the Antarctic moss Andreaea regularis. Oecologia. 2003;135:327–331. doi: 10.1007/s00442-003-1191-x. [DOI] [PubMed] [Google Scholar]
- Newsham KK, Robinson SA. Responses of plants in polar regions to UVB exposure: a meta-analysis. Global Change Biology. 2009;15:2574–2589. [Google Scholar]
- Newsham KK, Hodgson DA, Murray AWA, Peat HJ, Lewis Smith RI. Response of two Antarctic bryophytes to stratospheric ozone depletion. Global Change Biology. 2002;8:972–983. [Google Scholar]
- Niemi R, Martikainen PJ, Silvola J, Sonninen E, Wulff A, Holopainen T. Responses of two Sphagnum moss species and Eriophorum vaginatum to enhanced UV-B in a summer of low UV intensity. New Phytologist. 2002a;156:509–515. doi: 10.1046/j.1469-8137.2002.00532.x. [DOI] [PubMed] [Google Scholar]
- Niemi R, Martikainen PJ, Silvola J, Wulff A, Turtola S, Holopainen T. Elevated UV-B radiation alters fluxes of methane and carbon dioxide in peatland microcosms. Global Change Biology. 2002b;8:361–371. [Google Scholar]
- Núñez-Olivera E, Martínez-Abaigar J, Tomás R, Beaucourt N, Arróniz-Crespo M. Influence of temperature on the effects of artificially enhanced UV-B radiation on aquatic bryophytes under laboratory conditions. Photosynthetica. 2004;42:201–212. [Google Scholar]
- Oliver MJ. Biochemical and molecular mechanisms of desiccation tolerance in bryophytes. In: Goffinet B, Shaw AJ, editors. Bryophyte biology. Cambridge: Cambridge University Press; 2009. pp. 269–297. [Google Scholar]
- Paul ND, Gwynn-Jones D. Ecological roles of solar UV radiation: xtowards an integrated approach. Trends in Ecology and Evolution. 2003;18:48–55. [Google Scholar]
- Pfundel EE, Pan RS, Dilley RA. Inhibition of violaxanthin deepoxidation by ultraviolet-B radiation in isolated-chloroplasts and intact leaves. Plant Physiology. 1992;98:1372–1380. doi: 10.1104/pp.98.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix GK, Gwynn-Jones D, Callaghan TV, Sleep D, Lee JA. Effects of global change on a sub-Arctic heath: effects of enhanced UV-B radiation and increased summer precipitation. Journal of Ecology. 2001;89:256–267. [Google Scholar]
- Proctor MCF. Physiological ecology. In: Goffinet B, Shaw AJ, editors. Bryophyte biology. Cambridge: Cambridge University Press; 2009. pp. 237–268. [Google Scholar]
- Proctor MCF, Smirnoff N. Ecophysiology of photosynthesis in bryophytes: major roles for oxygen photoreduction and non-photochemical quenching? Physiologia Plantarum. 2011;141:130–140. doi: 10.1111/j.1399-3054.2010.01424.x. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, et al. Desiccation-tolerance in bryophytes: a review. Bryologist. 2007;110:595–621. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Robinson SA, Turnbull JD, Lovelock CE. Impact of changes in natural ultraviolet radiation on pigment composition, physiological and morphological characteristics of the Antarctic moss, Grimmia antarctici. Global Change Biology. 2005;11:476–489. [Google Scholar]
- Rozema J, Boelen P, Blokker P. Depletion of stratospheric ozone over the Antarctic and Arctic: responses of plants of polar terrestrial ecosystems to enhanced UV-B, an overview. Environmental Pollution. 2005;137:428–442. doi: 10.1016/j.envpol.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Rozema J, Boelen P, Solheim B, et al. Stratospheric ozone depletion: high arctic tundra plant growth on Svalbard is not affected by enhanced UV-B after 7 years of UV-B supplementation in the field. Plant Ecology. 2006;182:121–135. [Google Scholar]
- Rydgren K, Okland RH. Short-term costs of sexual reproduction in the clonal moss Hylocomium splendens. Bryologist. 2003;106:212–220. [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM, editors. Ecophysiology of photosynthesis. Berlin: Springer; 1995. pp. 49–70. [Google Scholar]
- Sonesson M, Carlsson BA, Callaghan TV, et al. Growth of two peat-forming mosses in subarctic mires: species interactions and effects of simulated climate change. Oikos. 2002;99:151–160. [Google Scholar]
- Turnbull JD, Leslie SJ, Robinson SA. Desiccation protects two Antarctic mosses from ultraviolet-B induced DNA damage. Functional Plant Biology. 2009;36:214–221. doi: 10.1071/FP08286. [DOI] [PubMed] [Google Scholar]
- Weatherhead B, Tanskanen A, Stevermer A, et al. ACIA. Arctic climate impact assessment. Cambridge: Cambridge University Press; 2005. Ozone and ultraviolet radiation; pp. 151–182. [Google Scholar]
- World Meteorological Organization. Scientific assessment of ozone depletion: 2006. Global Ozone Research and Monitoring Project-Report No. 50. 2007 NOAA, NASA, UNEP, WMO, EC. Available at http://www.esrl.noaa.gov/csd/assessments/2006/report.html . [Google Scholar]
- Woodin SJ, Van der Wal R, Sommerkorn M, Gornall JL. Differential allocation of carbon in mosses and grasses governs ecosystem sequestration: a 13C tracer study in the high Arctic. New Phytologist. 2009;184:944–949. doi: 10.1111/j.1469-8137.2009.03022.x. [DOI] [PubMed] [Google Scholar]